Green Photocatalysis: A Comprehensive Review of Plant-Based Materials for Sustainable Water Purification
Abstract
1. Introduction
2. Mechanism of Photocatalysis
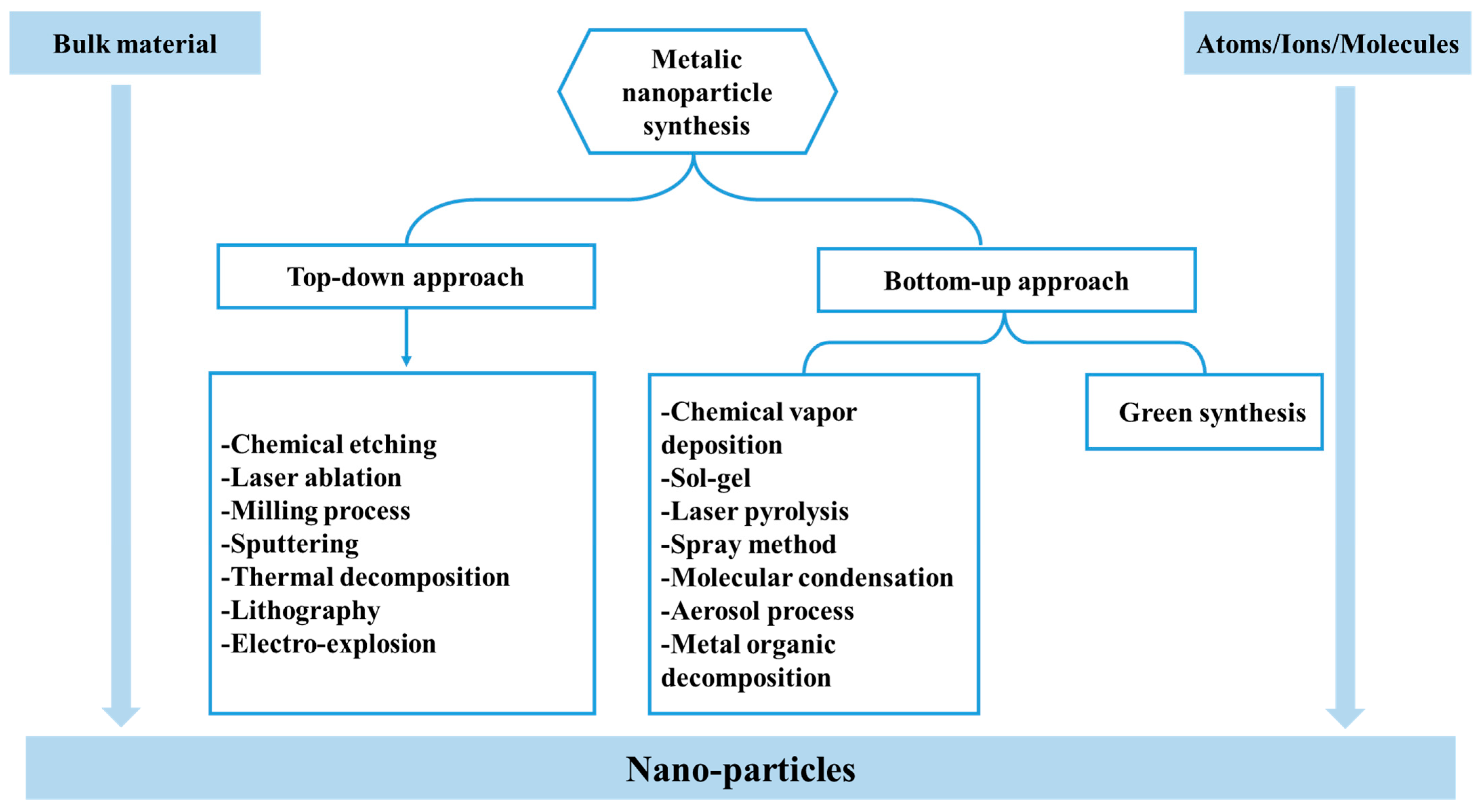
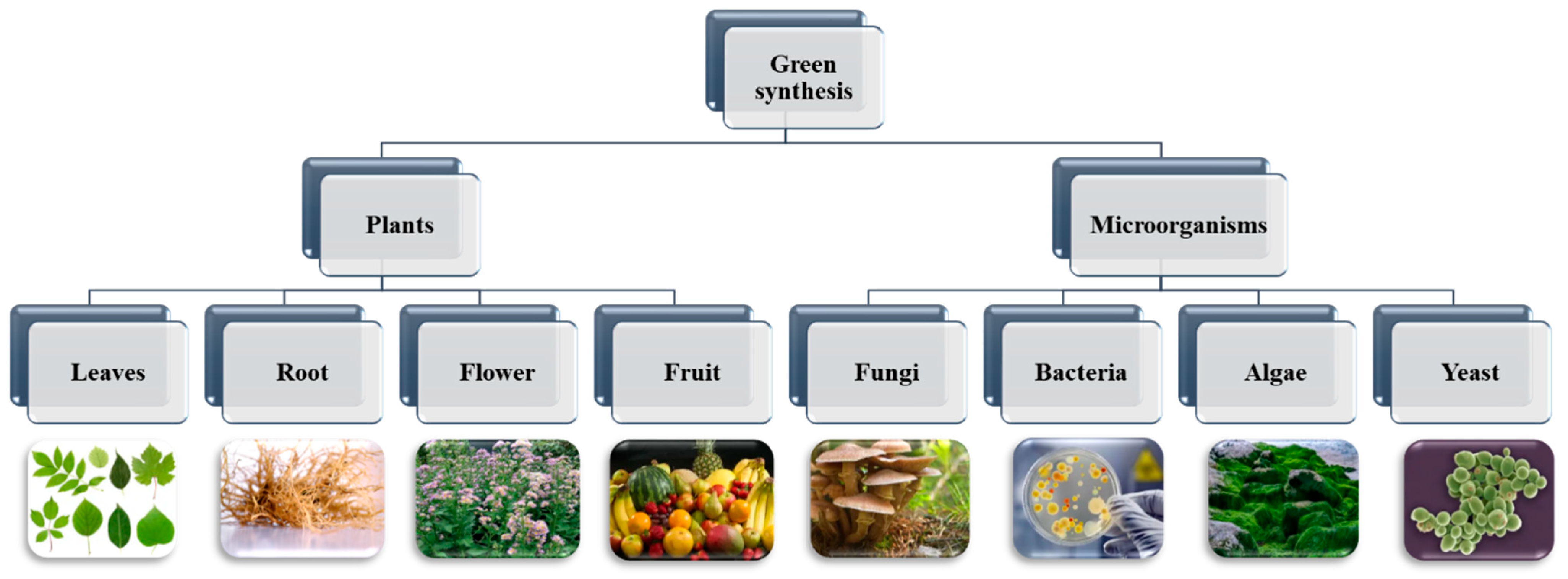
3. Plant-Mediated Green Synthesis of Nanoparticles
| Aspect | Advantages | Limitations |
|---|---|---|
| Eco-friendliness | Use non-toxic, biodegradable plant extracts; environmentally sustainable. | Some processes may still involve non-green conditions (e.g., high temperatures, long synthesis time). |
| Phytochemical content | Rich in natural compounds (e.g., flavonoids, phenolics, tannins) that serve as reducing and stabilizing agents. | Phytochemical composition varies by species, plant part, region, and season, affecting reproducibility. |
| Simplicity and cost-efficiency | Requires no sterile conditions, complex culturing, or expensive equipment. | Industrial scalability is limited; plant materials may require storage or preservation. |
| Safety | No use of hazardous chemicals; safe for researchers and the environment. | Lack of precise control over reaction conditions may result in inconsistent product quality. |
| Photocatalytic performance | Produces TiO2 NPs with small particle sizes, reduced bandgap, and high dye degradation efficiency (>90%). | Some materials show reduced reusability or stability after one photocatalytic cycle. |
| Versatility | Enables synthesis of various NP shapes (spherical, tubular, etc.) with good surface properties. | Irregular particle shapes and low crystallinity may occur, limiting some functional applications. |
| Mechanistic understanding | Offers natural and sustainable synthesis pathways. | The exact reaction mechanisms are still not fully understood, limiting optimization strategies. |
4. Green Synthesis of Oxide-Based Photocatalysts Using Plant Extracts
4.1. Titanium Dioxyde NPs (TiO2)
4.2. Zinc Oxide NPs (ZnO)
4.3. Tungsten Oxide (WO3)
4.4. Copper Oxide NPs (CuO/Cu2O)
4.5. Iron Oxide NPs (Fe2O3)
5. Quantitative Assessment of Environmental Impact
6. Conclusions and Future Perspective
- Industrial-scale expansion: Develop scalable synthesis routes that ensure reproducibility, cost-efficiency, and quality control for large-scale production.
- Advanced material design: Engineer composite and heterostructured catalysts (e.g., doped systems, hybrid nanocomposites, or heterojunctions) to extend light absorption into the visible spectrum and suppress charge recombination.
- Real-world applicability: Test photocatalysts in actual wastewater systems containing complex pollutant mixtures, variable pH, and competing ions to evaluate their robustness and practical efficiency.
- Mechanistic insights: Elucidate the molecular role of phytochemicals in nucleation, growth, and surface functionalization to better tune particle size, band structure, and catalytic activity.
- Catalysis–membrane integration: Explore the development of photocatalytic membranes that combine degradation and separation processes, thereby reducing secondary contamination, improving water quality, and enhancing process sustainability.
- Multifunctional applications: Explore additional roles such as antimicrobial activity, energy harvesting, or pollutant sensing, thereby broadening the technological relevance of these nanomaterials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, I.; Burakov, A.E.; Melezhik, A.V.; Babkin, A.V.; Burakova, I.V.; Neskomornaya, E.A.; Galunin, E.V.; Tkachev, A.G.; Kuznetsov, D.V. Removal of Copper (II) and Zinc (II) Ions in Water on a Newly Synthesized Polyhydroquinone/Graphene Nanocomposite Material: Kinetics, Thermodynamics and Mechanism. ChemistrySelect 2019, 4, 12708–12718. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; AlOthman, Z.A.; Alwarthan, A.; Al-Mohaimeed, A.M. Preparation of a Carboxymethylcellulose-Iron Composite for Uptake of Atorvastatin in Water. Int. J. Biol. Macromol. 2019, 132, 244–253. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; AlOthman, Z.A.; Badjah, A.Y. Kinetics, Thermodynamics, and Modeling of Amido Black Dye Photodegradation in Water Using Co/TiO2 Nanoparticles. Photochem. Photobiol. 2018, 94, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Basheer, A.A. Advances in the Smart Materials Applications in the Aerospace Industries. Aircr. Eng. Aero. Technol. 2020, 92, 1027–1035. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Ivanova, N.; Gugleva, V.; Dobreva, M.; Pehlivanov, I.; Stefanov, S.; Andonova, V. Bottom-Up and Top-Down Approaches for MgO. In Intech, Tourism; IntechOpen: London, UK, 2016; p. 13. [Google Scholar] [CrossRef]
