Invasive Meningococcal Disease in the Post–COVID-19 Era in South America
Abstract
1. Introduction
2. Methods
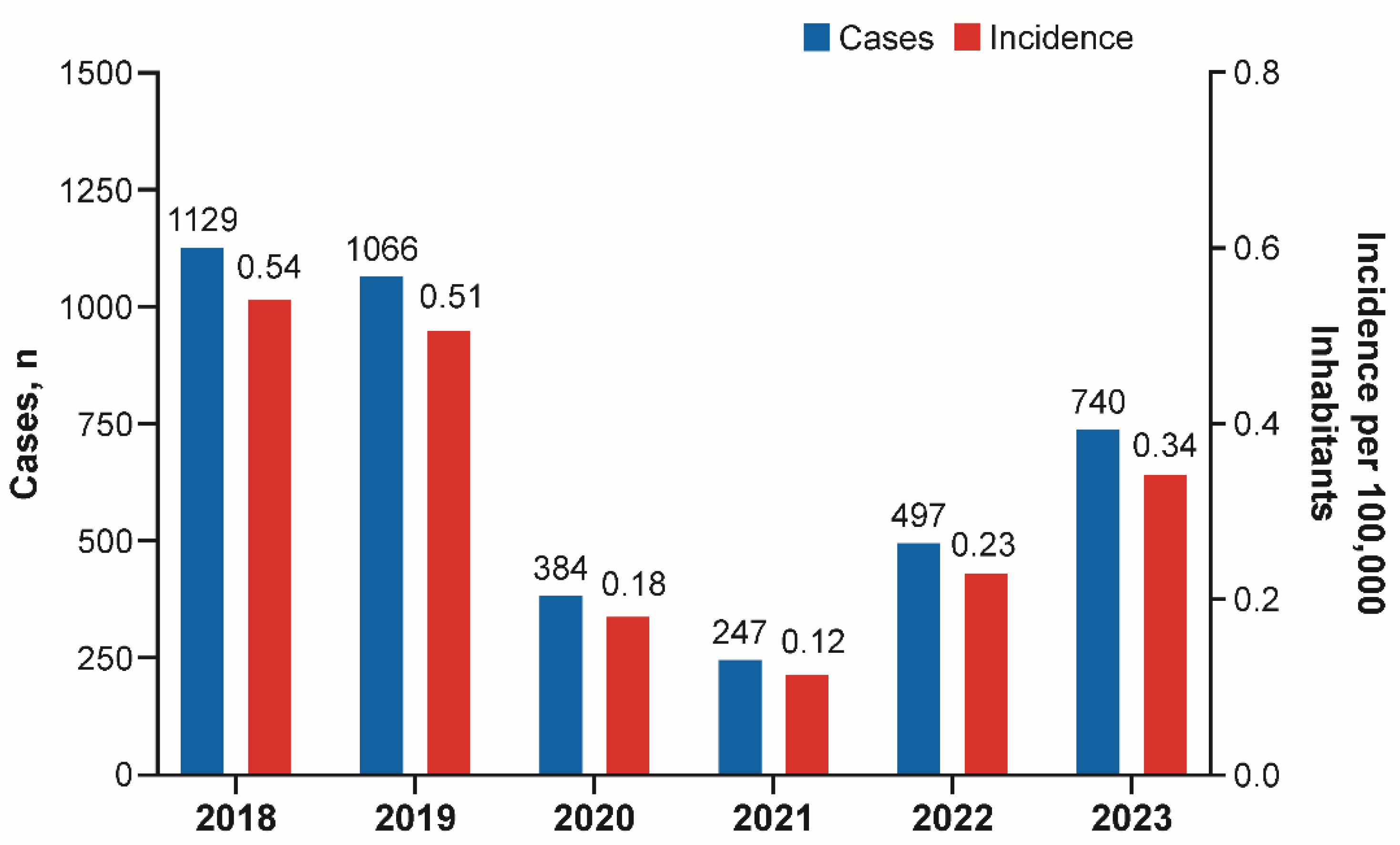
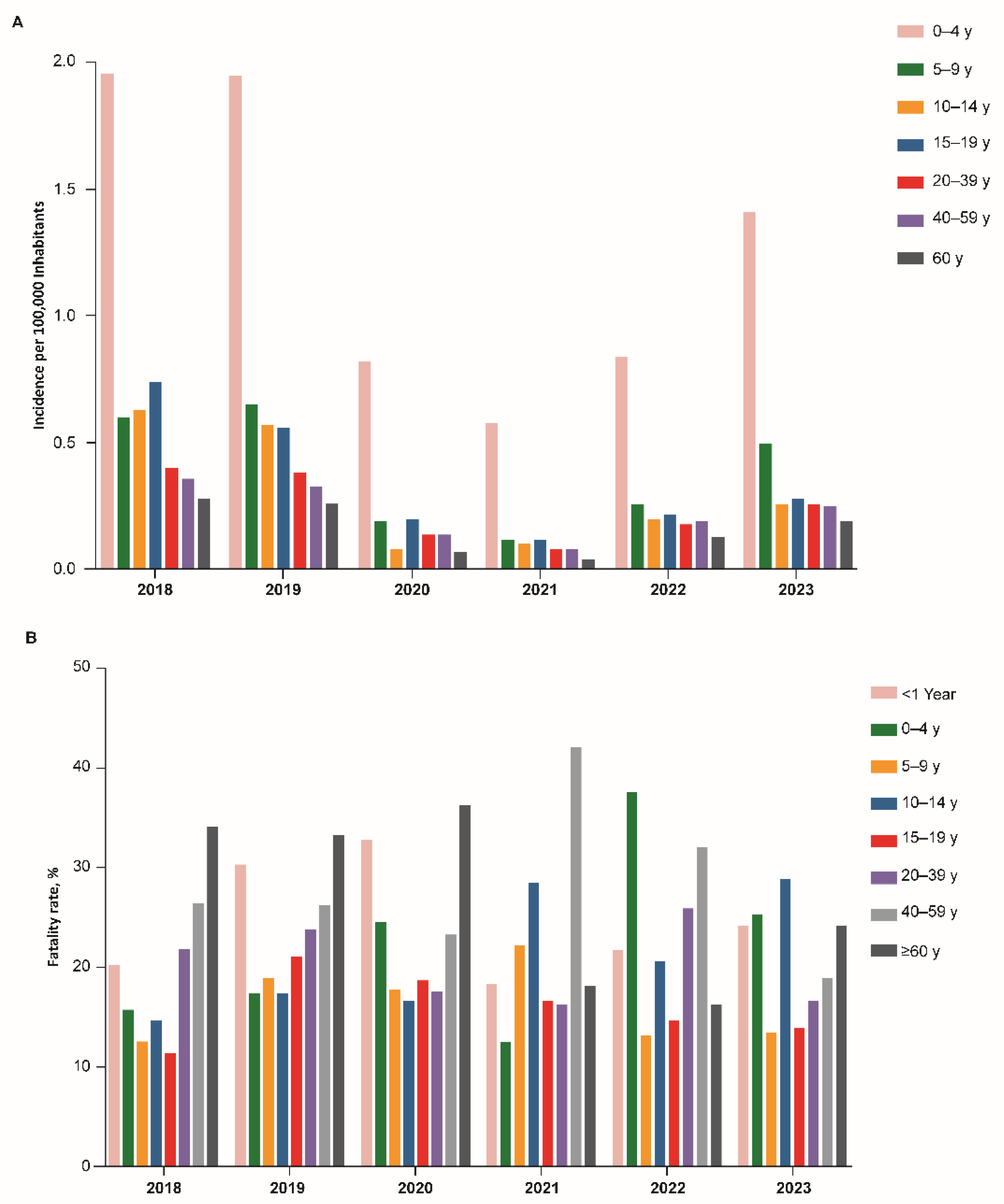
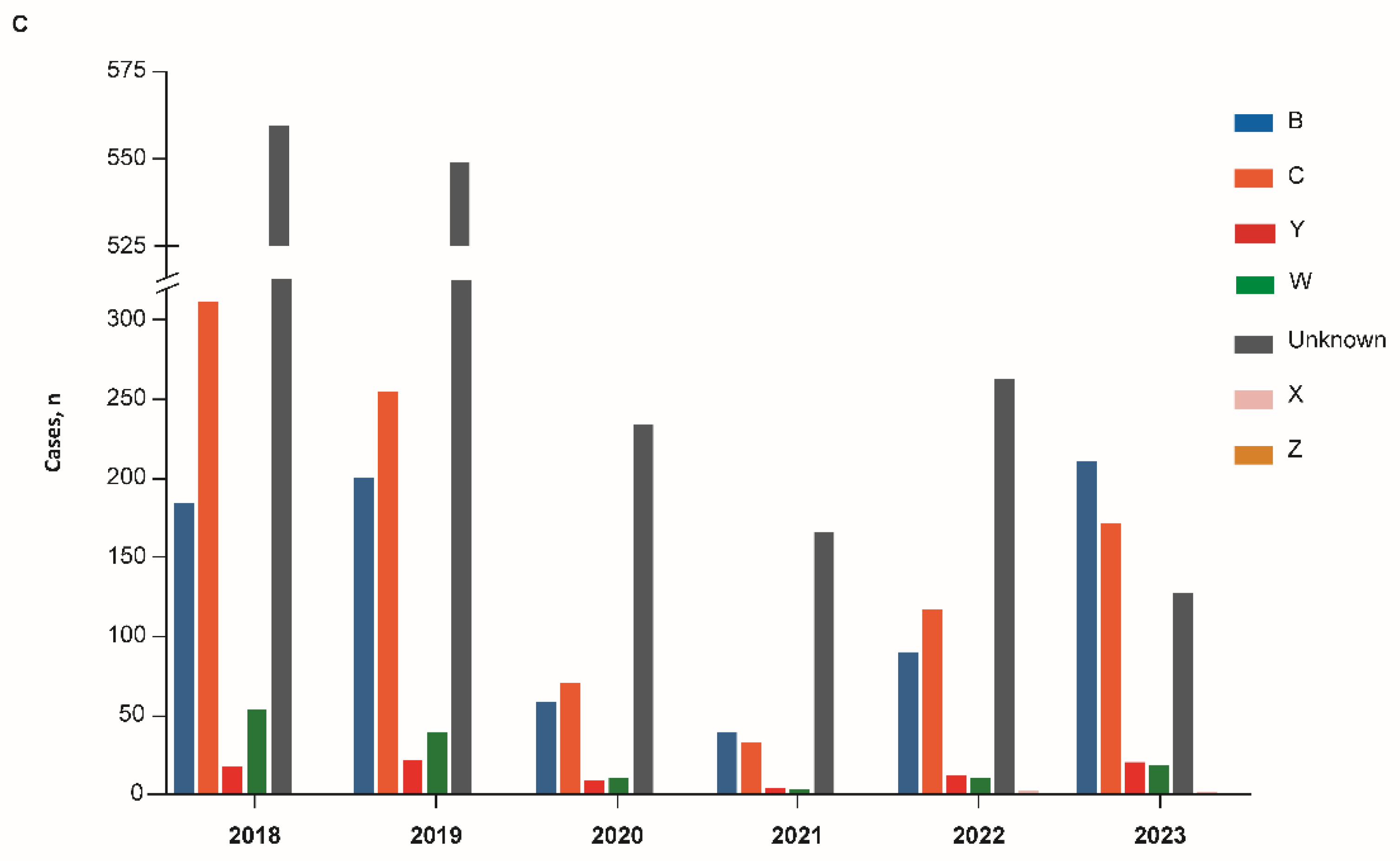
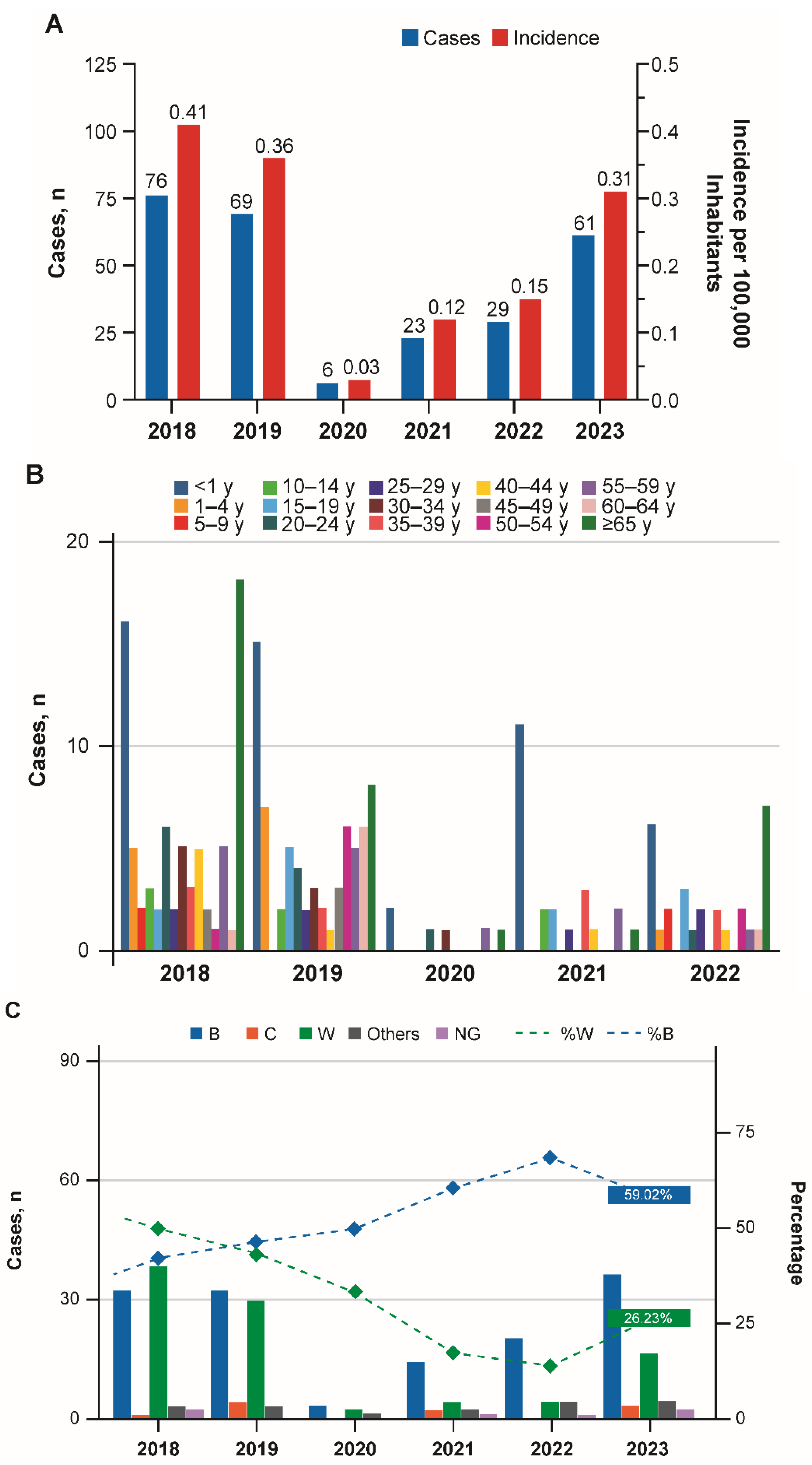
3. Results
3.1. Argentina
3.2. Brazil
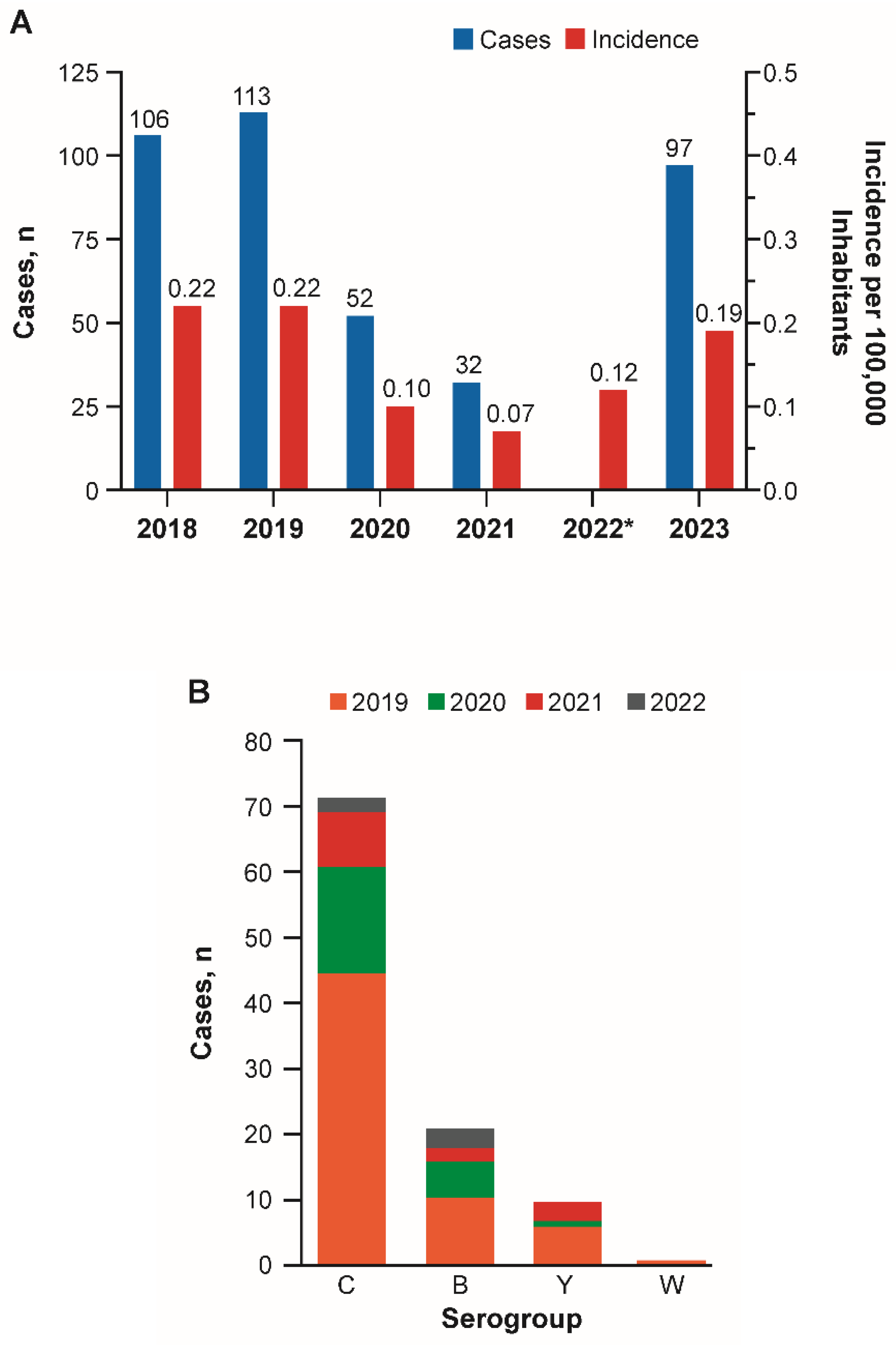
3.3. Chile
3.4. Colombia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IMD | Invasive meningococcal disease |
| IRIS | Invasive Respiratory Infection Surveillance |
| MenA | Monovalent polysaccharide conjugate serogroup A vaccine |
| MenACWY | Polysaccharide conjugate serogroups A, C, W, and Y vaccine |
| MenB | Protein-based serogroup B vaccine |
| MenC | Monovalent polysaccharide conjugate serogroup C vaccine |
| NIP | National immunization program |
| RSV | Respiratory syncytial virus |
| SIREVA II | Surveillance Network System of Agents Responsible for Bacterial Pneumonia and Meningitis |
| WHO | World Health Organization |
References
- Deghmane, A.-E.; Taha, S.; Taha, M.-K. Global epidemiology and changing clinical presentations of invasive meningococcal disease: A narrative review. Infect. Dis. 2022, 54, 1–7. [Google Scholar] [CrossRef]
