-
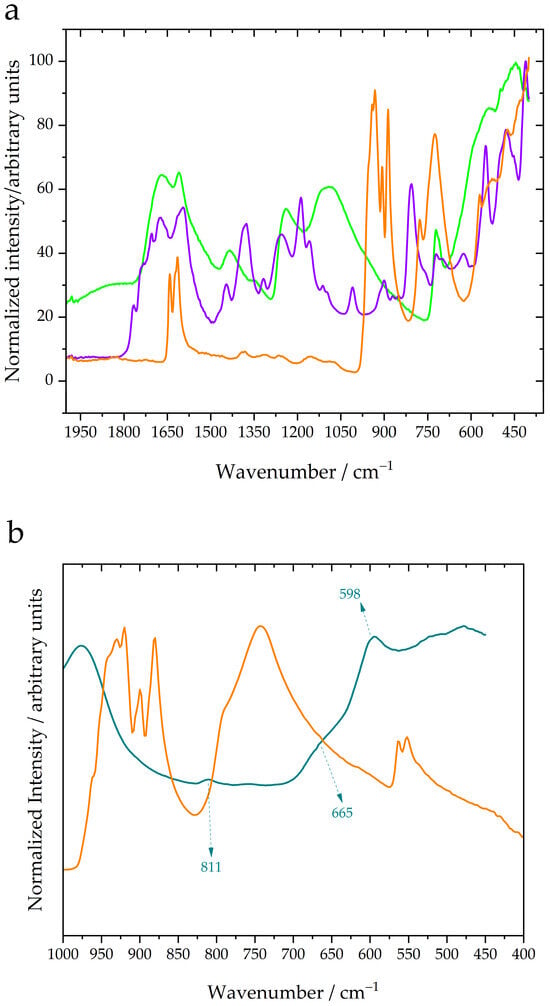
 Green Photocatalysis: A Comprehensive Review of Plant-Based Materials for Sustainable Water Purification
Green Photocatalysis: A Comprehensive Review of Plant-Based Materials for Sustainable Water Purification -
 An Iron-Dependent Alcohol Dehydrogenase Is Involved in Ethanol Metabolism of Aromatoleum aromaticum
An Iron-Dependent Alcohol Dehydrogenase Is Involved in Ethanol Metabolism of Aromatoleum aromaticum -
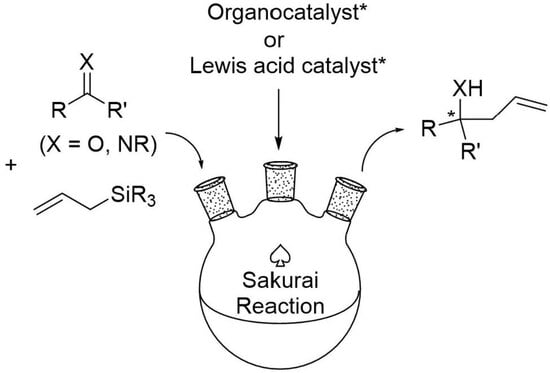
 Transesterification/Esterification Reaction Catalysed by Functional Hybrid MOFs for Efficient Biodiesel Production
Transesterification/Esterification Reaction Catalysed by Functional Hybrid MOFs for Efficient Biodiesel Production
Journal Description
Reactions
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within ESCI (Web of Science), Scopus, EBSCO, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 18.8 days after submission; acceptance to publication is undertaken in 3.5 days (median values for papers published in this journal in the second half of 2025).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
- Journal Cluster of Chemical Reactions and Catalysis: Catalysts, Chemistry, Electrochem, Inorganics, Molecules, Organics, Oxygen, Photochem, Reactions, Sustainable Chemistry.
Latest Articles
E-Mail Alert
News
MDPI Launches the Michele Parrinello Award for Pioneering Contributions in Computational Physical Science

MDPI INSIGHTS: The CEO's Letter #31 - MDPI 30 Years, 500 Journals, UK Summit, Z-Forum Conference, APE

Topics
Deadline: 20 May 2026
Deadline: 31 May 2026
Deadline: 25 August 2026
Deadline: 30 September 2026
Conferences
Special Issues
Deadline: 31 March 2026
Deadline: 1 May 2026
Deadline: 15 June 2026
Deadline: 15 July 2026