- Kumar, S.; Bhushan, P.; Bhattacharya, S. Fabrication of Nanostructures with Bottom-Up Approach and Their Utility in Diagnostics, Therapeutics, and Others. In Nanostructures for the Engineering of Cells, Tissues and Organs; Springer: Singapore, 2018; pp. 167–198. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, Characteristics, and Applications in Analytical and Other Sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Ganachari, S.V.; Martínez, L.; Kharissova, O.; Kharisov, B. Synthesis Techniques for Preparation of Nanomaterials. In Handbook of Ecomaterials; Martínez, L., Kharissova, O., Kharisov, B., Eds.; Springer: Cham, Switzerland, 2019; Volume 1, pp. 83–103. [Google Scholar] [CrossRef]
- Razavi, M.T.L.; Salahinejad, E.; Fahmy, M.; Yazdimamaghani, M.; Vashaee, D. Green Chemical and Biological Synthesis of Nanoparticles and Their Biomedical Applications. In Green Processes for Nanotechnology; Basiuk, V., Basiuk, E., Eds.; Springer: Cham, Switzerland, 2015; pp. 207–235. [Google Scholar] [CrossRef]
- Andronic, L.; Enesca, A. Black TiO2 Synthesis by Chemical Reduction Methods for Photocatalysis Applications. Front. Chem. 2020, 8, 565489. [Google Scholar] [CrossRef]
- Yahaya, M.Z.; Azam, M.A.; Teridi, M.A.M.; Singh, P.K.; Mohamad, A.A. Recent Characterisation of Sol-Gel Synthesised TiO2 Nanoparticles. In IntechOpen Limited; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Thapa, R.; Maiti, S.; Rana, T.H.; Maiti, U.N.; Chattopadhyay, K.K. Anatase TiO2 Nanoparticles Synthesis via Simple Hydrothermal Route: Degradation of Orange II, Methyl Orange and Rhodamine B. J. Mol. Catal. A Chem. 2012, 363–364, 223–229. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Q.; Duan, H.; Ge, M. Scalable Synthesis of High-Purity TiO2 Whiskers: Via Ion Exchange Method Enables Versatile Applications. RSC Adv. 2019, 9, 23735–23743. [Google Scholar] [CrossRef]
- Buraso, W.; Lachom, V.; Siriya, P.; Laokul, P. Synthesis of TiO2 Nanoparticles via a Simple Precipitation Method and Photocatalytic Performance. Mater. Res. Express 2018, 5, 115016. [Google Scholar] [CrossRef]
- Aguilar, T.; Carrillo-Berdugo, I.; Gómez-Villarejo, R.; Gallardo, J.J.; Martínez-Merino, P.; Piñero, J.C.; Alcántara, R.; Fernández-Lorenzo, C.; Navas, J. A Solvothermal Synthesis of TiO2 Nanoparticles in a Non-Polar Medium to Prepare Highly Stable Nanofluids with Improved Thermal Properties. Nanomaterials 2018, 8, 816. [Google Scholar] [CrossRef]
- Ojeda, M.; Chen, B.; Leung, D.Y.C.; Xuan, J.; Wang, H. A Hydrogel Template Synthesis of TiO2 Nanoparticles for Aluminium-Ion Batteries. Energy Proc. 2017, 105, 3997–4002. [Google Scholar] [CrossRef]
- Widiyandari, H.; Purwanto, A.; Gunawan, V.; Widyanto, S.A. Synthesis of Titanium Dioxide (TiO2) Fine Particle by Flame Spray Pyrolysis (FSP) Method Using Liquid Petroleum Gas (LPG) as Fuel. Reaktor 2018, 17, 226–229. [Google Scholar] [CrossRef]
- Sofronov, D.; Rucki, M.; Demidov, O.; Doroshenko, A.; Sofronova, E.; Shaposhnyk, A.; Kapustnik, O.; Mateychenko, P.; Kucharczyk, W. Formation of TiO2 Particles during Thermal Decomposition of Ti(NO3)4, TiOF2 and TiOSO4. J. Mater. Res. Technol. 2020, 9, 12201–12212. [Google Scholar] [CrossRef]
- Bandgar, A.; Sabale, S.; Pawar, S.H. Studies on Influence of Reflux Time on Synthesis of Nanocrystalline TiO2 Prepared by Peroxotitanate Complex Solutions. Ceram. Int. 2012, 38, 1905–1913. [Google Scholar] [CrossRef]
- Reinke, M.; Ponomarev, E.; Kuzminykh, Y.; Hoffmann, P. Combinatorial Characterization of TiO2 Chemical Vapor Deposition Utilizing Titanium Isopropoxide. ACS Comb. Sci. 2015, 17, 413–420. [Google Scholar] [CrossRef]
- Kitamura, Y.; Okinaka, N.; Shibayama, T.; Mahaney, O.O.P.; Kusano, D.; Ohtani, B.; Akiyama, T. Combustion Synthesis of TiO2 Nanoparticles as Photocatalyst. Powder Technol. 2007, 176, 93–98. [Google Scholar] [CrossRef]
- Moon, J.T.; Lee, S.K.; Joo, J.B. Controllable One-Pot Synthesis of Uniform Colloidal TiO2 Particles in a Mixed Solvent Solution for Photocatalysis. Beilstein J. Nanotechnol. 2018, 9, 1715–1727. [Google Scholar] [CrossRef]
- Yasin, S.A.; Zeebaree, S.Y.S.; Zeebaree, A.Y.S.; Zebari, O.I.H.; Saeed, I.A. The Efficient Removal of Methylene Blue Dye Using CuO/PET Nanocomposite in Aqueous Solutions. Catalysts 2021, 11, 241. [Google Scholar] [CrossRef]
- Wu, V.K.; Bai, G.-R.; Eastman, J.A.; Zhou, G. Synthesis of TiO2 Nanoparticles Using Chemical Vapor Condensation. Mater. Res. Soc. Symp. Proc. 2005, 879, 712. [Google Scholar] [CrossRef]
- Ellouzi, I.; Oualid, H.A. Efficient and Eco-Friendly Mechanical Milling Preparation of Anatase/Rutile TiO2-Glucose Composite with Energy Gap Enhancement. Proceedings 2019, 3, 3. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Chen, Z.; Li, Y.; Yu, Z.; Liu, Q.; Li, J.; Feng, C.; Zhang, D. Sonochemical Synthesis of TiO2 Nanoparticles on Graphene for Use as Photocatalyst. Ultrason. Sonochem. 2011, 18, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Negrea, D.; Ducu, C.; Moga, S.; Malinovschi, V.; Monty, C.J.A.; Vasile, B.; Dorobantu, D.; Enachescu, M. Solar Physical Vapor Deposition Preparation and Microstructural Characterization of TiO2 Based Nanophases for Dye-Sensitized Solar Cell Applications. J. Nanosci. Nanotechnol. 2012, 12, 8746–8750. [Google Scholar] [CrossRef]