- Martinón-Torres, F. Deciphering the burden of meningococcal disease: Conventional and under-recognized elements. J. Adolesc. Health 2016, 59, S12–S20. [Google Scholar] [CrossRef]
- Arteta-Acosta, C.; Villena Martinez, R.; Santolaya de Pablo, M.E. Sequelae at hospital discharge in 61 children with invasive meningococcal disease, Chile, 2009–2019. Pediatr. Infect. Dis. J. 2022, 41, 607–613. [Google Scholar] [CrossRef]
- Olbrich, K.J.; Muller, D.; Schumacher, S.; Beck, E.; Meszaros, K.; Koerber, F. Systematic review of invasive meningococcal disease: Sequelae and quality of life impact on patients and their caregivers. Infect. Dis. Ther. 2018, 7, 421–438. [Google Scholar] [CrossRef]
- Wang, B.; Santoreneos, R.; Giles, L.; Haji Ali Afzali, H.; Marshall, H. Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine 2019, 37, 2768–2782. [Google Scholar] [CrossRef]
- Pardo de Santayana, C.; Tin Tin Htar, M.; Findlow, J.; Balmer, P. Epidemiology of invasive meningococcal disease worldwide from 2010–2019: A literature review. Epidemiol. Infect. 2023, 151, e57. [Google Scholar] [CrossRef] [PubMed]
- Guedes, S.; Bertrand-Gerentes, I.; Evans, K.; Coste, F.; Oster, P. Invasive meningococcal disease in older adults in North America and Europe: Is this the time for action? A review of the literature. BMC Public Health 2022, 22, 380. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Ma, J.H.; Warren, K.; Tsang, R.S.; Low, D.E.; Jamieson, F.B.; Alexander, D.C. Extensive genomic variation within clonal complexes of Neisseria meningitidis. Genome Biol. Evol. 2011, 3, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.M.; Lucidarme, J.; Gray, S.J.; Newbold, L.S.; Ure, R.; Brehony, C.; Harrison, O.B.; Bray, J.E.; Jolley, K.A.; Bratcher, H.B.; et al. Genomic epidemiology of age-associated meningococcal lineages in national surveillance: An observational cohort study. Lancet Infect. Dis. 2015, 15, 1420–1428. [Google Scholar] [CrossRef]
- Jafri, R.Z.; Ali, A.; Messonnier, N.E.; Tevi-Benissan, C.; Durrheim, D.; Eskola, J.; Fermon, F.; Klugman, K.P.; Ramsay, M.; Sow, S.; et al. Global epidemiology of invasive meningococcal disease. Popul. Health Metr. 2013, 11, 11–17. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Invasive Meningococcal Disease—Annual Epidemiological Report for 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/invasive-meningococcal-disease-annual-epidemiological-report-2022 (accessed on 25 June 2025).
- Peterson, M.E.; Li, Y.; Bita, A.; Moureau, A.; Nair, H.; Kyaw, M.H.; Meningococcal Surveillance Group; Abad, R.; Bailey, F.; Garcia, I.F.; et al. Meningococcal serogroups and surveillance: A systematic review and survey. J. Glob. Health 2019, 9, 010409. [Google Scholar] [CrossRef]
- Rivacoba, M.C.; Villena, R.; Hormazabal, J.C.; Benadof, D.; Paya, E.; Valdivieso, F.; Canals, A.; Arteta-Acosta, C.; Santolaya, M.E. Hypervirulent strains of Neisseria meningitidis and clinical manifestations in children with invasive meningococcal disease. Pediatr. Infect. Dis. J. 2023, 42, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Safadi, M.A.P.; Martinon-Torres, F.; Weckx, L.Y.; Moreira, E.D.J.; da Fonseca Lima, E.J.; Mensi, I.; Calabresi, M.; Toneatto, D. Immunogenicity and safety of concomitant administration of meningococcal serogroup B (4CMenB) and