- Andriyanti, W.; Nurfiana, F.; Sari, A.N.; Kundari, N.A.; Aziz, I. Synthesis TiO2-Ag Thin Film by DC Sputtering Method for Dye Degradation. J. Phys. Conf. Ser. 2020, 1436, 012008. [Google Scholar] [CrossRef]
- Anandgaonker, P.; Kulkarni, G.; Gaikwad, S.; Rajbhoj, A. Synthesis of TiO2 Nanoparticles by Electrochemical Method and Their Antibacterial Application. Arab. J. Chem. 2019, 12, 1815–1822. [Google Scholar] [CrossRef]
- Nath, A.; Laha, S.S.; Khare, A. Synthesis of TiO2 Nanoparticles via Laser Ablation at Titanium-Water Interface. Integr. Ferroelectr. Int. J. 2010, 121, 58–64. [Google Scholar] [CrossRef]
- Bouhadoun, S.; Guillard, C.; Sorgues, S.; Hérissan, A.; Colbeau-Justin, C.; Dapozze, F.; Habert, A.; Maurel, V.; Herlin-Boime, N. Laser Synthesized TiO2-Based Nanoparticles and Their Efficiency in the Photocatalytic Degradation of Linear Carboxylic Acids. Sci. Technol. Adv. Mater. 2017, 18, 805–815. [Google Scholar] [CrossRef]
- Huang, B.; Li, L.; Jiang, Y.P.; Hao, S.X. Preparation of Nano-Titanium Dioxide by Microemulsion Method. Adv. Mater. Res. 2015, 1073–1076, 8–11. [Google Scholar] [CrossRef]
- Cabello, G.; Davoglio, R.A.; Pereira, E.C. Microwave-Assisted Synthesis of Anatase TiO2 Nanoparticles with Catalytic Activity in Oxygen Reduction. J. Electroanal. Chem. 2017, 794, 36–42. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Kavianipour, M.; Aghigh, S.M.; Ghoranneviss, M. On the Formation of TiO2 Nanoparticles via Submerged Arc Discharge Technique: Synthesis, Characterization and Photocatalytic Properties. J. Cluster Sci. 2010, 21, 753–766. [Google Scholar] [CrossRef]
- Tsai, C.; Hsi, H.; Fan, K. Synthesized Oxygen-Vacant TiO2 Nanopowders with Thermal Plasma Torch Evaporation Condensation Process. World Congr. Eng. Comput. Sci. 2010, II, 20–23. [Google Scholar]
- Tripathy, S.K.; Sahoo, T.; Mohapatra, M.; Anand, S.; Yu, Y.T. Polyol-Assisted Synthesis of TiO2 Nanoparticles in a Semi-Aqueous Solvent. J. Phys. Chem. Solids 2009, 70, 147–152. [Google Scholar] [CrossRef]
- Tokoi, Y.; Suzuki, T.; Nakayama, T.; Suematsu, H.; Jiang, W.; Niihara, K. Synthesis of TiO2 Nanosized Powder by Pulsed Wire Discharge. Jpn. J. Appl. Phys. 2008, 47, 760–763. [Google Scholar] [CrossRef]
- Thakur, B.K.; Kumar, A.; Kumar, D. Green Synthesis of Titanium Dioxide Nanoparticles Using Azadirachta indica Leaf Extract and Evaluation of Their Antibacterial Activity. South Afr. J. Bot. 2019, 124, 223–227. [Google Scholar] [CrossRef]
- Landage, K.S.; Arbade, C.J.B.G.; Khanna, P. Biological Approach to Synthesize TiO2 Nanoparticles Using Staphylococcus aureus for Antibacterial and Anti-Biofilm Applications. J. Microbiol. Exp. Res. 2020, 8, 36–43. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-Mediated Biosynthesis and Characterization of TiO2 Nanoparticles and Their Activity against Pathogenic Bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 91, 23–29. [Google Scholar] [CrossRef]
- Peiris, M.M.K.; Guansekera, T.D.C.P.; Jayaweera, P.M.; Fernando, S.S.N. TiO2 Nanoparticles from Baker’s Yeast: A Potent Antimicrobial. J. Microbiol. Biotechnol. 2018, 28, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail Review on Chemical, Physical and Green Synthesis, Classification, Characterizations and Applications of Nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Mallah, S.; El Mchaouri, M.; Boumya, W.; Machrouhi, A.; Elmoubarki, R.; Barka, N.; Elhalil, A. Recent advances and modification strategies of TiO2 semiconductors for the photocatalytic remediation of water-containing organic pollutants. Euro-Mediterr. J. Environ. Integr. 2025, 1–36. [Google Scholar] [CrossRef]
- Tan, S.N.; Yuen, M.L.; Ramli, R.A. Photocatalysis of Dyes: Operational Parameters, Mechanisms, and Degradation Pathway. Green Anal. Chem. 2025, 12, 100230. [Google Scholar] [CrossRef]
- Nagaraj, G.; Dhayal Raj, A.; Albert Irudayaraj, A.; Josephine, R.L. Tuning the Optical Band Gap of Pure TiO2 via Photon Induced Method. Optik 2019, 179, 889–894. [Google Scholar] [CrossRef]
- Jafarova, V.N.; Orudzhev, G.S. Structural and Electronic Properties of ZnO: A First-Principles Density-Functional Theory Study within LDA(GGA) and LDA(GGA)+U Methods. Solid State Commun. 2021, 325, 114166. [Google Scholar] [CrossRef]
- Mubeen, A.; Majid, A.; Alkhedher, M.; Tag-ElDin, E.M.; Bulut, N. Structural and Electronic Properties of SnO Downscaled to Monolayer. Materials 2022, 15, 5578. [Google Scholar] [CrossRef]
- Oudah, M.H.; Hasan, M.H.; Abd, A.N. Synthesis of Copper Oxide Thin Films by Electrolysis Method Based on Porous Silicon for Solar Cell Applications. IOP Conf. Ser. Mater. Sci. Eng. 2020, 757, 012051. [Google Scholar] [CrossRef]
- Gul, A.R.; Shaheen, F.; Rafique, R.; Bal, J. Grass-Mediated Biogenic Synthesis of Silver Nanoparticles and Their Drug Delivery Evaluation: A Biocompatible Anti-Cancer Therapy. Chem. Eng. J. 2020, 407, 127202. [Google Scholar] [CrossRef]