serogroup C (MenC-CRM) vaccines in infants: A phase 3b, randomized controlled trial. Vaccine 2017, 35, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Begum, N.; Ruiz-Garcia, Y.; Martinon-Torres, F.; Bekkat-Berkani, R.; Meszaros, K. Range of invasive meningococcal disease sequelae and health economic application—A systematic and clinical review. BMC Public Health 2022, 22, 1078. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.P.; Crowe, S.J.; Ortega-Sanchez, I.R.; Bahta, L.; Campos-Outcalt, D.; Loehr, J.; Morgan, R.L.; Poehling, K.A.; McNamara, L.A. Use of the Pfizer pentavalent meningococcal vaccine among persons aged ≥10 years: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Haidara, F.C.; Umesi, A.; Sow, S.O.; Ochoge, M.; Diallo, F.; Imam, A.; Traore, Y.; Affleck, L.; Doumbia, M.F.; Daffeh, B.; et al. Meningococcal ACWYX conjugate vaccine in 2-to-29-year-olds in Mali and Gambia. N. Engl. J. Med. 2023, 388, 1942–1955. [Google Scholar] [CrossRef]
- World Health Organization. List of Prequalified Vaccines. Available online: https://extranet.who.int/gavi/PQ_Web/Browse.aspx?nav=3 (accessed on 25 June 2025).
- Aguinaga-Ontoso, I.; Guillen-Aguinaga, S.; Guillen-Aguinaga, L.; Alas-Brun, R.; Aguinaga-Ontoso, E.; Rayon-Valpuesta, E.; Guillen-Grima, F. Has COVID-19 affected DTP3 vaccination in the Americas? Vaccines 2024, 12, 238. [Google Scholar] [CrossRef]
- Avila Aguero, M.L.; Castillo, J.B.D.; Falleiros-Arlant, L.H.; Berezin, E.; de Moraes, J.C.; Torres-Martinez, C.; Lopez, E.L.; Castillo, M.E.; Laris-Gonzalez, A.; Solorzano, F.; et al. Risks of low vaccination coverage and strategies to prevent the resurgence of vaccine-preventable diseases in infants in the COVID-19 pandemic scenario: Recommendations for Latin America and the Caribbean by the group of experts on infant immunization for Latin America. Expert Rev. Vaccines 2023, 22, 1091–1101. [Google Scholar] [CrossRef]
- Shaw, D.; Abad, R.; Amin-Chowdhury, Z.; Bautista, A.; Bennett, D.; Broughton, K.; Cao, B.; Casanova, C.; Choi, E.H.; Chu, Y.W.; et al. Trends in invasive bacterial diseases during the first 2 years of the COVID-19 pandemic: Analyses of prospective surveillance data from 30 countries and territories in the IRIS Consortium. Lancet Digit. Health 2023, 5, e582–e593. [Google Scholar] [CrossRef]
- Alderson, M.R.; Arkwright, P.D.; Bai, X.; Black, S.; Borrow, R.; Caugant, D.A.; Dinleyici, E.C.; Harrison, L.H.; Lucidarme, J.; McNamara, L.A.; et al. Surveillance and control of meningococcal disease in the COVID-19 era: A Global Meningococcal Initiative review. J. Infect. 2022, 84, 289–296. [Google Scholar] [CrossRef]
- Brueggemann, A.B.; Jansen van Rensburg, M.J.; Shaw, D.; McCarthy, N.D.; Jolley, K.A.; Maiden, M.C.J.; van der Linden, M.P.G.; Amin-Chowdhury, Z.; Bennett, D.E.; Borrow, R.; et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: A prospective analysis of surveillance data. Lancet Digit. Health 2021, 3, e360–e370. [Google Scholar] [CrossRef]
- Rybak, A.; Levy, C.; Angoulvant, F.; Auvrignon, A.; Gembara, P.; Danis, K.; Vaux, S.; Levy-Bruhl, D.; van der Werf, S.; Bechet, S.; et al. Association of nonpharmaceutical interventions during the COVID-19 pandemic with invasive pneumococcal disease, pneumococcal carriage, and respiratory viral infections among children in France. JAMA Netw. Open 2022, 5, e2218959. [Google Scholar] [CrossRef] [PubMed]
- Steens, A.; Knol, M.J.; Freudenburg-de Graaf, W.; de Melker, H.E.; van der Ende, A.; van Sorge, N.M. Pathogen- and type-specific changes in invasive bacterial disease epidemiology during the first year of the COVID-19 pandemic in the Netherlands. Microorganisms 2022, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.A.; Campbell, H.; Ribeiro, S.; Bertran, M.; Walsh, L.; Walker, A.; Willerton, L.; Lekshmi, A.; Bai, X.; Lucidarme, J.; et al. Epidemiological and strain characteristics of invasive meningococcal disease prior to, during and after COVID-19 pandemic restrictions in England. J. Infect. 2023, 87, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Findlow, J.; Htar, M.T.T.; Villena, R.; Balmer, P. Invasive meningococcal disease in the post-COVID world: Patterns of disease rebound. Vaccines 2025, 13, 165. [Google Scholar] [CrossRef]
- Villena, R.; Safadi, M.A.; Gentile, A.; Pujadas, M.; De la Maza, V.; George, S.; Torres, J.P. Epidemiology of meningococcal disease in four South American countries and rationale of vaccination in adolescents from the region: Position paper of the Latin American Society of Pediatric Infectious Diseases (SLIPE). Vaccines 2023, 11, 1841. [Google Scholar] [CrossRef]
- Antimicrobial Service Argentina. Category: SIREVA Network—Argentina. Available online: http://antimicrobianos.com.ar/category/resistencia/sireva/ (accessed on 27 September 2024).
- Ministry of Health Argentina. National Epidemiological Bulletin. Available online: https://www.argentina.gob.ar/salud/boletin-epidemiologico-nacional/boletines-2024 (accessed on 1 July 2024).