- Soni, V.; Raizada, P.; Singh, P.; Cuong, H.N.; S, R.; Saini, A.; Saini, R.V.; Van Le, Q.; Nadda, A.K.; Le, T.-T.; et al. Sustainable and Green Trends in Using Plant Extracts for the Synthesis of Biogenic Metal Nanoparticles toward Environmental and Pharmaceutical Advances: A Review. Environ. Res. 2021, 202, 111622. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- He, Y.; Wei, F.; Ma, Z.; Zhang, H.; Yang, Q.; Yao, B.; Huang, Z.; Li, J.; Zeng, C.; Zhang, Q. Green Synthesis of Silver Nanoparticles Using Seed Extract of Alpinia katsumadai, and Their Antioxidant, Cytotoxicity, and Antibacterial Activities. RSC Adv. 2017, 7, 39842–39851. [Google Scholar] [CrossRef]
- Rizwana, H.; Bokahri, N.A.; Alkhattaf, F.S.; Albasher, G.; Aldehaish, H.A. Antifungal, Antibacterial, and Cytotoxic Activities of Silver Nanoparticles Synthesized from Aqueous Extracts of Mace-Arils of Myristica fragrans. Molecules 2021, 26, 7709. [Google Scholar] [CrossRef]
- Rashidipour, M.; Heydari, R. Biosynthesis of Silver Nanoparticles Using Extract of Olive Leaf: Synthesis and In Vitro Cytotoxic Effect on MCF-7 Cells. J. Nanostruct. Chem. 2014, 4, 112. [Google Scholar] [CrossRef]
- Babu, S.A.; Prabu, H.G. Synthesis of AgNPs Using the Extract of Calotropis procera Flower at Room Temperature. Mater. Lett. 2011, 65, 1675–1677. [Google Scholar] [CrossRef]
- Behravan, M.; Hossein Panahi, A.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile Green Synthesis of Silver Nanoparticles Using Berberis vulgaris Leaf and Root Aqueous Extract and Its Antibacterial Activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef]
- Rani, N.; Singh, P.; Kumar, S.; Kumar, P.; Bhankar, V.; Kumar, K. Plant-Mediated Synthesis of Nanoparticles and Their Applications: A Review. Mater. Res. Bull. 2023, 163, 112233. [Google Scholar] [CrossRef]
- Langa, C.; Hintsho-Mbita, N.C. Plant and Bacteria Mediated Synthesis of TiO2 NPs for Dye Degradation in Water: A Review. Chem. Phys. Impact 2023, 7, 100293. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Huang, Y.; Parsons, J.G.; Zhao, L.; Lopez-Moreno, L.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Plant-Based Green Synthesis of Metallic Nanoparticles: Scientific Curiosity or a Realistic Alternative to Chemical Synthesis? Nanotechnol. Environ. Eng. 2016, 1, 4. [Google Scholar] [CrossRef]
- Küünal, S.; Rauwel, P.; Rauwel, E. Plant Extract Mediated Synthesis of Nanoparticles. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.H. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. J. Nanomater. 2014, 2014, 417305. [Google Scholar] [CrossRef]
- Zakharova, G.S.; Natal’ya, V.; Tat’yana, I.G.; Pervova, M.G.; Murzakaev, A.M.; Enyashin, A.N. Morphology-Controlling Hydrothermal Synthesis of h-WO3 for Photocatalytic Degradation of 1,2,4-Trichlorobenzene. J. Alloy. Compd. 2023, 938, 168620. [Google Scholar] [CrossRef]
- Wellia, D.V.; Syuadi, A.F.; Rahma, R.M.; Syafawi, A.; Habibillah, M.R.; Arief, S.; Kurnia, K.A.; Saepurahman; Kusumawati, Y.; Saefumillah, A. Rind of Aloe vera (L.) Burm. f Extract for the Synthesis of Titanium Dioxide Nanoparticles: Properties and Application in Model Dye Pollutant Degradation. Case Stud. Chem. Environ. Eng. 2024, 9, 100627. [Google Scholar] [CrossRef]
- Singh, D.; Anuradha; Mathur, D.; Kumar, S.; Pani, B.; Kumar, A.; Kanojia, R.; Gupta, R.; Singh, L. Bio-Genic Synthesis of Calcium Coated Zinc Oxide Nanoparticles from Beetroot Extract and Their Photo-Degradation Study on Methylene Blue and Rhodamine B. Plant Nano Biol. 2023, 4, 100031. [Google Scholar] [CrossRef]
- Atri, A.; Echabaane, M.; Bouzidi, A.; Harabi, I.; Soucase, B.M.; Ben Chaâbane, R. Green Synthesis of Copper Oxide Nanoparticles Using Ephedra Alata Plant Extract and a Study of Their Antifungal, Antibacterial Activity and Photocatalytic Performance under Sunlight. Heliyon 2023, 9, e13484. [Google Scholar] [CrossRef]
- Al-Fa’ouri, A.M.; Abu-Kharma, M.H.; Awwad, A.M.; Abugazleh, M.K. Investigation of Optical and Structural Properties of Copper Oxide Nanoparticles Synthesized via Green Method Using Bougainvillea Leaves Extract. Nano-Struct. Nano-Objects 2023, 36, 101051. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, P. Green Synthesis of TiO2 Nanoparticles Using Tinospora cordifolia Plant Extract and Its Potential Application for Photocatalysis and Antibacterial Activity. Inorg. Chem. Commun. 2023, 156, 111221. [Google Scholar] [CrossRef]
- Shimi, A.K.; Ahmed, H.M.; Wahab, M.; Katheria, S.; Wabaidur, S.M.; Eldesoky, G.E.; Islam, M.A.; Rane, K.P. Synthesis and Applications of Green Synthesized TiO2 Nanoparticles for Photocatalytic Dye Degradation and Antibacterial Activity. J. Nanomater. 2022, 2022, 7060388. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Brar, S.K.; Daghrir, R.; Tyagi, R.D.; Picard, P.; Surampalli, R.Y.; Drogui, P. Photocatalytic Degradation of Carbamazepine in Wastewater by Using a New Class of Whey-Stabilized Nanocrystalline TiO2 and ZnO. Sci. Total Environ. 2014, 485–486, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Bekele, E.T.; Gonfa, B.A.; Zelekew, O.A.; Belay, H.H.; Sabir, F.K. Synthesis of Titanium Oxide Nanoparticles Using Root Extract of Kniphofia foliosa as a Template: Characterization and Its Application on Drug Resistant Bacteria. J. Nanomater. 2020, 2020, 2817037. [Google Scholar] [CrossRef]