- Ministry of Health Brazil. Meningitis—Confirmed Cases Notified in the Notifiable Diseases Information System—Brazil. Available online: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinannet/cnv/meninbr.def (accessed on 27 September 2024).
- Ministry of Health Brazil. Projection of the Population of the Federation Units by Sex, Simple Age or Age Group: 2000–2070 (2024 Edition). Available online: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?ibge/cnv/projpop2024uf.def (accessed on 27 January 2025).
- Ministry of Health Brazil. Vaccination Schedule. Available online: https://www.gov.br/saude/pt-br/vacinacao/calendario (accessed on 25 June 2025).
- Tan, L.L.J.; Safadi, M.A.P.; Horn, M.; Regojo Balboa, C.; Moya, E.; Schanbaum, J.; Pimenta, P.; Lambert, E.; Soumahoro, L.; Sohn, W.Y.; et al. Pandemic’s influence on parents’ attitudes and behaviors toward meningococcal vaccination. Hum. Vaccines Immunother. 2023, 19, 2179840. [Google Scholar] [CrossRef]
- Ministry of Health Brazil. Immunizations—Coverage—Brazil. Available online: http://tabnet.datasus.gov.br/cgi/dhdat.exe?bd_pni/cpnibr.def (accessed on 30 September 2024).
- Instituto de Salud Pública de Chile. Informe de Resultados de Vigilancia de Laboratorio Enfermedad Invasora Neisseria meningitidis 2019. Available online: https://www.ispch.cl/sites/default/files/Informe%20Neisseria%20meningitidis%20%20SE%201-30-2019%20%281%29%20%282%29.pdf (accessed on 25 June 2025).
- Instituto de Salud Pública de Chile. Informe de Resultados de Vigilancia de Laboratorio Enfermedad Invasora Neisseria meningitidis 2020. Available online: https://www.ispch.gob.cl/wp-content/uploads/2021/02/Informe-Neisseria-meningitidis-SE-1-53-2020-v1-1.pdf (accessed on 29 February 2024).
- Instituto de Salud Pública de Chile. Informe de Resultados de Vigilancia de Laboratorio Enfermedad Invasora Neisseria meningitidis. Chile. 2023. Available online: https://www.ispch.cl/wp-content/uploads/2024/03/Informe-Neisseria-meningitidis-SE-1-52-2023_08febrero2024.pdf (accessed on 6 June 2024).
- Instituto de Salud Pública de Chile. Vigilancia de Laboratorio Enfermedad Invasora Neisseria meningitidis 2012–2022. Available online: https://www.ispch.cl/wp-content/uploads/2023/04/BoletinNeisseriaMeningitidis01-05042023A-1-1-2.pdf (accessed on 25 June 2025).
- Instituto de Salud Pública de Chile. Programa Nacional de Immunizaciones. Available online: https://www.minsal.cl/programa-nacional-de-inmunizaciones/ (accessed on 1 July 2024).
- Ministry of Health of Chile. Preliminary Coverages. Available online: https://vacunas.minsal.cl/coberturas-preliminares/ (accessed on 27 September 2024).
- National Insititute of Health Colombia. Publications—Event Reports. Available online: https://www.ins.gov.co/buscador-eventos/Informesdeevento/Forms/AllItems.aspx (accessed on 30 September 2024).
- National Institute of Health Colombia. Event Report, Bacterial Meningitis and Meningococcal Disease, Epidemiological Periods XIII 2023. Available online: https://www.ins.gov.co/buscador-eventos/Informesdeevento/MENINGITIS%20BACTERIANA%20PE%20XIII%202023.pdf (accessed on 30 June 2025).
- Organisation for Economic Co-Operation and Development. COVID-19 in Latin America and the Caribbean: An Overview of Government Responses to the Crisis. Available online: https://www.oecd.org/en/publications/covid-19-in-latin-america-and-the-caribbean-an-overview-of-government-responses-to-the-crisis_0a2dee41-en.html (accessed on 25 June 2025).
- Presa, J.; Carrico, R.; Fergie, J.E.; Hanenberg, S.; Marshall, G.S.; Rivard, K.; Shaw, J.; Zimet, G.D.; Peyrani, P.; Cane, A. Preventing Meningococcal Disease in US Adolescents and Young Adults Through Vaccination. Infect. Dis. Ther. 2025, 14, 1381–1403. [Google Scholar] [CrossRef]
- Christensen, H.; May, M.; Bowen, L.; Hickman, M.; Trotter, C.L. Meningococcal carriage by age: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 853–861. [Google Scholar] [CrossRef]
- Graña, M.G.; Cavada, G.; Vasquez, M.; Shen, J.; Maervoet, J.; Klint, J.; Gómez, J.A. Modeling the public health impact of different meningococcal vaccination strategies with 4CMenB and MenACWY versus the current toddler MenACWY National Immunization Program in Chile. Hum. Vaccines Immunother. 2021, 17, 5603–5613. [Google Scholar] [CrossRef]
- UNICEF. COVID-19 Pandemic Leads to Major Backsliding on Childhood Vaccinations, New WHO, UNICEF Data Shows. Available online: https://www.unicef.org/press-releases/covid-19-pandemic-leads-major-backsliding-childhood-vaccinations-new-who-unicef-data (accessed on 21 December 2022).