- Ambika, S.; Sundrarajan, M. [EMIM] BF4 Ionic Liquid-Mediated Synthesis of TiO2 Nanoparticles Using Vitex negundo Linn Extract and Its Antibacterial Activity. J. Mol. Liq. 2016, 221, 986–992. [Google Scholar] [CrossRef]
- Rajkumari, J.; Magdalane, C.M.; Siddhardha, B.; Madhavan, J.; Ramalingam, G.; Al-Dhabi, N.A.; Arasu, M.V.; Ghilan, A.; Duraipandiayan, V.; Kaviyarasu, K. Synthesis of Titanium Oxide Nanoparticles Using Aloe barbadensis Mill and Evaluation of Its Antibiofilm Potential against Pseudomonas aeruginosa PAO1. J. Photochem. Photobiol. B 2019, 201, 111667. [Google Scholar] [CrossRef]
- Abisharani, J.M.; Devikala, S.; Dinesh Kumar, R.; Arthanareeswari, M.; Kamaraj, P. Green Synthesis of TiO2 Nanoparticles Using Cucurbita pepo Seeds Extract. Mater. Today Proc. 2019, 14, 302–307. [Google Scholar] [CrossRef]
- Nabi, G.; Ain, Q.-U.; Tahir, M.B.; Riaz, K.N.; Iqbal, T.; Rafique, M.; Hussain, S.; Raza, W.; Aslam, I.; Rizwan, M. Green synthesis of TiO2 nanoparticles using lemon peel extract: Their optical and photocatalytic properties. J. Mater. Sci. 2020, 55, 12345–12358. [Google Scholar] [CrossRef]
- Nabi, G.; Majid, A.; Riaz, A.; Alharbi, T.; Kamran, M.A.; Al-Habardi, M. Green synthesis of spherical TiO2 nanoparticles using Citrus limetta extract: Excellent photocatalytic water decontamination agent for RhB dye. Inorg. Chem. Commun. 2021, 129, 108618. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Pushpamalini, T.; Keerthana, M.; Sangavi, R.; Nagaraj, A.; Kamaraj, P. Comparative analysis of green synthesis of TiO2 nanoparticles using four different leaf extracts. Mater. Today Proc. 2021, 40, S180–S184. [Google Scholar] [CrossRef]
- Dimitrov, O.; Zaharieva, K.; Stambolova, I.; Eneva, R.; Engibarov, S.; Lazarkevich, I.; Gocheva, Y.; Stoyanova, D.; Shipochka, M.; Markov, P.; et al. Photocatalytic and antimicrobial activity of TiO2 particles, obtained by Mentha spicata leaves–mediated synthesis. Catal. Today 2025, 460, 115466. [Google Scholar] [CrossRef]
- Rajendhiran, R.; Deivasigamani, V.; Palanisamy, J.; Masan, S.; Pitchaiya, S. Terminalia catappa and Carissa carandas assisted synthesis of TiO2 nanoparticles—A green synthesis approach. Mater. Today Proc. 2021, 45, 2232–2238. [Google Scholar] [CrossRef]
- Subhapriya, S.; Gomathipriya, P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef]
- Ngoepe, N.M.; Mathipa, M.M.; Hintsho-Mbita, N.C. Biosynthesis of titanium dioxide nanoparticles for the photodegradation of dyes and removal of bacteria. Optik 2020, 224, 165728. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Singh, D.; Anuradha; Mathur, D.; Kumar, A.; Kumar, S.; Pani, B.; Kanojia, R.; Ratan, J. Photocatalytic properties of biologically synthesized uncoated and calcium coated ZnO nanoparticles using cucumber juice. Rasayan J. Chem. 2022, 95–102. [Google Scholar] [CrossRef]
- Pham, T.A.T.; Tran, V.A.; Le, V.D.; Nguyen, M.V.; Troung, D.D.; Do, X.T.; Vu, A.-T. Facile Preparation of ZnO Nanoparticles and Ag/ZnO Nanocomposite and Their Photocatalytic Activities under Visible Light. Int. J. Photoenergy 2020, 2020, 8897667. [Google Scholar] [CrossRef]
- Venkatesan, S.; Suresh, S.; Arumugam, J.; Ramu, P.; Pugazhenthiran, N.; Jothilakshmi, R.; Prabu, K.M. Sunlight assisted degradation of methylene blue dye by zinc oxide nanoparticles green synthesized using Vitex negundo plant leaf extract. Results Chem. 2024, 7, 101315. [Google Scholar] [CrossRef]
- Sedefoglu, N. Green synthesis of ZnO nanoparticles by Myrtus communis plant extract with investigation of effect of precursor, calcination temperature and study of photocatalytic performance. Ceram. Int. 2024, 50, 9884–9895. [Google Scholar] [CrossRef]
- Debanath, M.K.; Karmakar, S. Study of Blueshift of Optical Band Gap in Zinc Oxide (ZnO) Nanoparticles Prepared by Low-Temperature Wet Chemical Method. Mater. Lett. 2013, 111, 116–119. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, M.; Mukherjee, A.; Chattopadhyay, D.; Acharya, K. Biosynthesis and Safety Evaluation of ZnO Nanoparticles. Bioprocess Biosyst. Eng. 2014, 37, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, G.; Chohan, J.S.; Paramasivam, P.; Maranan, R.; Nagaraj, M. Green marvel: Harnessing spinach leaves’ power for enhanced photodegradation of various effluents with biogenic ZnO nanoparticles. Desal. Water Treat. 2024, 319, 100566. [Google Scholar] [CrossRef]
- Deenadayalan, J.; Chen, S.-S.; Das, B.; Pasawan, M. Mineralization and toxicity reduction of chlortetracycline using an environmentally friendly ZnO photocatalyst prepared from Camellia sinensis leaf extract. J. Environ. Chem. Eng. 2025, 13, 115345. [Google Scholar] [CrossRef]
- Nachimuthu, S.; Thangavel, S.; Kannan, K.; Selvakumar, V.; Muthusamy, K.; Siddiqui, M.R.; Wabaidur, S.M.; Parvathiraja, C. Lawsonia inermis mediated synthesis of ZnO/Fe2O3 nanorods for photocatalysis—Biological treatment for the enhanced effluent treatment, antibacterial and antioxidant activities. Chem. Phys. Lett. 2022, 804, 139907. [Google Scholar] [CrossRef]
- Venkatesan, S.; Suresh, S.; Ramu, P.; Arumugam, J.; Thambidurai, S.; Pugazhenthiran, N. Methylene Blue Dye Degradation Potential of Zinc Oxide Nanoparticles Bioreduced Using Solanum trilobatum Leaf Extract. Results Chem. 2022, 4, 100637. [Google Scholar] [CrossRef]
- Supin, K.K.; Namboothiri, P.M.P.; Vasundhara, M. Enhanced Photocatalytic Activity in ZnO Nanoparticles Developed Using Novel Lepidagathis ananthapuramensis Leaf Extract. RSC Adv. 2023, 13, 1497–1515. [Google Scholar] [CrossRef]
- Venkatesan, S.; Suresh, S.; Ramu, P.; Kandasamy, M.; Arumugam, J.; Thambidurai, S.; Prabu, K.M.; Pugazhenthiran, N. Biosynthesis of Zinc Oxide Nanoparticles Using Euphorbia milii Leaf Constituents: Characterization and Improved Photocatalytic Degradation of Methylene Blue Dye under Natural Sunlight. J. Indian Chem. Soc. 2022, 99, 100436. [Google Scholar] [CrossRef]
- Aldeen, T.S.; Mohamed, H.E.A.; Maaza, M. ZnO Nanoparticles Prepared via a Green Synthesis Approach: Physical Properties, Photocatalytic and Antibacterial Activity. J. Phys. Chem. Solids 2022, 160, 110313. [Google Scholar] [CrossRef]
- Anggraini, F.; Fatimah, I.; Ramanda, G.D.; Nurlaela, N.; Wijayanti, H.K.; Sagadevan, S.; Oh, W.-C.; Doong, R.-A. Unveiling the green synthesis of WO3 nanoparticles by using beetroot (Beta vulgaris) extract for photocatalytic oxidation of rhodamine B. Chemosphere 2025, 370, 143890. [Google Scholar] [CrossRef] [PubMed]
- Hkiri, K.; Mohamed, H.E.A.; Harrisankar, N.; Gibaud, A.; van Steen, E.; Maaza, M. Environmental water treatment with green synthesized WO3 nanoflakes for cationic and anionic dyes removal: Photocatalytic studies. Catal. Commun. 2024, 187, 106851. [Google Scholar] [CrossRef]
- Ke, J.; Zhou, H.; Liu, J.; Duan, X.; Zhang, H.; Liu, S.; Wang, S. Crystal transformation of 2D tungstic acid H2WO4 to WO3 for enhanced photocatalytic water oxidation. J. Colloid Interface Sci. 2018, 514, 576–583. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Vijaya, J.J.; Kaviyarasu, K.; Kombaiah, K.; Kennedy, L.J.; Ramalingam, R.J.; Munusamy, M.A.; Al-Lohedan, H.A. Green Synthesis of Ag Nanoparticles Using Tamarind Fruit Extract for the Antibacterial Studies. J. Photochem. Photobiol. B Biol. 2017, 169, 178–185. [Google Scholar] [CrossRef]
- Antony, A.J.; Bennie, R.B.; Joel, C.; Basavegowda, N.; Hatshan, M.R.; Kumar, Y.A. Green synthesis of metastable h-WO3 nanoparticles for photocatalytic degradation of ofloxacin in waste water. J. Alloys Compd. 2025, 1019, 179305. [Google Scholar] [CrossRef]
- Rupashree, M.P.; Soppin, K.; Pratibha, S.; Chethan, B. Cost Effective Photocatalytic and Humidity Sensing Performance of Green Tea Mediated Copper Oxide Nanoparticles. Inorg. Chem. Commun. 2021, 134, 108974. [Google Scholar] [CrossRef]
- Arunkumar, B.; Johnson Jeyakumar, S.; Jothibas, M. A Sol-Gel Approach to the Synthesis of CuO Nanoparticles Using Lantana camara Leaf Extract and Their Photocatalytic Activity. Optik 2019, 183, 698–705. [Google Scholar] [CrossRef]
- Malka, E.; Perelshtein, I.; Lipovsky, A.; Shalom, Y.; Naparstek, L.; Perkas, N.; Patick, T.; Lubart, R.; Nitzan, Y.; Banin, E.; et al. Eradication of Multi-Drug Resistant Bacteria by a Novel Zn-Doped CuO Nanocomposite. Small 2013, 9, 4069–4076. [Google Scholar] [CrossRef]
- Udayabhanu, P.C.; Nethravathi, M.A.; Pavan Kumar, D.; Suresh, K.; Lingaraju, H.; Rajanaika, H.; Nagabhushan, S.C.; Sharma, S.C. Tinospora cordifolia Mediated Facile Green Synthesis of Cupric Oxide Nanoparticles and Their Photocatalytic, Antioxidant and Antibacterial Properties. Mater. Sci. Semicond. Process. 2015, 33, 81–88. [Google Scholar] [CrossRef]
- Allaa, S.K.; Verma, A.D.; Kumar, V.; Mandal, R.K.; Sinha, I.; Prasad, N.K. Solvothermal Synthesis of CuO-MgO Nanocomposite Particles and Their Catalytic Applications. RSC Adv. 2016, 6, 61927–61933. [Google Scholar] [CrossRef]
- Novikova, A.A.; Moiseeva, D.Y.; Karyukov, E.V.; Kalinichenko, A.A. Facile Preparation Photocatalytically Active CuO Plate-Like Nanoparticles from Brochantite. Mater. Lett. 2016, 167, 165–169. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Veeramani, V.; Saravanan, M.; Rajalakshmi, G.; Kaliannan, T.; Al-Misned, F.A.; Pugazhendhi, A. Green Chemistry Route of Biosynthesized Copper Oxide Nanoparticles Using Psidium guajava Leaf Extract and Their Antibacterial Activity and Effective Removal of Industrial Dyes. J. Environ. Chem. Eng. 2021, 9, 105033. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Černík, M. Green Synthesis of Copper Oxide Nanoparticles Using Gum Karaya as a Biotemplate and Their Antibacterial Application. Int. J. Nanomed. 2013, 8, 889–898. [Google Scholar] [CrossRef]