- Cohen, R.; Ashman, M.; Taha, M.K.; Varon, E.; Angoulvant, F.; Levy, C.; Rybak, A.; Ouldali, N.; Guiso, N.; Grimprel, E. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect. Dis. Now 2021, 51, 418–423. [Google Scholar] [CrossRef]
- Bloom, D.E.; Bonanni, P.; Martinon-Torres, F.; Richmond, P.C.; Safadi, M.A.P.; Salisbury, D.M.; Charos, A.; Schley, K.; Findlow, J.; Balmer, P. Meningococcal disease in the post-COVID-19 era: A time to prepare. Infect. Dis. Ther. 2023, 12, 2649–2663. [Google Scholar] [CrossRef]
- Houghton, N.; Bascolo, E.; Del Riego, A. Socioeconomic inequalities in access barriers to seeking health services in four Latin American countries. Rev. Panam. Salud Publica = Pan Am. J. Public Health 2020, 44, e11. [Google Scholar] [CrossRef]
- Roberti, J.; Leslie, H.H.; Doubova, S.V.; Ranilla, J.M.; Mazzoni, A.; Espinoza, L.; Calderon, R.; Arsenault, C.; Garcia-Elorrio, E.; Garcia, P.J. Inequalities in health system coverage and quality: A cross-sectional survey of four Latin American countries. Lancet Glob. Health 2024, 12, e145–e155. [Google Scholar] [CrossRef]
- Ruano, A.L.; Rodríguez, D.; Rossi, P.G.; Maceira, D. Understanding inequities in health and health systems in Latin America and the Caribbean: A thematic series. Int. J. Equity Health 2021, 20, 94. [Google Scholar] [CrossRef]
- Ministry of Health of Brazil. Programa Nacional de Imunizações. Available online: https://www.gov.br/saude/pt-br/vacinacao/calendario (accessed on 6 May 2024).
- Coronell-Rodriguez, W.; Caceres, D.C.; Cintra, O.; Guzman-Holst, A. Epidemiology of Invasive Meningococcal Disease in Colombia: A Retrospective Surveillance Database Analysis. Infect Dis Ther 2023, 12, 2709–2724. [Google Scholar] [CrossRef]
| Year | Total Cases, n | Serogroup, n | NG | Other Groups, n | |||
|---|---|---|---|---|---|---|---|
| B | C | W | Y | ||||
| 2018 | 64 | 34 | 7 | 22 | 0 | 1 | - |
| 2019 | 63 | 29 | 9 | 20 | 3 | 0 | 2 |
| 2020 | 17 | 12 | 0 | 3 | 1 | 1 | - |
| 2021 | 14 | 8 | 2 | 4 | 0 | 0 | - |
| 2023 | 37 | 23 | 11 | 1 | 2 | 0 | - |
| Year | Total Cases, n | Incidence *,† | Serogroup, %* | ||||
|---|---|---|---|---|---|---|---|
| B | C | W | Y | Other | |||
| 2018 | 96 | 0.22 | 59 | 9 | 31 | 0 | 1 |
| 2019 | 97 | 0.22 | 51 | 14 | 27 | 4 | 2 |
| 2020 | 30 | 0.06 | 78 | 0 | 17 | 6 | 0 |
| 2021 | 20 | 0.03 | 47 | 30 | 24 | 0 | 0 |
| 2022 | 56 | 0.13 | 65 | 12 | 15 | 6 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáfadi, M.A.P.; Falconi, J.F.; Abalos, M.G.; Serra, L.; Gentile, A.; Diaz, A.; Cortes, C.P.; Villena, R. Invasive Meningococcal Disease in the Post–COVID-19 Era in South America. Vaccines 2025, 13, 1079. https://doi.org/10.3390/vaccines13111079
Sáfadi MAP, Falconi JF, Abalos MG, Serra L, Gentile A, Diaz A, Cortes CP, Villena R. Invasive Meningococcal Disease in the Post–COVID-19 Era in South America. Vaccines. 2025; 13(11):1079. https://doi.org/10.3390/vaccines13111079
Chicago/Turabian StyleSáfadi, Marco A. P., Juan Francisco Falconi, Maria Gabriela Abalos, Lidia Serra, Angela Gentile, Alejandro Diaz, Claudia P. Cortes, and Rodolfo Villena. 2025. "Invasive Meningococcal Disease in the Post–COVID-19 Era in South America" Vaccines 13, no. 11: 1079. https://doi.org/10.3390/vaccines13111079
APA StyleSáfadi, M. A. P., Falconi, J. F., Abalos, M. G., Serra, L., Gentile, A., Diaz, A., Cortes, C. P., & Villena, R. (2025). Invasive Meningococcal Disease in the Post–COVID-19 Era in South America. Vaccines, 13(11), 1079. https://doi.org/10.3390/vaccines13111079