- Varaprasad, K.; Pariguana, M.; Raghavendra, G.M.; Jayaramudu, T.; Sadiku, E.R. Development of Biodegradable Metal Oxide/Polymer Nanocomposite Films Based on Poly-ε-Caprolactone and Terephthalic Acid. Mater. Sci. Eng. C 2017, 70, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajadi, S.M.; Maham, M. Tamarix gallica Leaf Extract Mediated Novel Route for Green Synthesis of CuO Nanoparticles and Their Application for N-Arylation of Nitrogen-Containing Heterocycles under Ligand-Free Conditions. RSC Adv. 2015, 5, 40628–40635. [Google Scholar] [CrossRef]
- Revathi, T.; Thambidurai, S. Cytotoxic, Antioxidant and Antibacterial Activities of Copper Oxide Incorporated Chitosan-Neem Seed Biocomposites. Int. J. Biol. Macromol. 2019, 139, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.E.; Alnoor, H.; Pozina, G.; Liu, X.; Willander, M.; Nur, O. Synthesis of Mg-Doped ZnO NPs via a Chemical Low-Temperature Method and Investigation of the Efficient Photocatalytic Activity for the Degradation of Dyes under Solar Light. Solid State Sci. 2020, 99, 106053. [Google Scholar] [CrossRef]
- Su, Q.; Zuo, C.; Liu, M.; Tai, X. A Review on Cu2O-Based Composites in Photocatalysis: Synthesis, Modification, and Applications. Molecules 2023, 28, 5576. [Google Scholar] [CrossRef]
- Ranjan, R.; Shukla, M. Curcumin-Mediated Synthesis of Cuprous Oxide Nanoparticles and Its Photocatalytic Application. Next Mater. 2025, 6, 100481. [Google Scholar] [CrossRef]
- Shukla, M.; Pal, S.; Sinha, I. Ionic Liquid Functionalized Cu2O Nanoparticles. J. Mol. Struct. 2022, 1262, 132961. [Google Scholar] [CrossRef]
- Ferreira, D.S.; Marques, G.N.; Ferreira, A.M.P.; Oliveira, M.M.; da Rocha, C.Q.; Moreira, A.J.; Fernandes, C.H.M.; Lanza, M.R.; Bernardi, M.I.B.; Mascaro, L.H.; et al. Synthesis of hematite (α-Fe2O3) nanoparticles using Syzygium cumini extract: Photodegradation of norfloxacin and enhanced recyclability. Mater. Chem. Phys. 2025, 332, 130281. [Google Scholar] [CrossRef]
- Matinise, N.; Fuku, X.G.; Kaviyarasu, K.; Mayedwa, N.; Maaza, M. ZnO Nanoparticles via Moringa oleifera Green Synthesis: Physical Properties & Mechanism of Formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar] [CrossRef]
- Garg, S.; Yuan, Y.; Mortazavi, M.; Waite, T.D. Caveats in the Use of Tertiary Butyl Alcohol as a Probe for Hydroxyl Radical Involvement in Conventional Ozonation and Catalytic Ozonation Processes. ACS EST Eng. 2022, 2, 1665–1676. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, H.; Kumar, P.; Rani, G.; Maken, S. Syzygium cumini leaf extract mediated green synthesis of ZnO nanoparticles: A sustained release for anticancer, antimicrobial, antioxidant, and anti-corrosive applications. J. Mol. Struct. 2025, 1325, 141017. [Google Scholar] [CrossRef]
- Riaz, T.; Munnwar, A.; Shahzadi, T.; Zaib, M.; Shahid, S.; Javed, M.; Iqbal, S.; Rizwan, K.; Waqas, M.; Khalid, B.; et al. Phyto-mediated synthesis of nickel oxide (NiO) nanoparticles using leaves’ extract of Syzygium cumini for antioxidant and dyes removal studies from wastewater. Inorg. Chem. Commun. 2022, 142, 109656. [Google Scholar] [CrossRef]
- Din, M.I.; Akbar, R.; Hussain, Z.; Khalid, R.; Arshad, M.; Hussain, T.; Khan, S.A. Biosynthesis of monodispersed stable copper nanoparticles using Syzygium cumini: Characterization and potential applications. Desalination Water Treat. 2023, 281, 130–136. [Google Scholar] [CrossRef]
- Attia, N.F.; Abd El-Monaem, E.M.; El-Aqapa, H.G.; Elashery, S.E.; Eltaweil, A.S.; El Kady, M.; El-Seedi, H.R. Iron Oxide Nanoparticles and Their Pharmaceutical Applications. Appl. Surf. Sci. Adv. 2022, 11, 100284. [Google Scholar] [CrossRef]
- Sunny, N.E.; Mathew, S.S.; Kumar, S.V.; Saravanan, P.; Rajeshkannan, R.; Rajasimman, M.; Vasseghian, Y. Effect of Green Synthesized Nano-Titanium Synthesized from Trachyspermum ammi Extract on Seed Germination of Vigna radiata. Chemosphere 2022, 300, 134600. [Google Scholar] [CrossRef]
- Ali, M.; Wang, X.; Haroon, U.; Chaudhary, H.J.; Kamal, A.; Ali, Q.; Munis, M.F.H. Antifungal Activity of Zinc Nitrate Derived Nano ZnO Fungicide Synthesized from Trachyspermum ammi to Control Fruit Rot Disease of Grapefruit. Ecotoxicol. Environ. Saf. 2022, 233, 113311. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, M.; Javed, M.S.; Hassan, A.M.; Choi, D.; Khawar, M.R.; Waqas, M.; Ayub, A.; Alothman, A.A.; Almuhous, N.A. Eco-benign synthesis of α-Fe2O3 mediated Trachyspermum ammi: A new insight to photocatalytic and bio-medical applications. J. Photochem. Photobiol. A Chem. 2024, 449, 115423. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.H.; Miah, M.Y.; Paul, S.C.; Das Aka, T.; Saha, O.; Rahaman, M.; Sharif, J.I.; Habiba, O.; Ashaduzzaman. Green Synthesis of Iron Oxide Nanoparticle Using Carica papaya Leaf Extract: Application for Photocatalytic Degradation of Remazol Yellow RR Dye and Antibacterial Activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef]
- Endres, T.H.; Yimer, A.A.; Beyene, T.T.; Muleta, G.G. An efficient green synthesis of Ag/Fe2O3 nanocomposite using Carica papaya peel extract for enhanced photocatalytic, antioxidant, and antibacterial activities. Results Chem. 2025, 15, 102184. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Ata, S.; Sultan, M.; Ali, A.; Abbas, A.; Jilani, K.; Kamal, S.; Sarim, F.M.; Khan, M.I.; et al. Green Synthesis of Iron Oxide Nanoparticles Using Pomegranate Seeds Extract and Photocatalytic Activity Evaluation for the Degradation of Textile Dye. J. Mater. Res. Technol. 2019, 8, 6115–6124. [Google Scholar] [CrossRef]
- Bishnoi, S.; Kumar, A.; Selvaraj, R. Facile Synthesis of Magnetic Iron Oxide Nanoparticles Using Inedible Cynometra ramiflora Fruit Extract Waste and Their Photocatalytic Degradation of Methylene Blue Dye. Mater. Res. Bull. 2018, 97, 121–127. [Google Scholar] [CrossRef]
- Sudhakar, C.; Poonkothai, M.; Selvankumar, T.; Selvam, K. Facile Synthesis of Iron Oxide Nanoparticles Using Cassia auriculata Flower Extract and Accessing Their Photocatalytic Degradation and Larvicidal Effect. J. Mater. Sci. Mater. Electron. 2022, 33, 11434–11445. [Google Scholar] [CrossRef]
- Qasim, S.; Zafar, A.; Saif, M.S.; Ali, Z.; Nazar, M.; Waqas, M.; Haq, A.U.; Tariq, T.; Hassan, S.G.; Iqbal, F.; et al. Green Synthesis of Iron Oxide Nanorods Using Withania coagulans Extract Improved Photocatalytic Degradation and Antimicrobial Activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111784. [Google Scholar] [CrossRef]
- Du, J.; AL-Huqail, A.; Cao, Y.; Yao, H.; Sun, Y.; Garaleh, M.; Massoud, E.E.S.; Ali, E.; Assilzadeh, H.; Escorcia-Gutierrez, J. Green synthesis of zinc oxide nanoparticles from Sida acuta leaf extract for antibacterial and antioxidant applications, and catalytic degradation of dye through the use of convolutional neural network. Environ. Res. 2024, 258, 119204. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R. Environmental Impact and Life Cycle Analysis of Green Nanomaterials. In Green Functionalized Nanomaterials for Environmental Applications; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 513–539. [Google Scholar] [CrossRef]


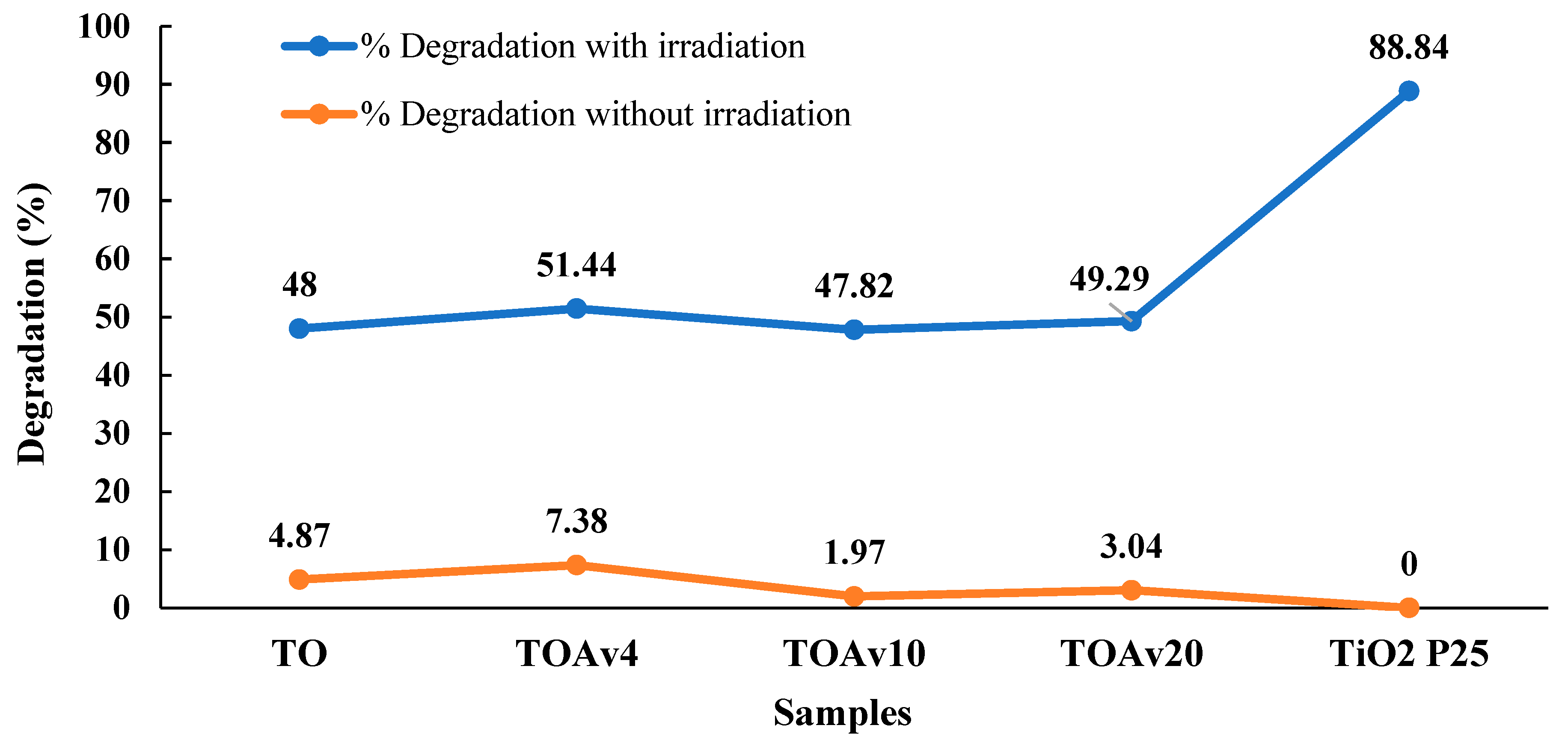
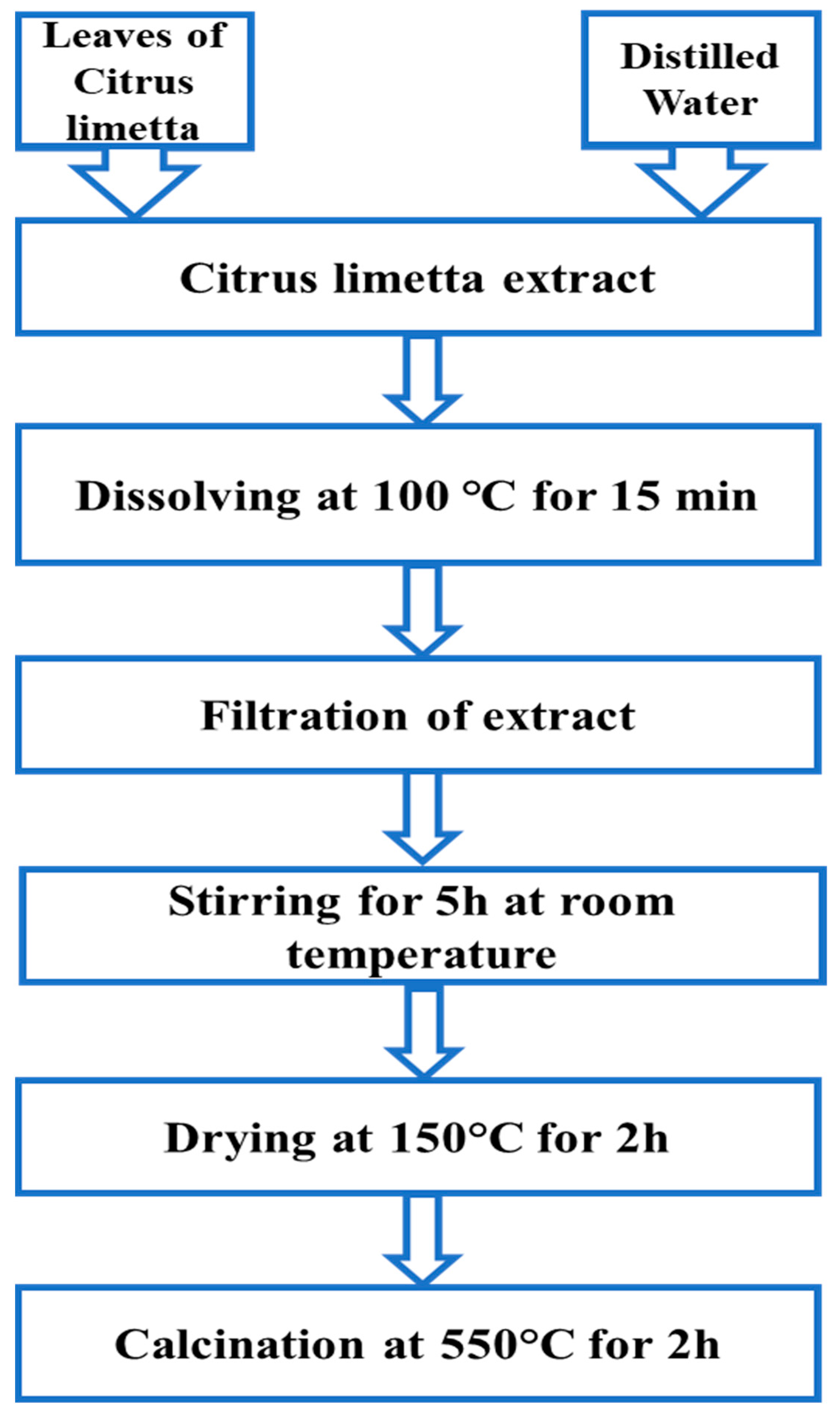
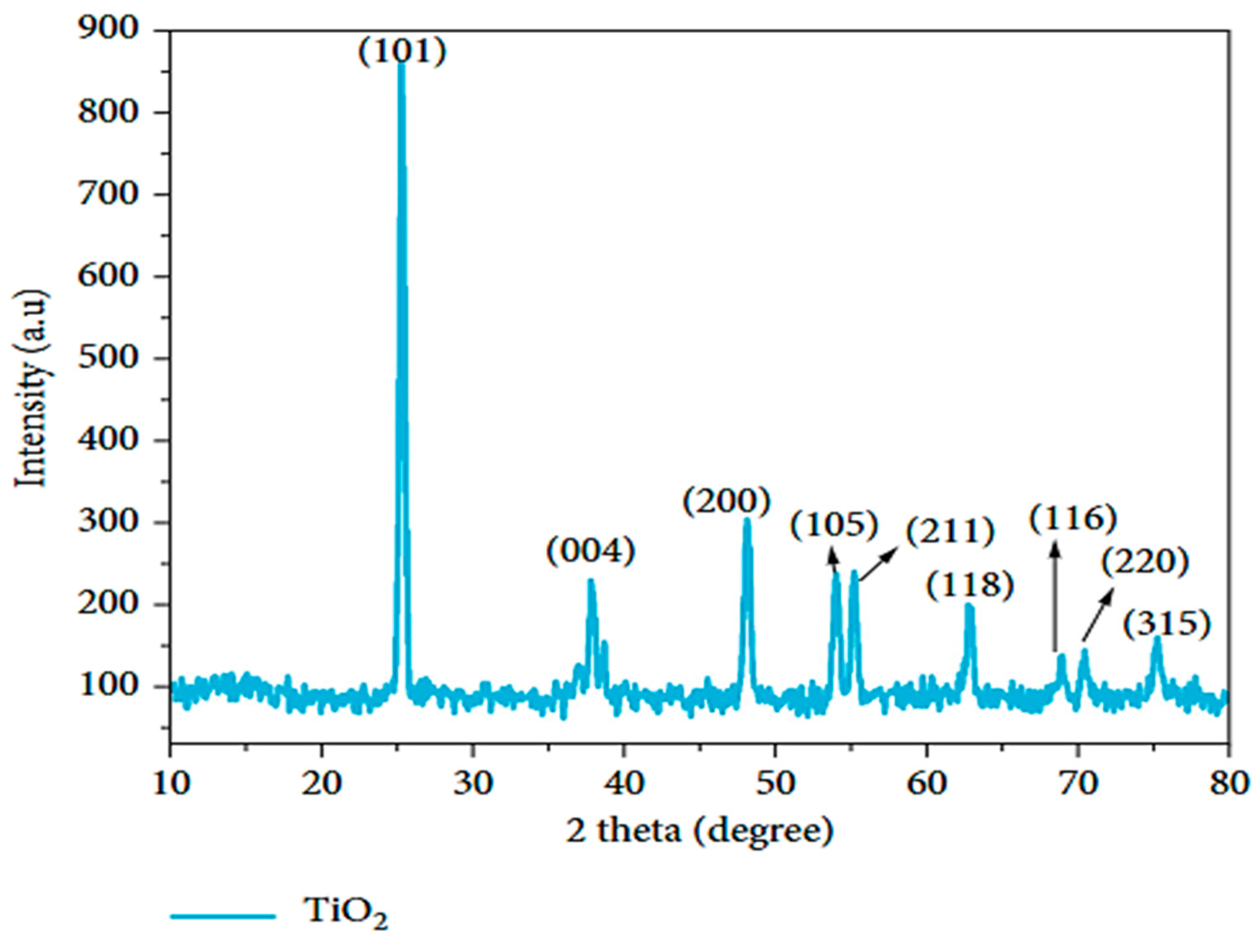
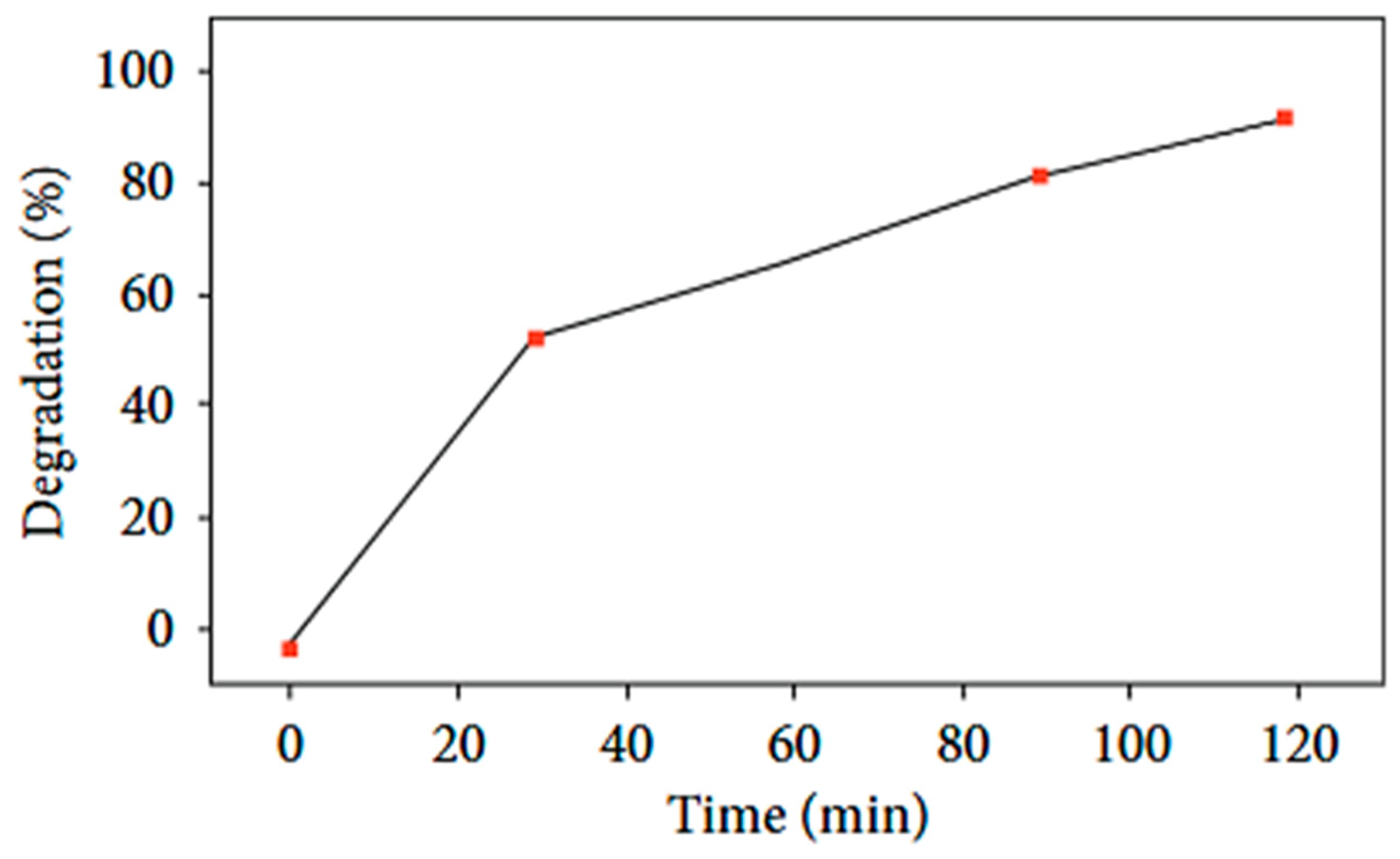
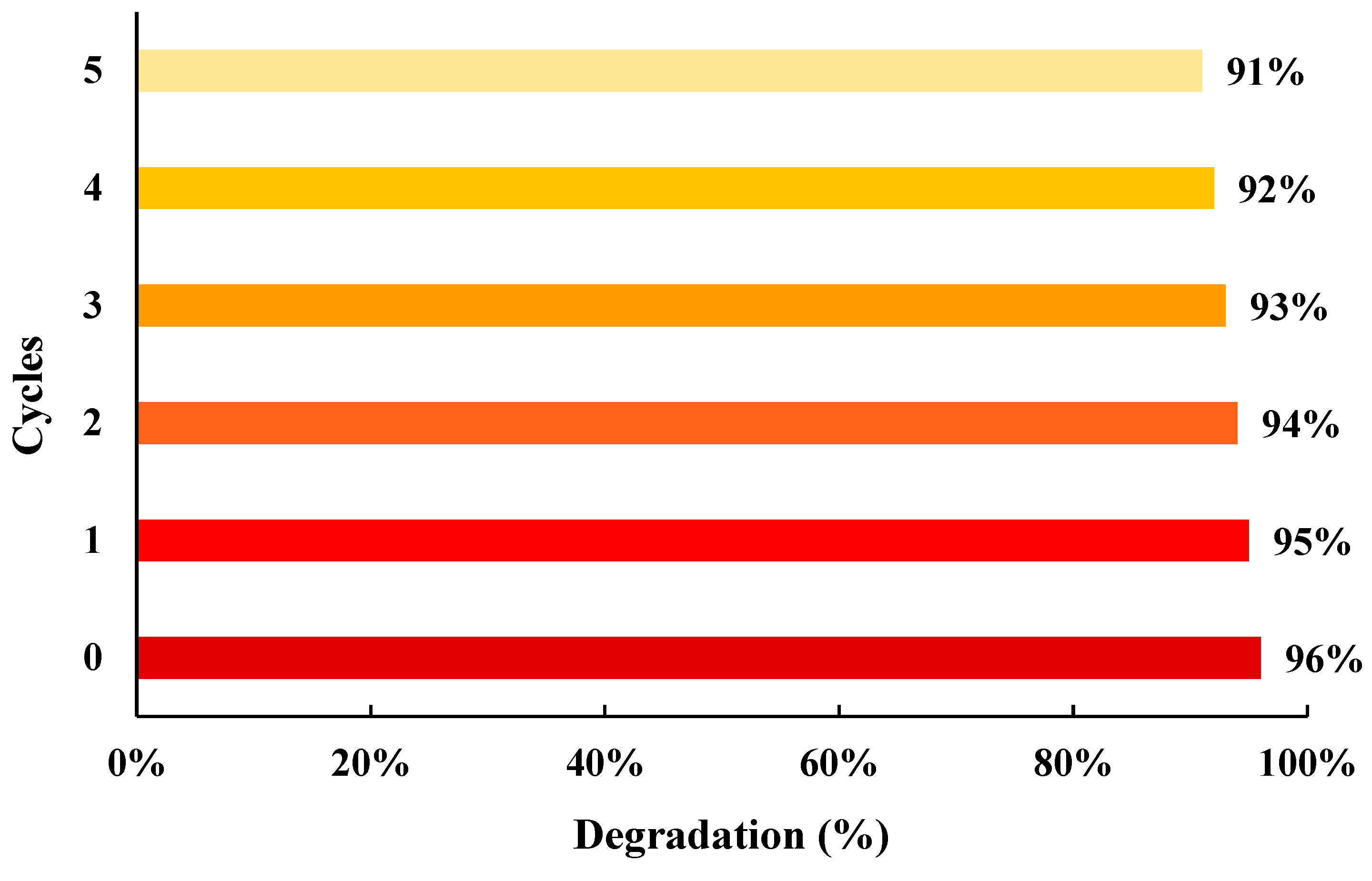
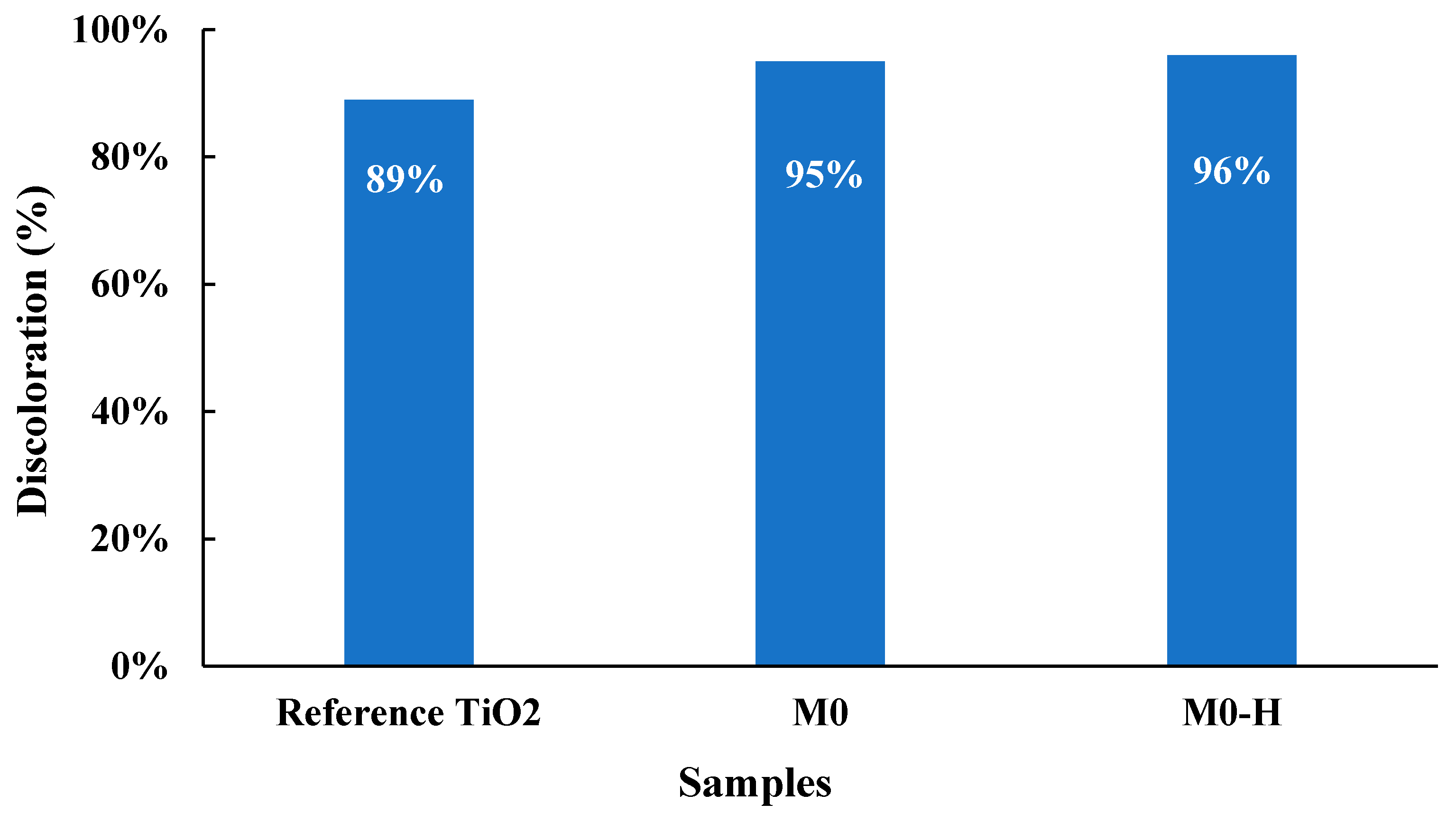
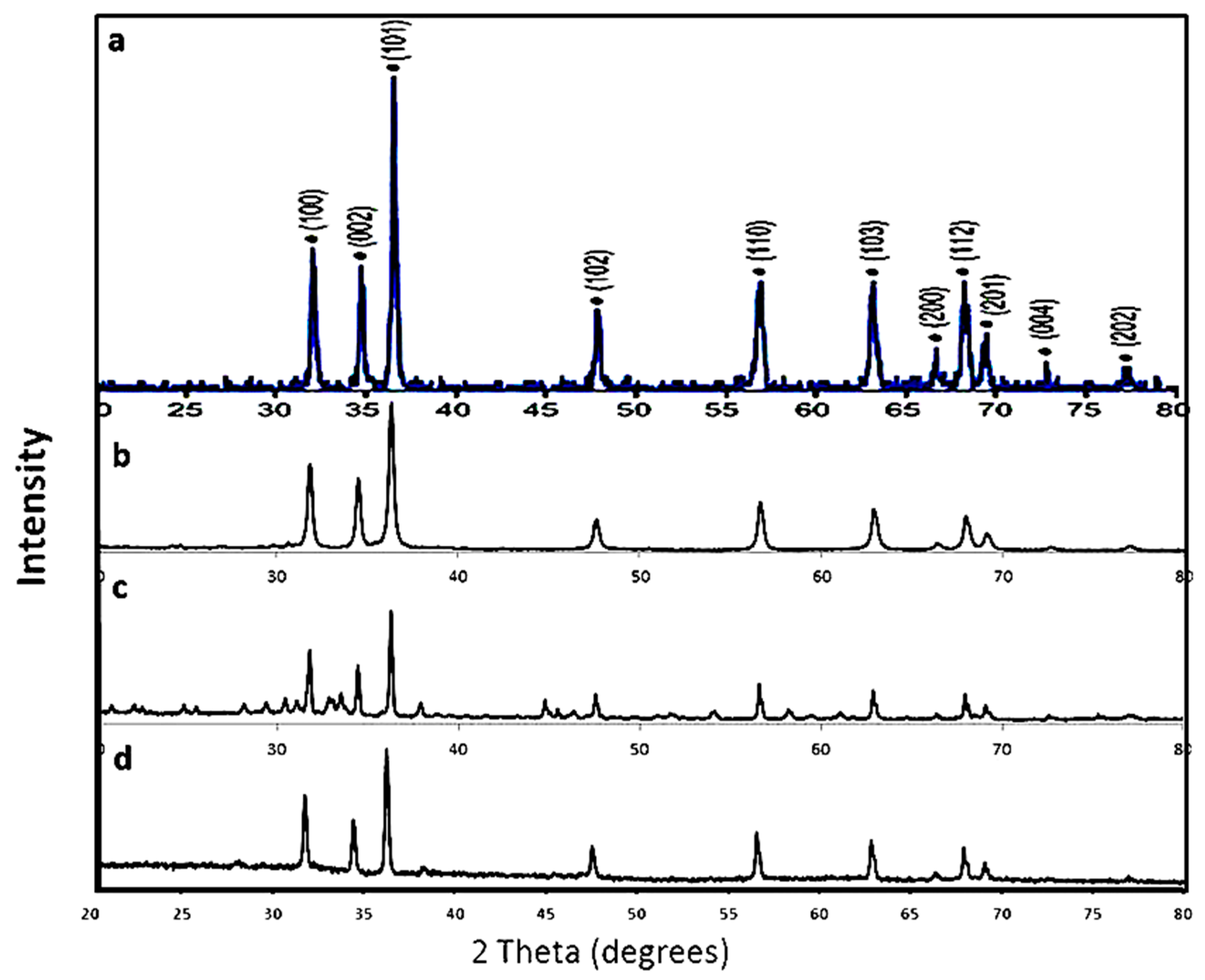
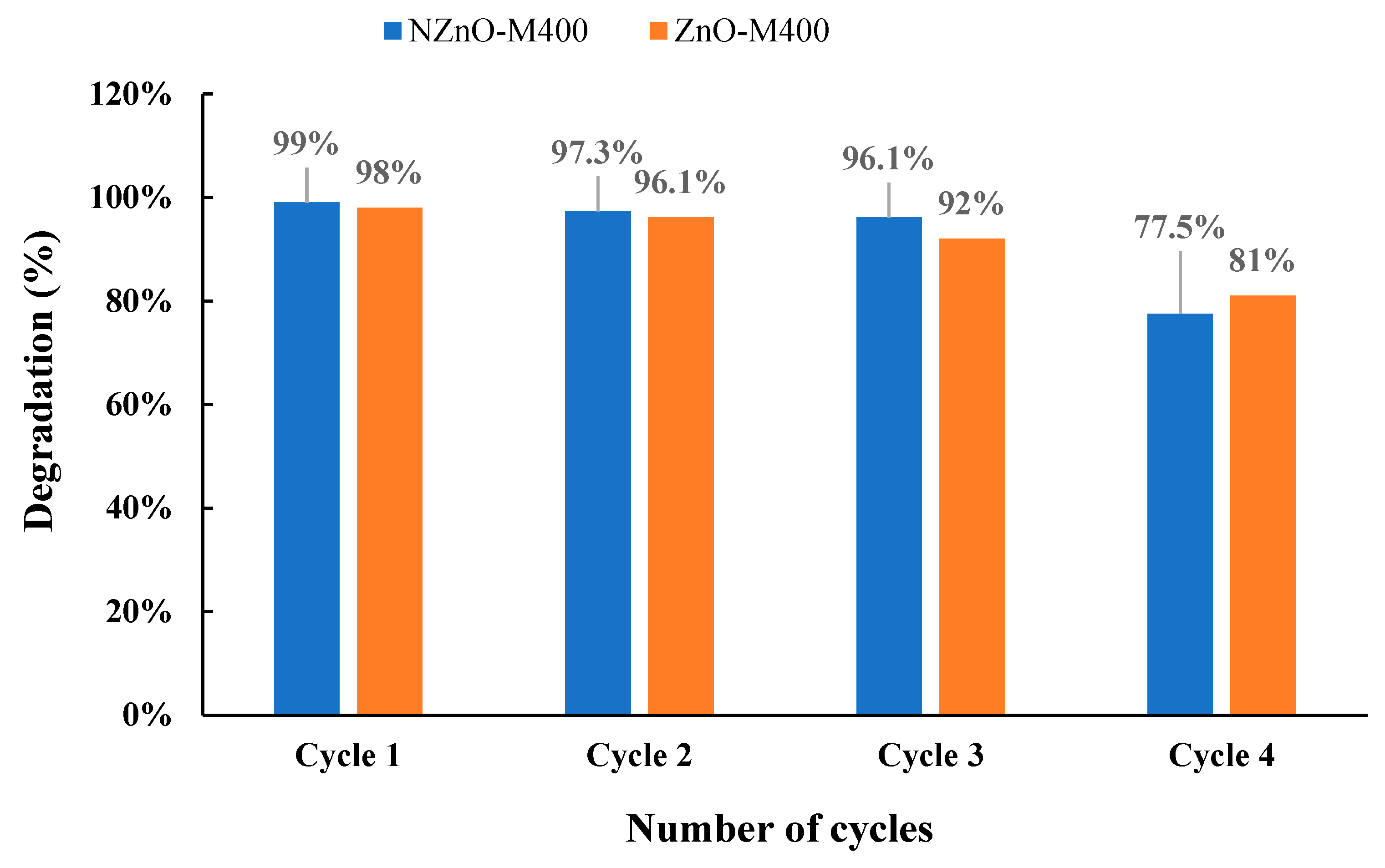

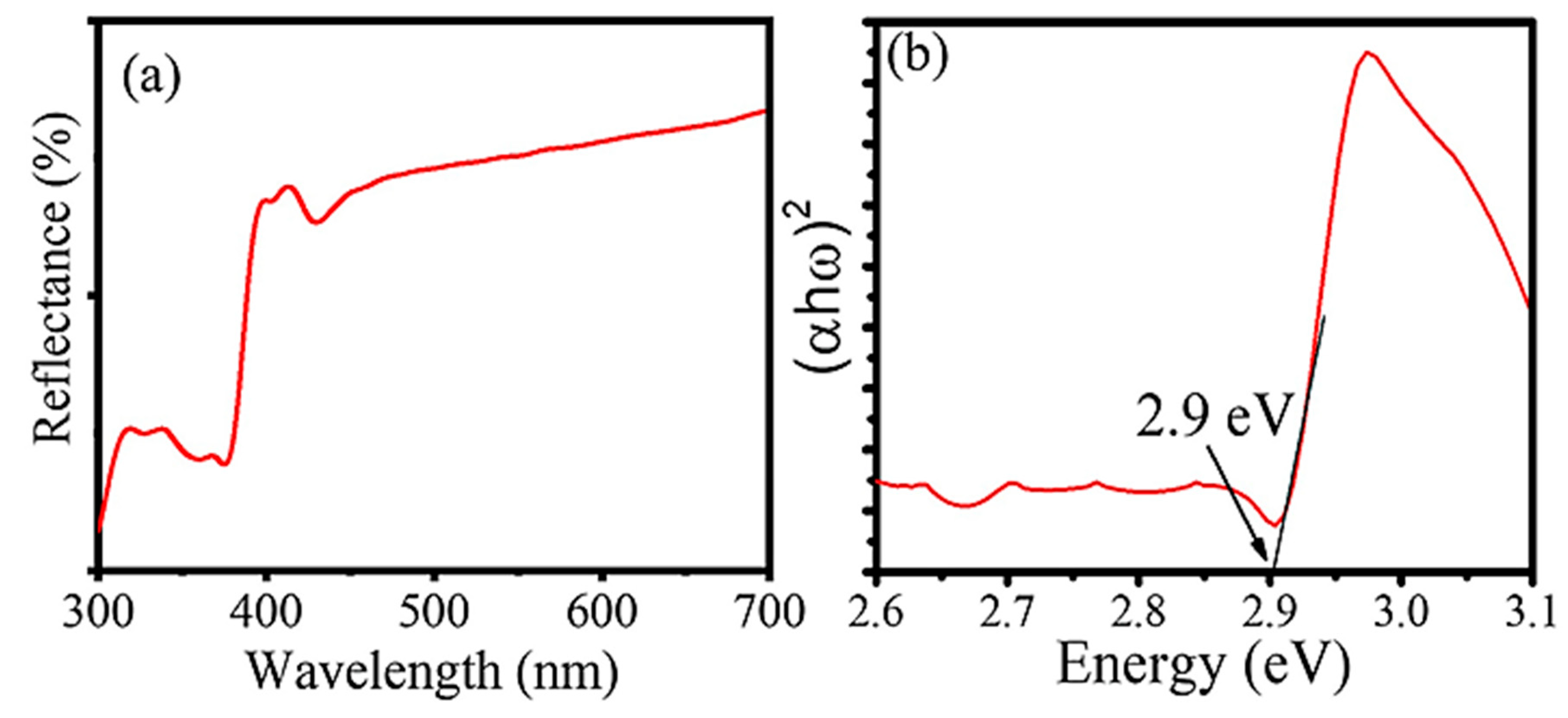
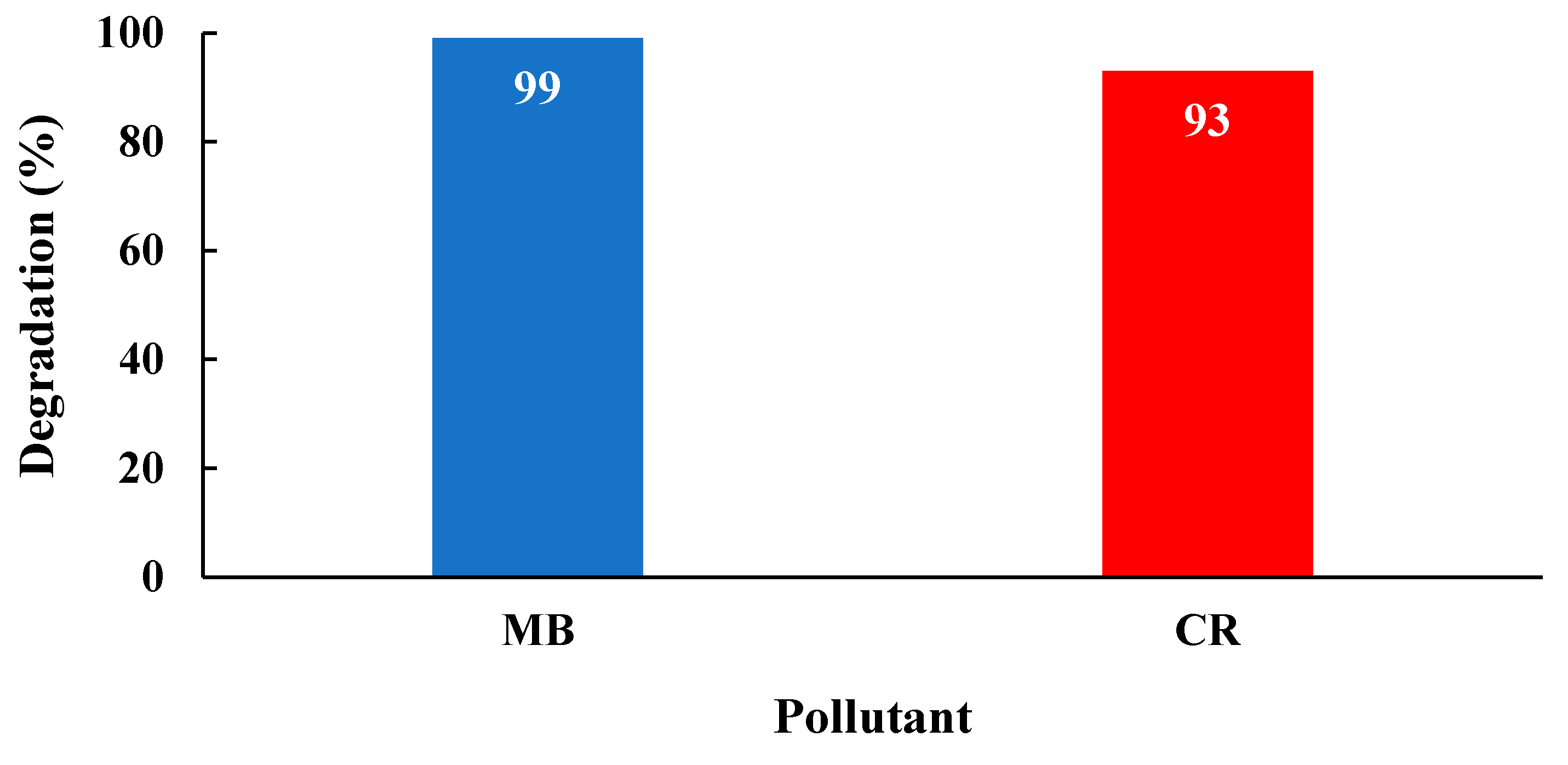
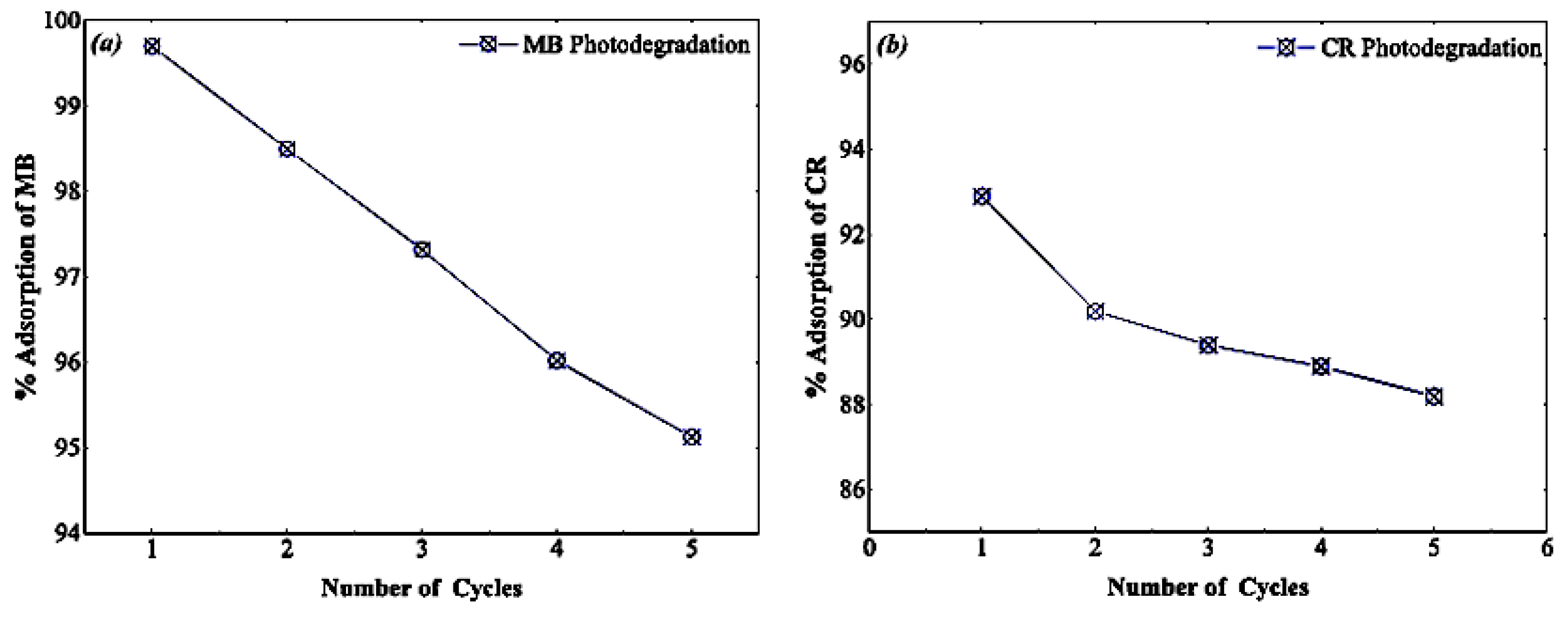
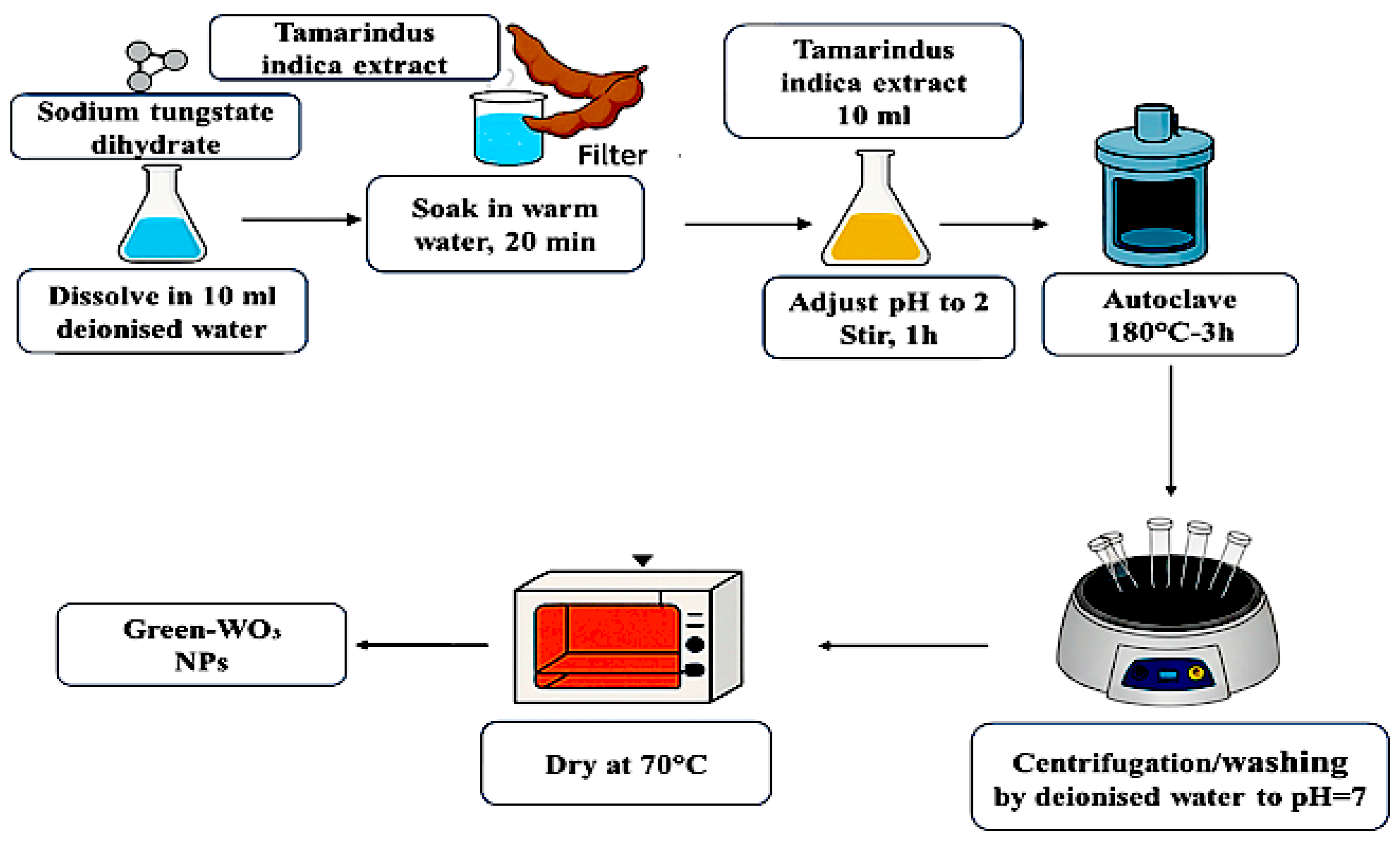
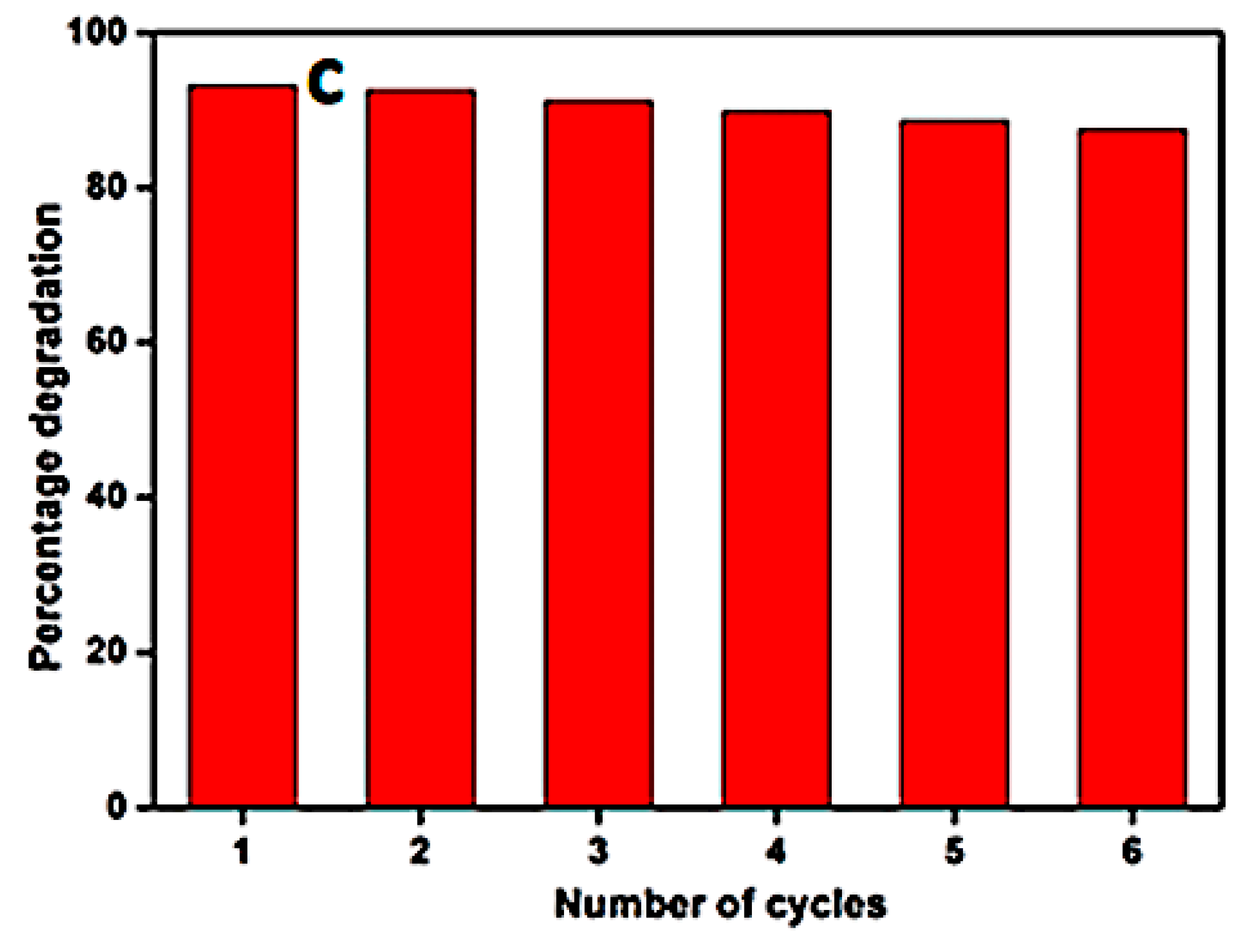
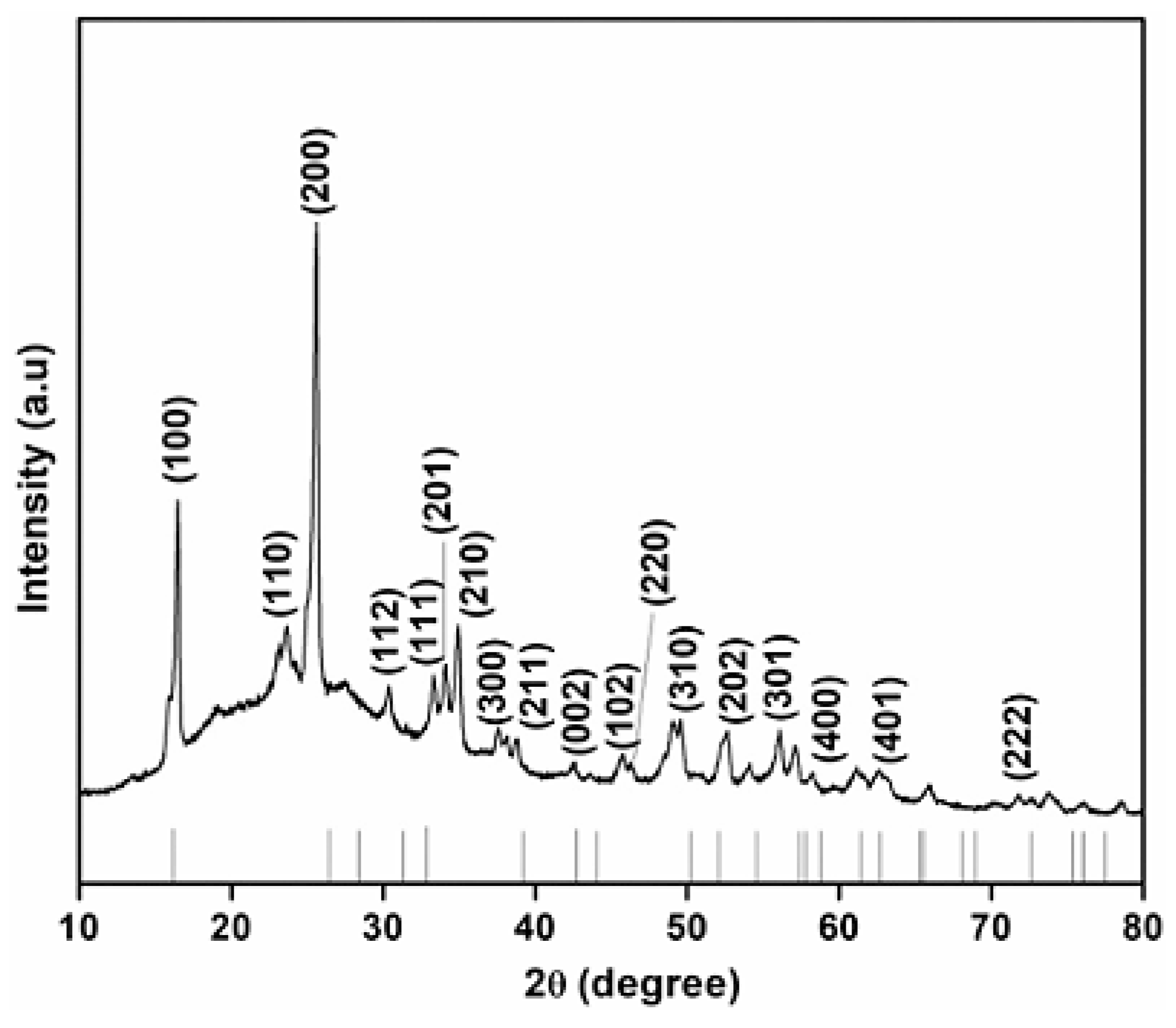

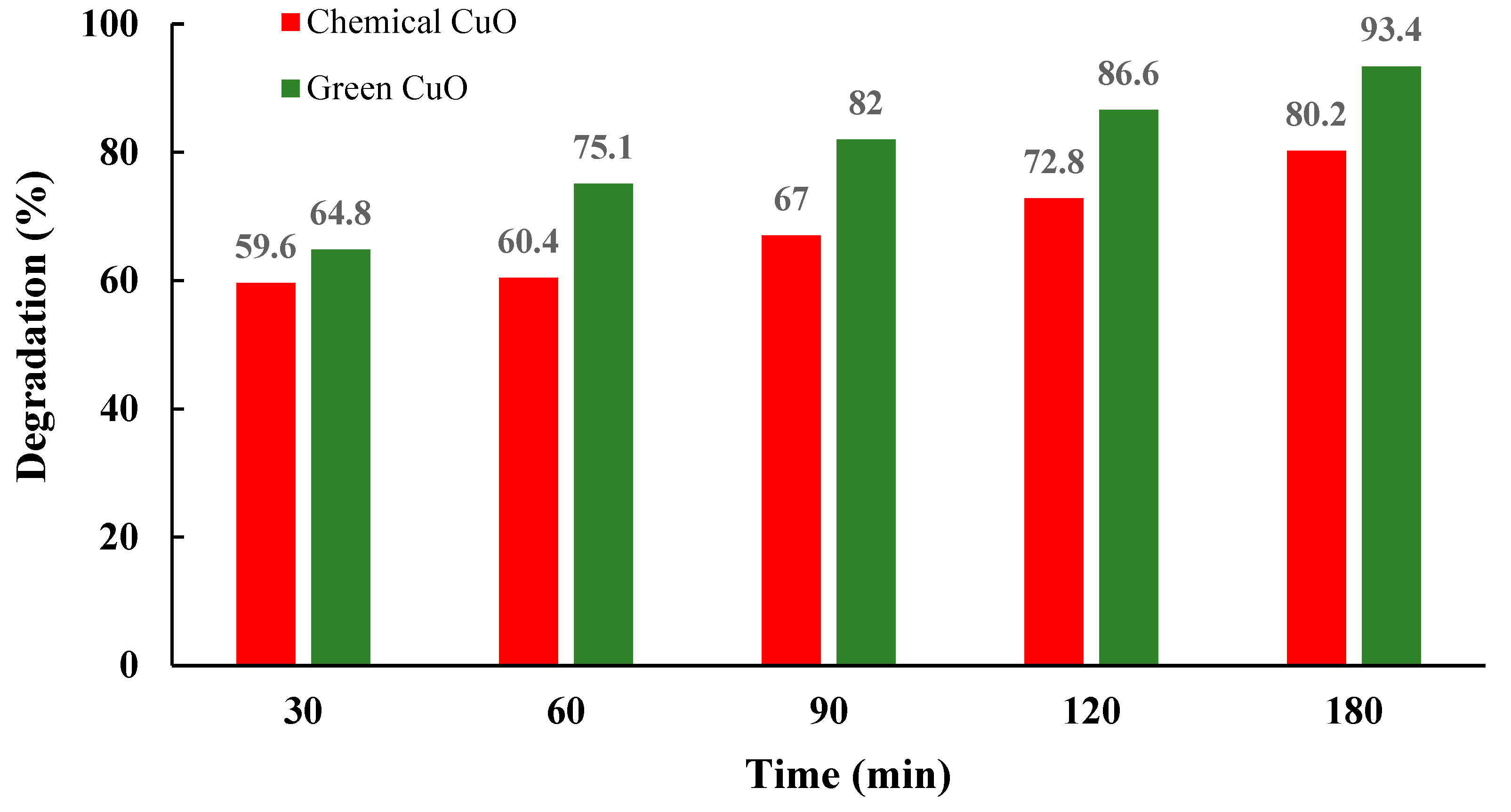
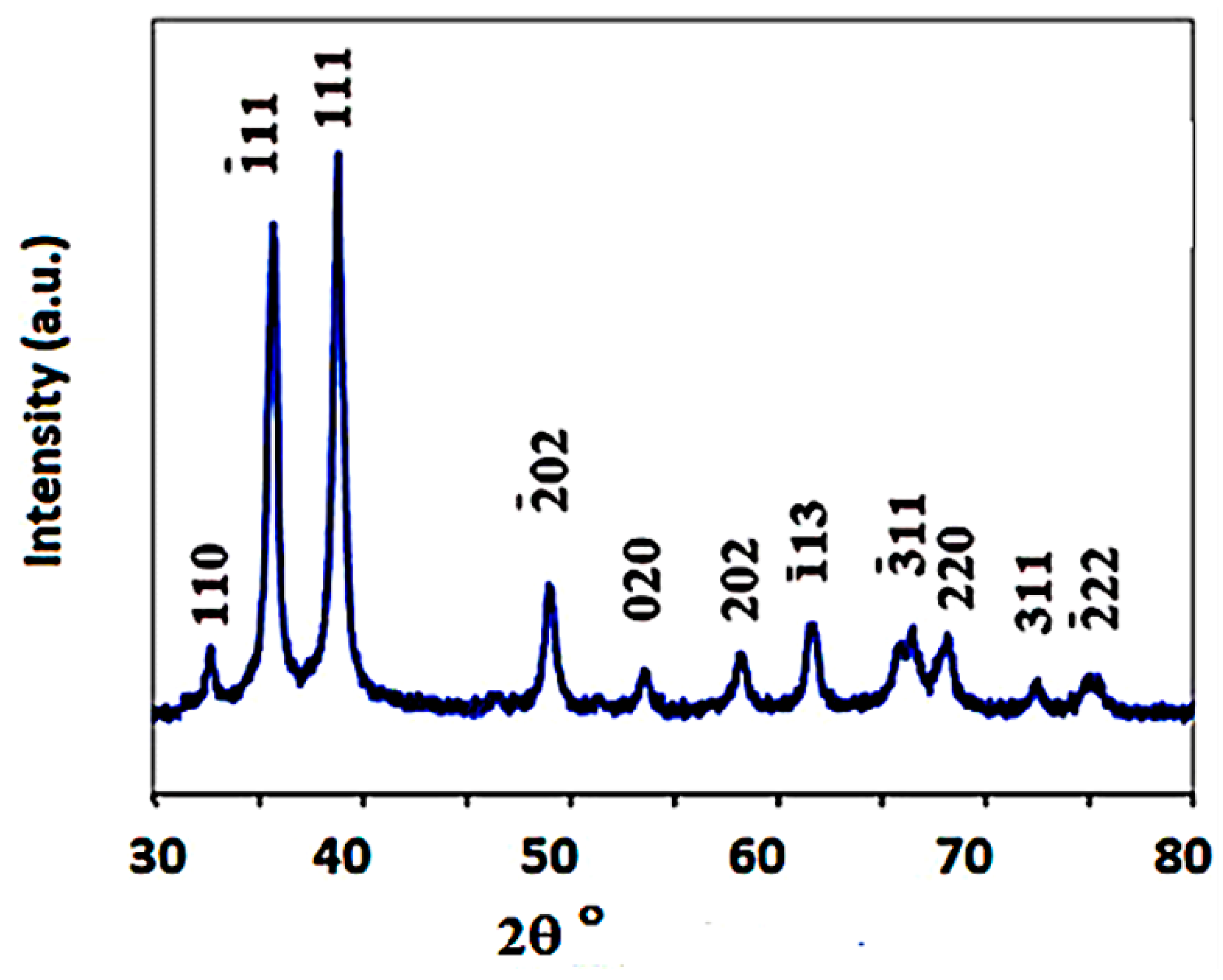
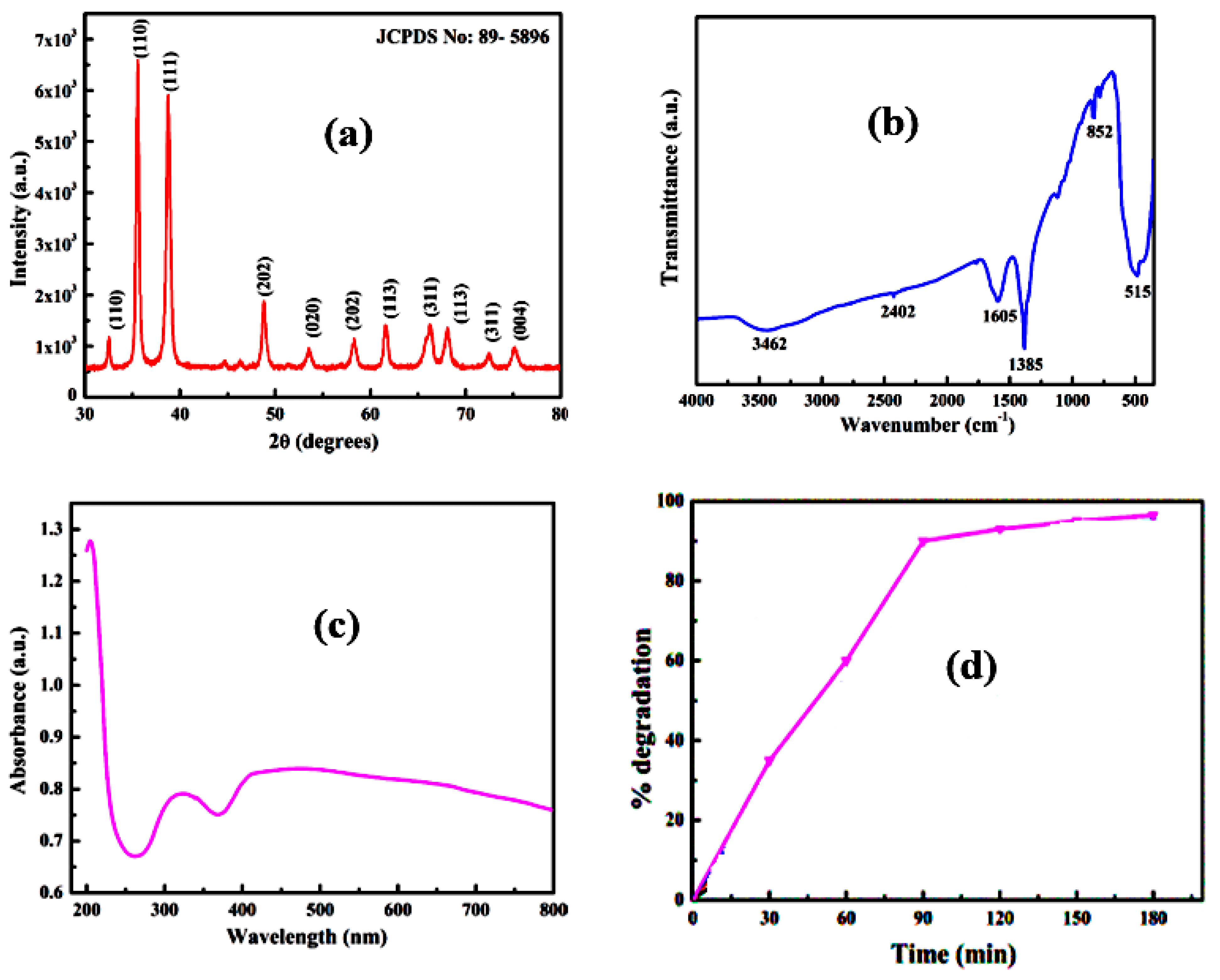
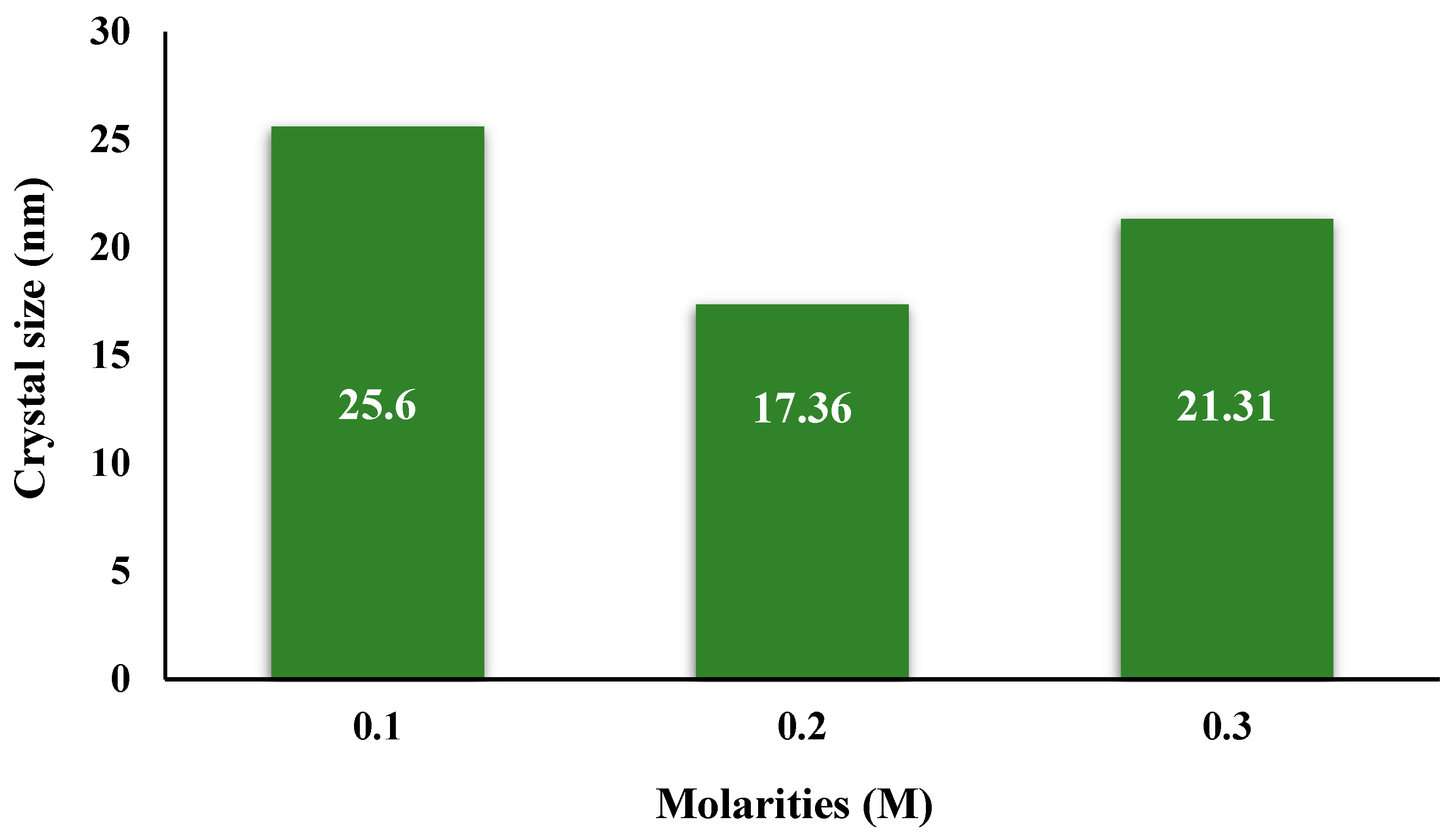
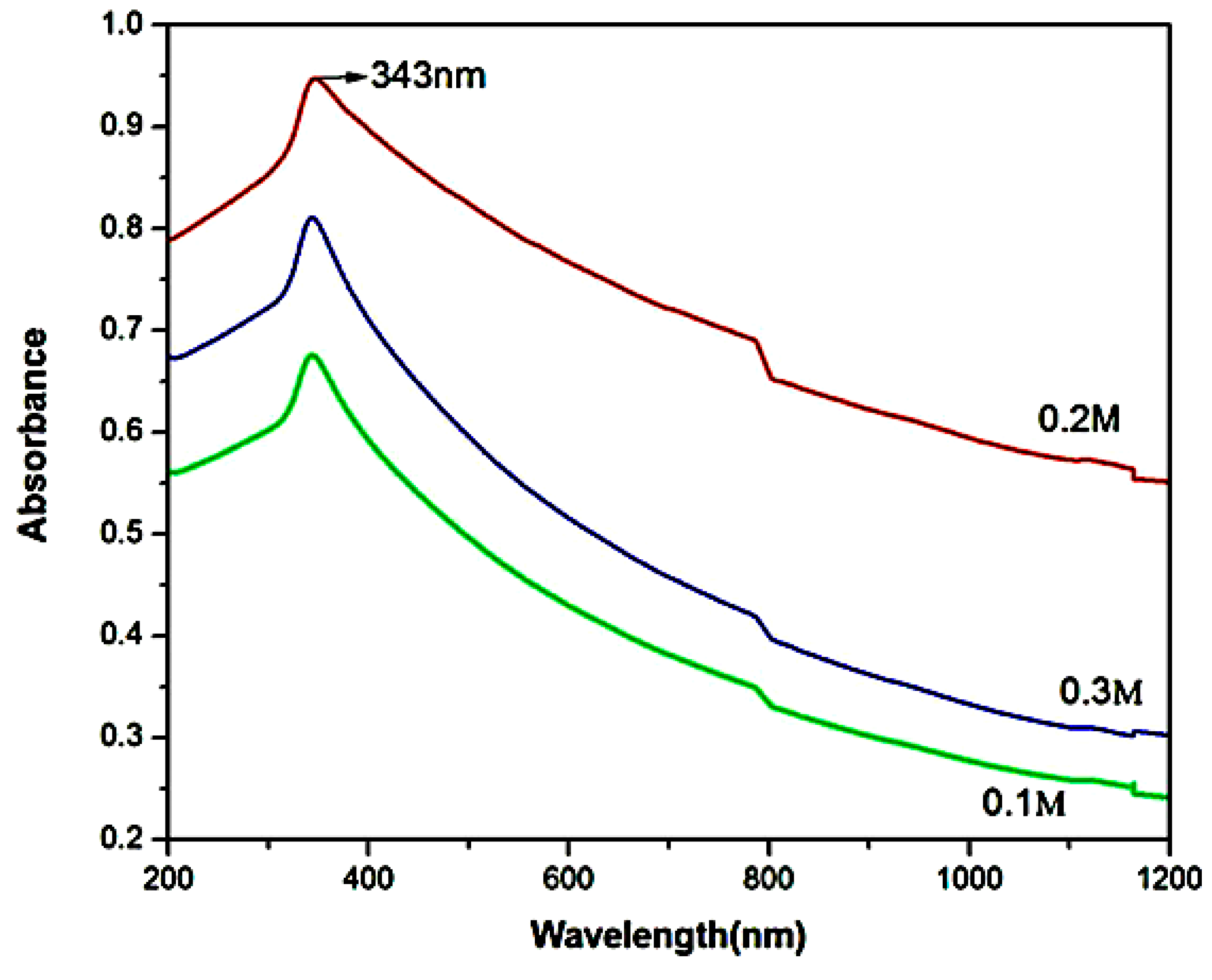

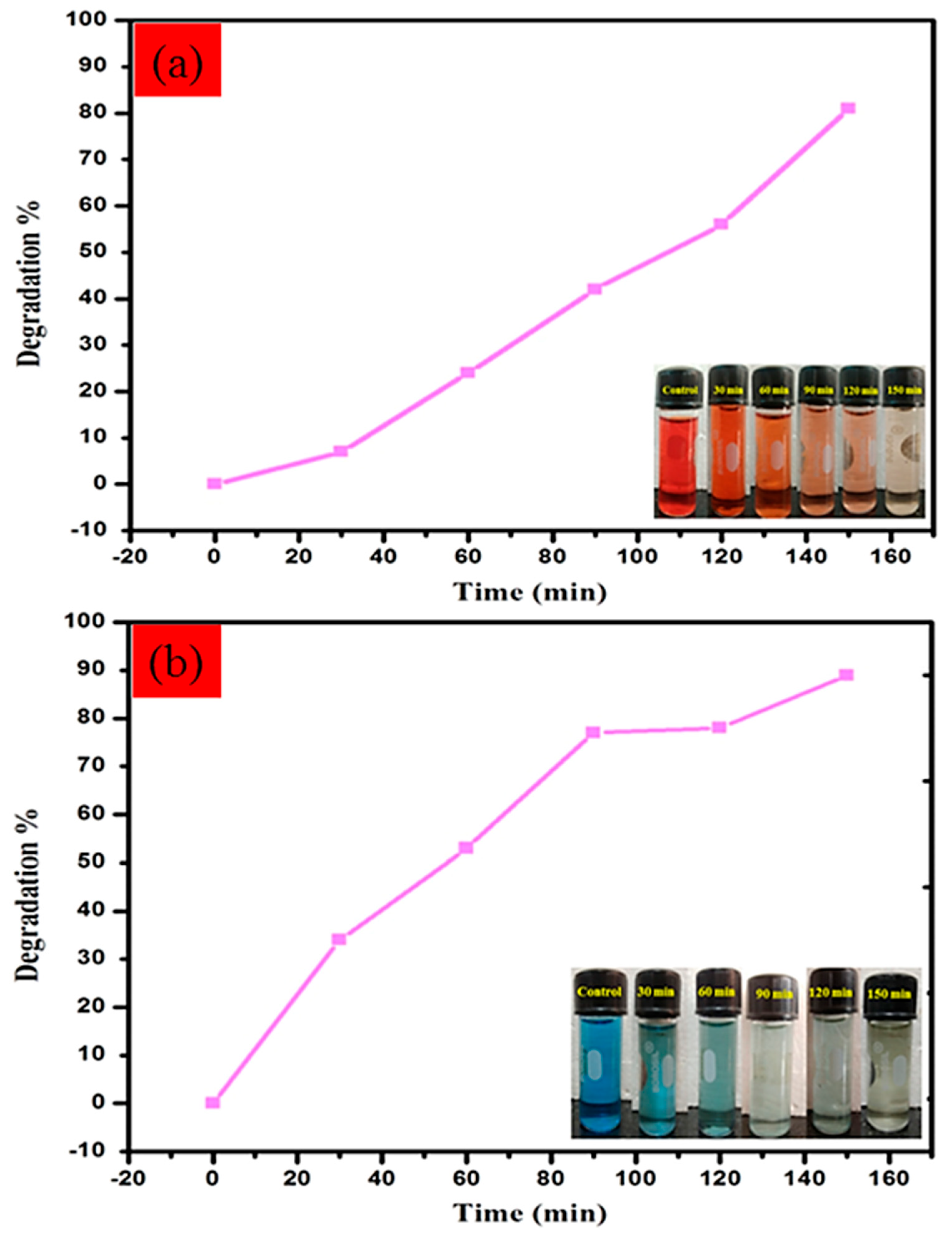
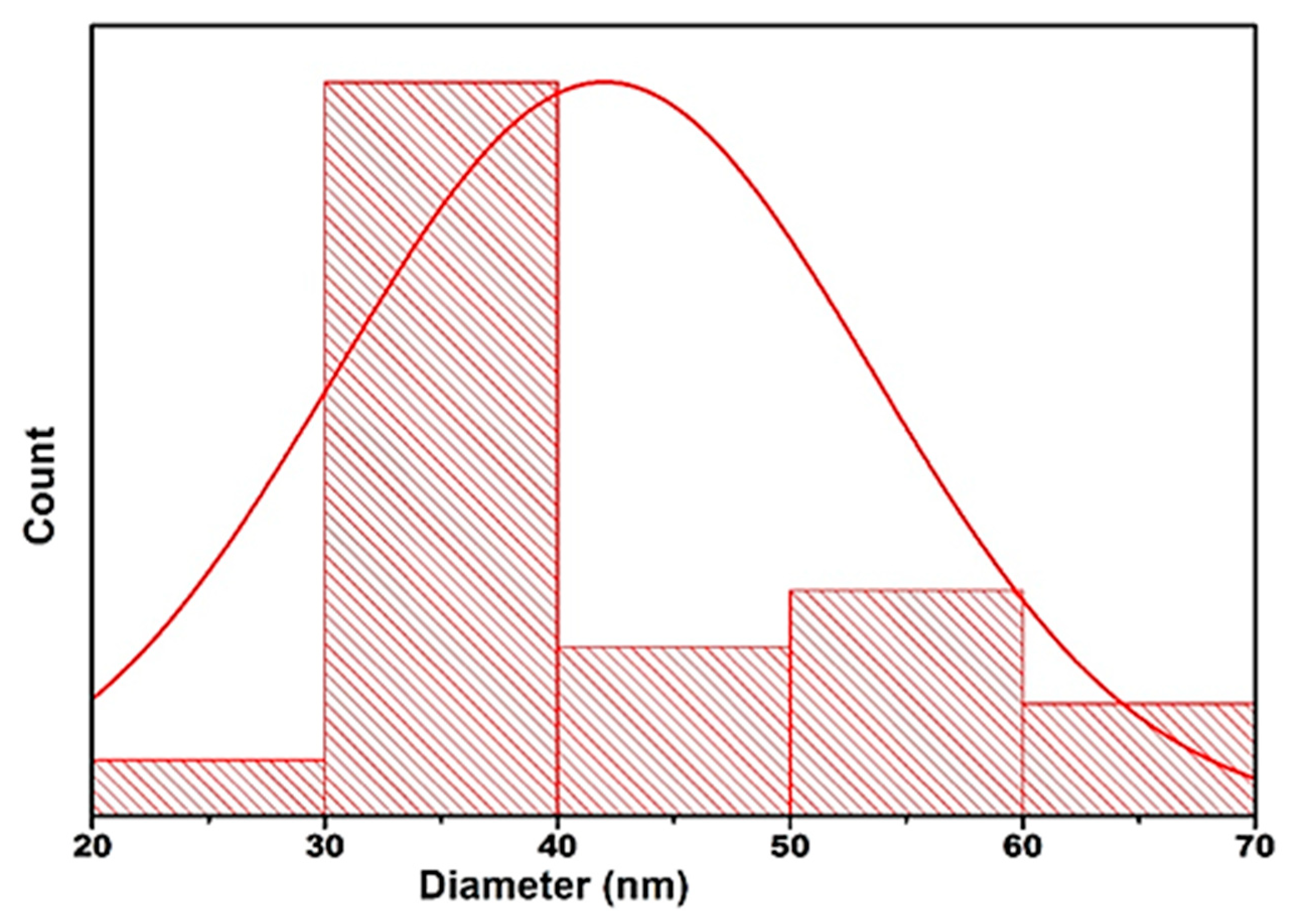
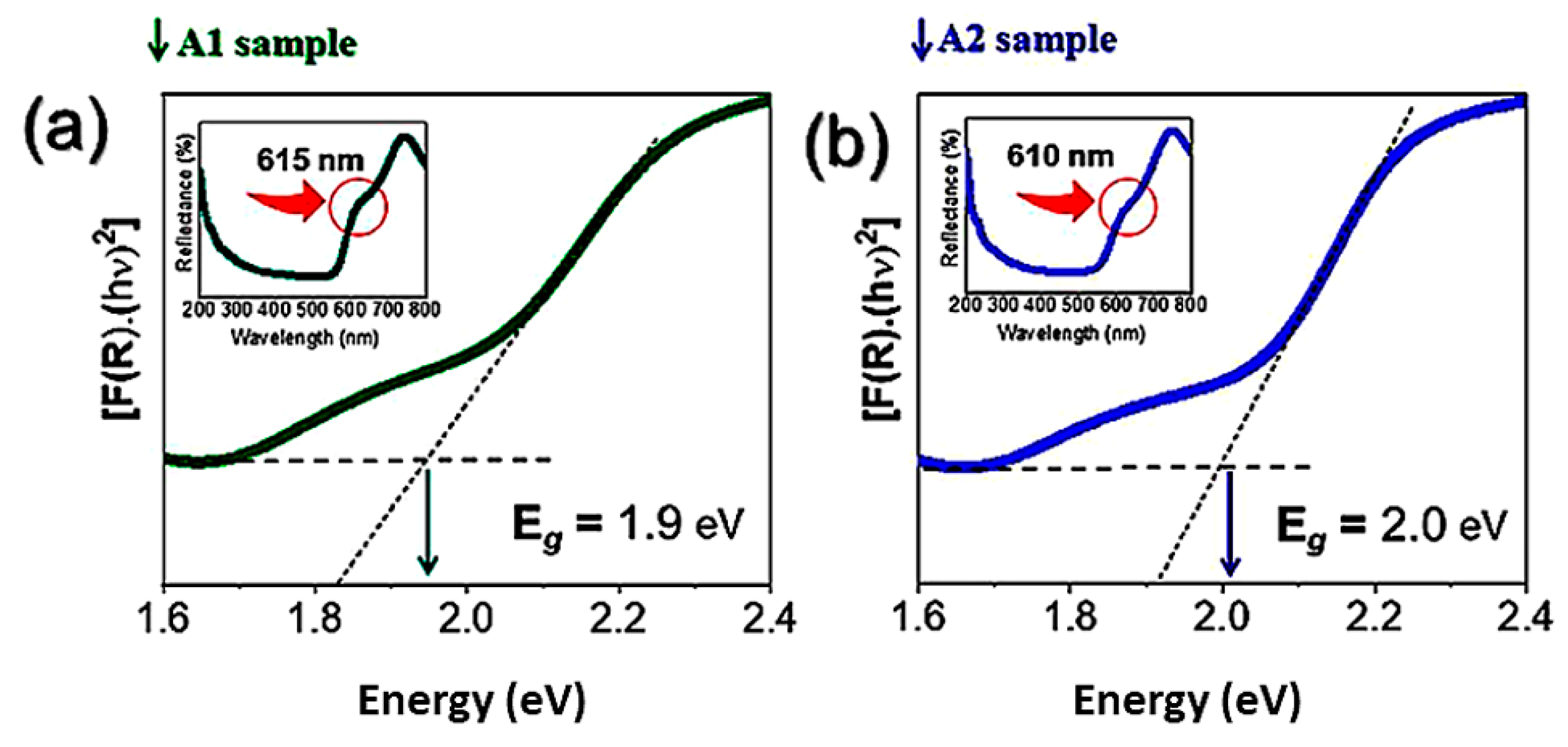
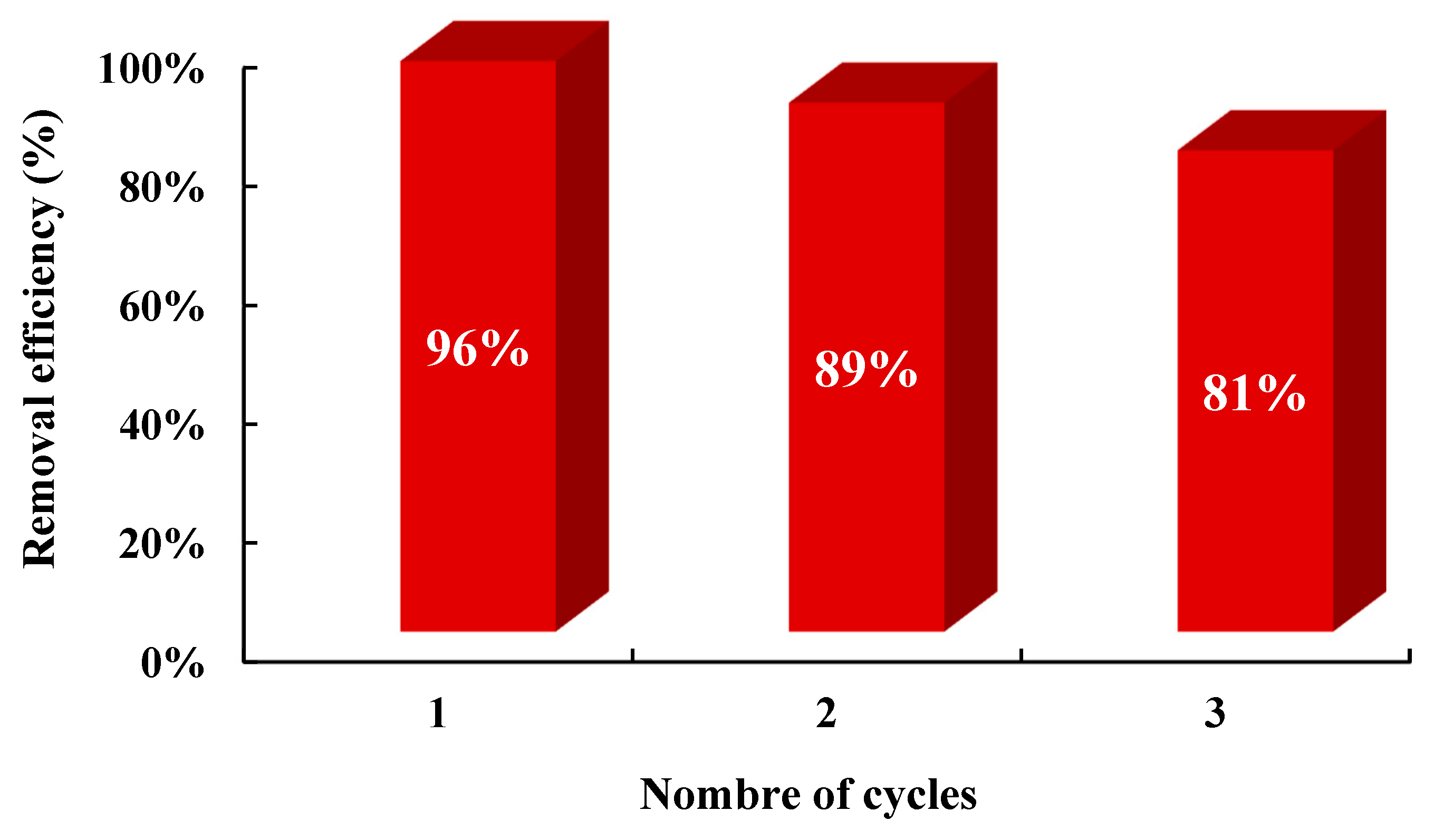
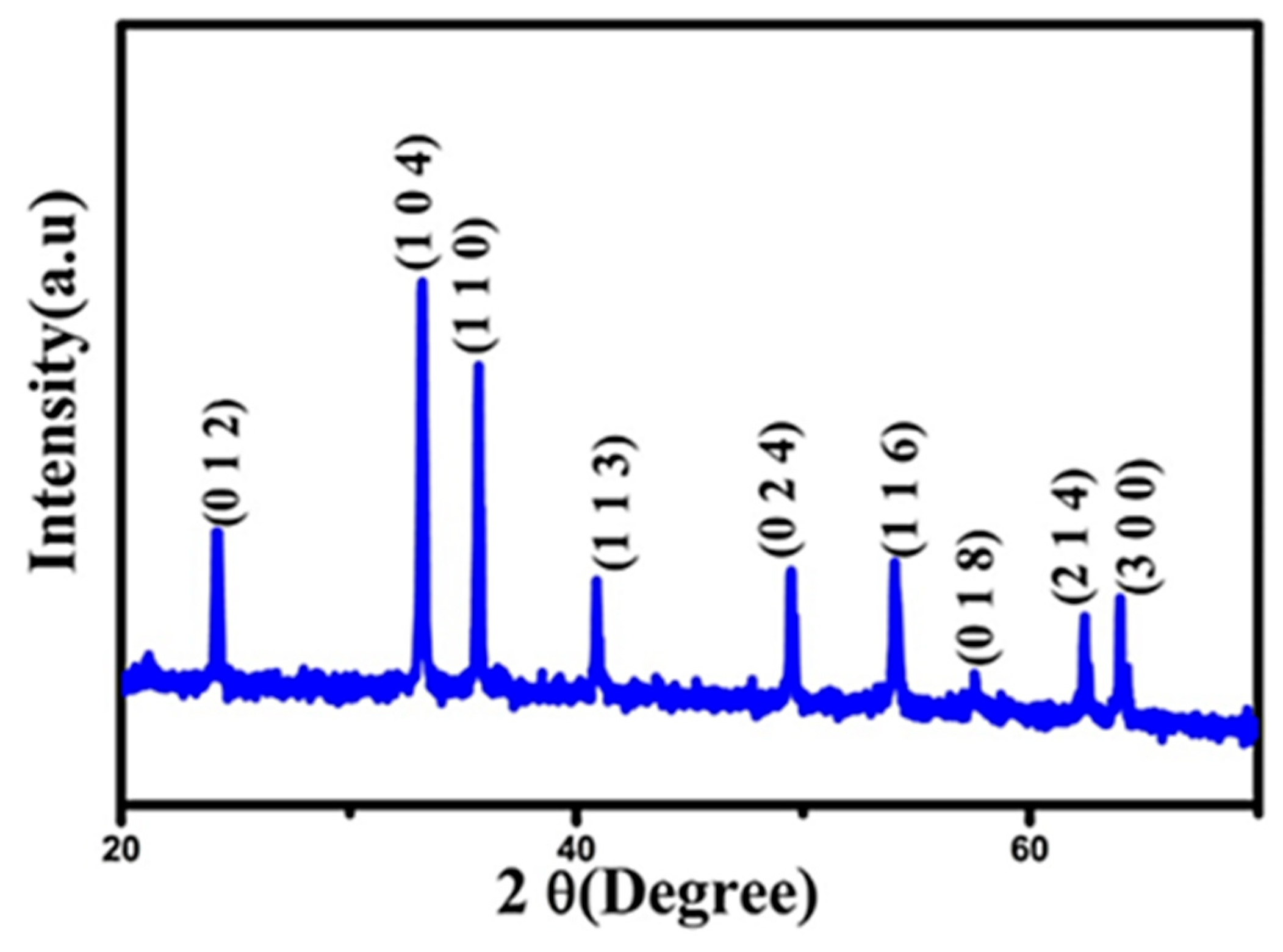
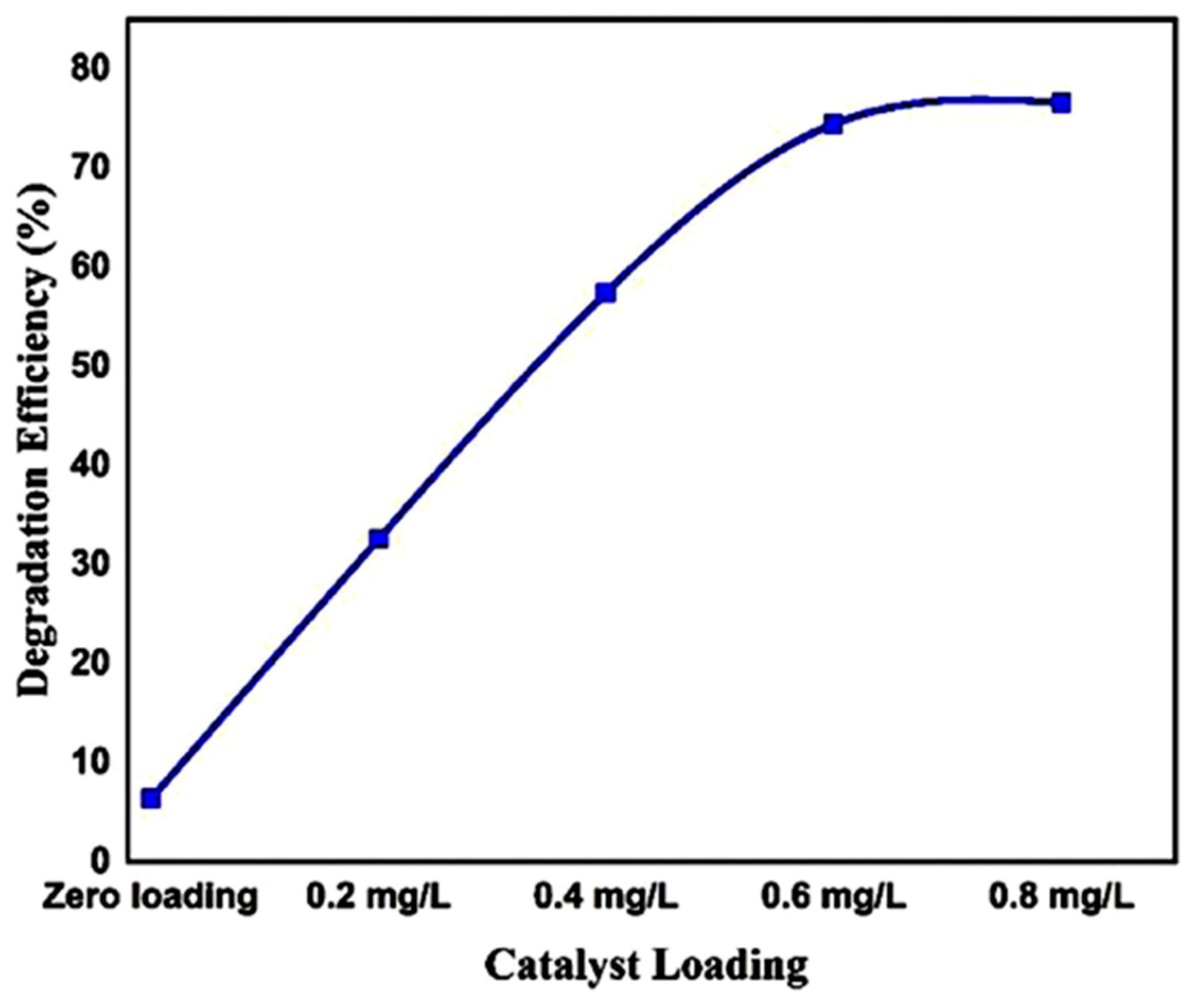
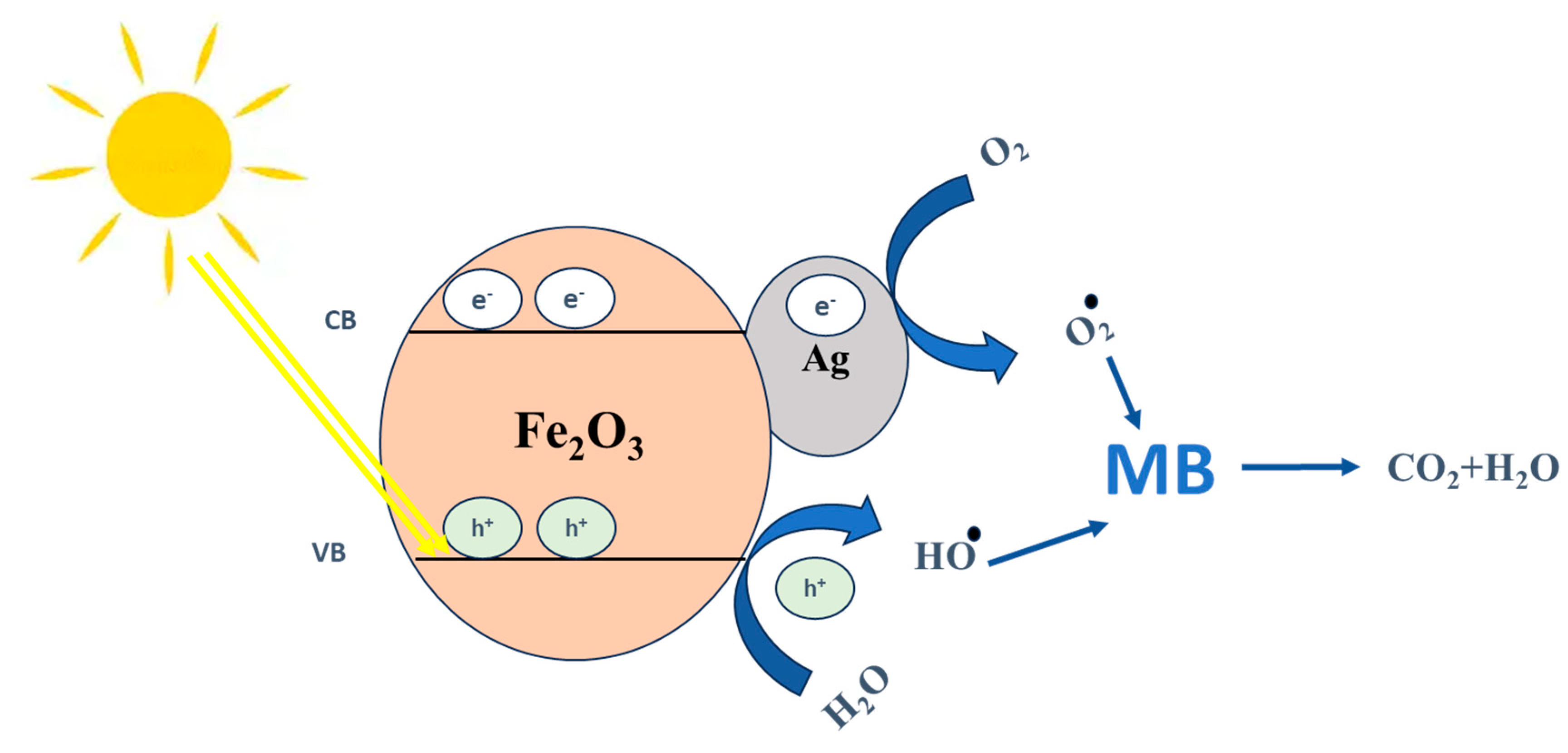

| 2θ | 25.33° | 37.90° | 47.89° | 53.90° | 54.94° | 62.74° | 70.18° | 74.96° |
| Crystal plane | (101) | (004) | (200) | (105) | (211) | (204) | (220) | (215) |
| Sample Code | Percent Extact (v/v) | Volume (mL) Ratio Precursor/Extract |
|---|---|---|
| TO | 0 | 5:0 |
| TOAv4 | 4% | 5:0.2 |
| TOAv10 | 10% | 5:0.5 |
| TOAv20 | 20% | 5:1 |
| Plant Extract | Metal Precursor | Size (nm) | BG (eV) | Ref. |
|---|---|---|---|---|
| Aloe vera | TiO2 | 14–24 | 3.15–3.18 | [65] |
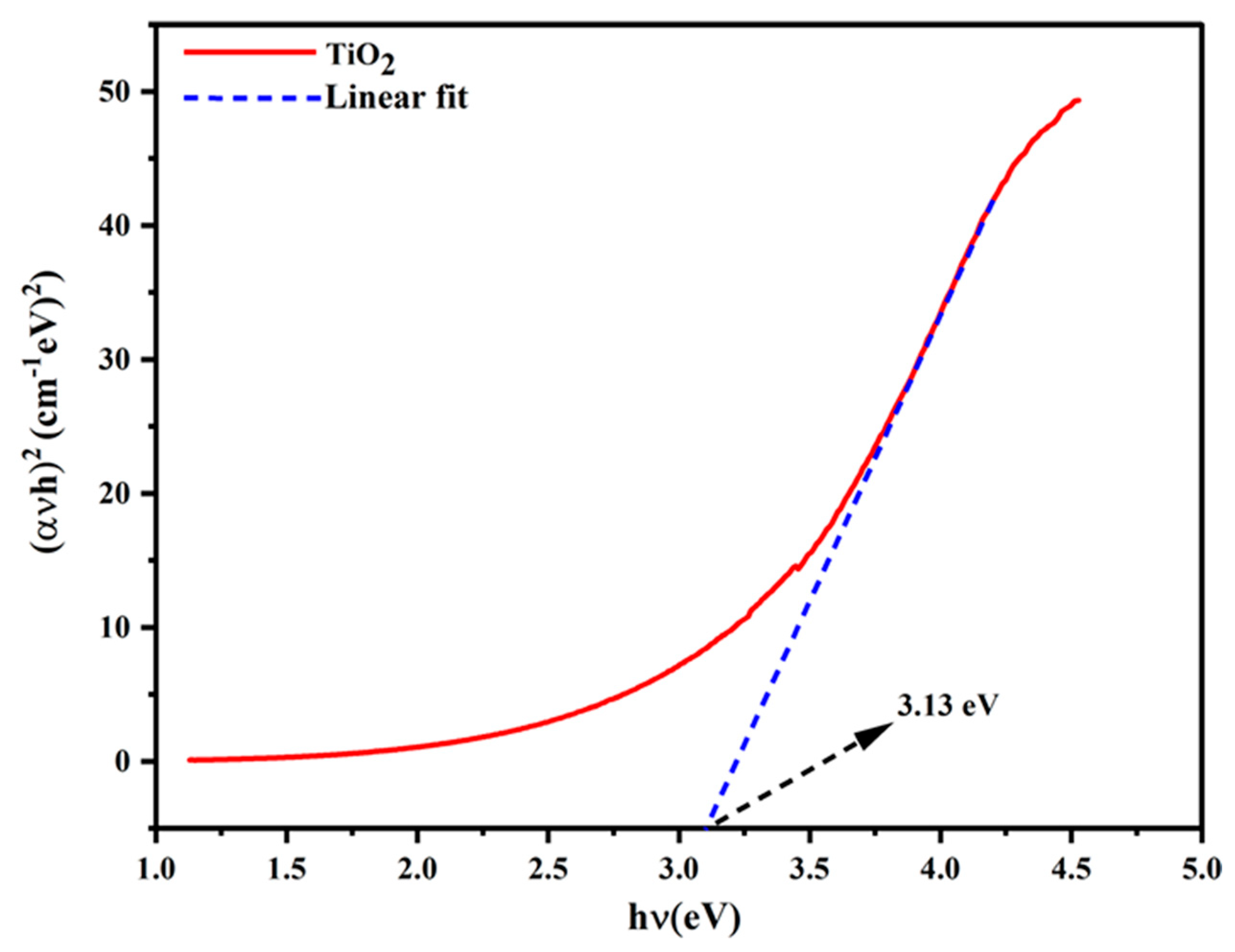
| Tinospora cordifolia | TiO2 | 15.02 | 3.13 | [69] |
| Mulberry | TiO2 | 24 | 3.16 | [70] |
| Lemon peel | TiO2 | 80–140 | 3.08 | [76] |
| Citrus limetta | TiO2 | 80–100 | 3.22 | [77] |
| Syzygium cumini | TiO2 | 10 | 3.48 | [78] |
| Piper betel | TiO2 | 6.6 | - | [79] |
| Ocimum tenuiflorum | TiO2 | 7.0 | - | [79] |
| Moringa oleifera | TiO2 | 6.6 | - | [79] |
| Mentha spicata | TiO2 | 11 | 3.22–3.25 | [80] |
| Terminalia catappa and carissa carandas | TiO2 | 10–21 | 3.21 | [81] |
| Trigonella foenum-graecum | TiO2 | 40–60 | - | [82] |
| Coriandrum sativum | TiO2 | 6.8 | - | [79] |
| Monsonia burkeana | TiO2 | 8.93 | 3.53 | [83] |
| Jatropha curcas | TiO2 | 13 | 3.28 | [84] |
| Photocatalyst | Plant Used | Pollutant | Experimental Conditions | % Degradation | Ref. |
|---|---|---|---|---|---|
| TiO2 | Aloe vera | MB | -Calcination temperature: T = 500 °C (1 h) -Irradiation source: UV light | 48%, 51.44%, 47.82%, 49.29% (without plant extract, with 4%, 10%, 20%, respectively) | [65] |
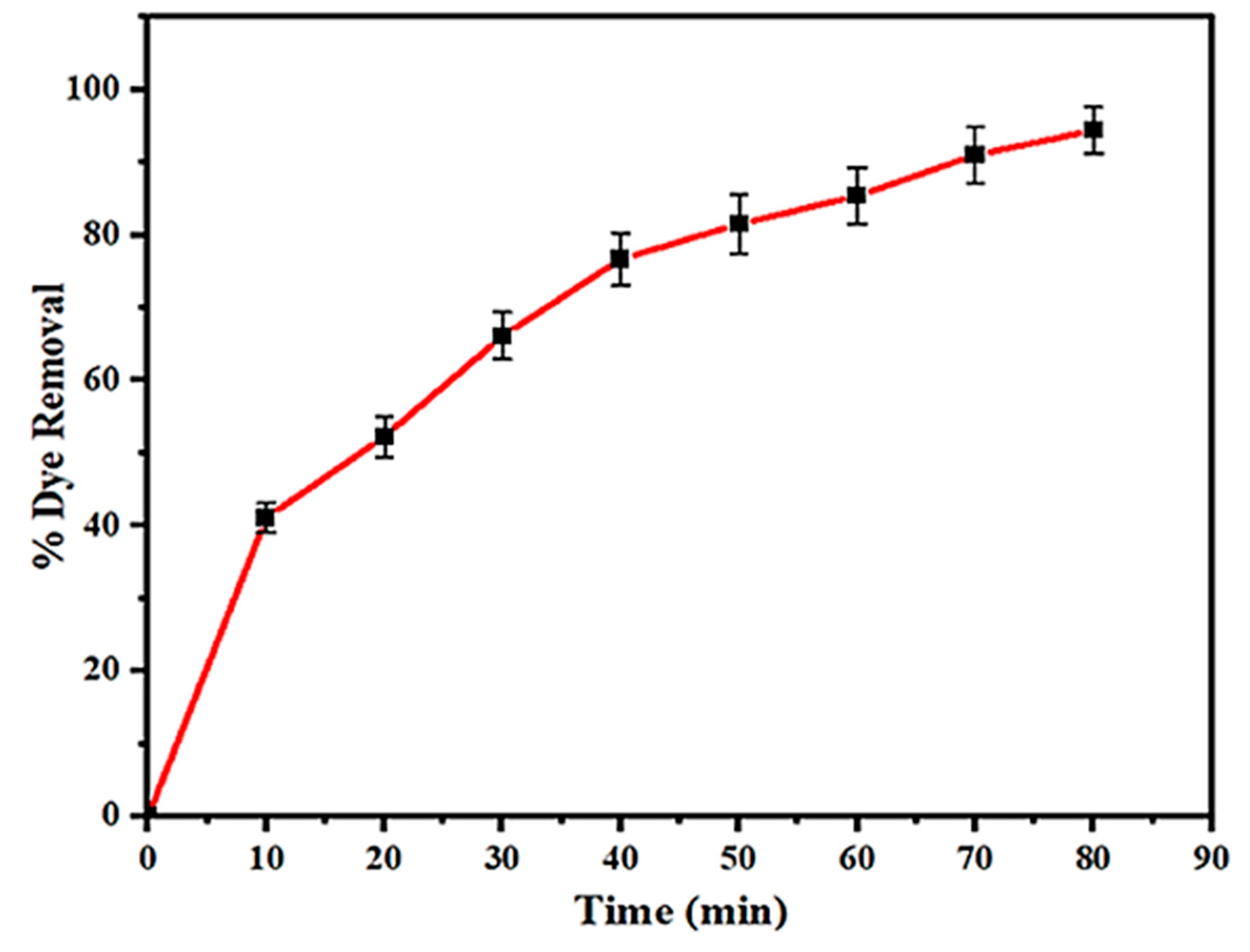
| TiO2 | Tinospora cordifolia | Acid Blue 113 dye | -Calcination temperature: T = 400 °C; -Irradiation source: UV light -Time of photocatalysis: 80 min; -Pollutant concentration: 50 mg/L -Amount of NPs: 2 g/L; -pH = 4 | 94.43% | [69] |
| TiO2 | Mulberry | MB | -Irradiation source: UV light; -Time of photocatalysis: 120 min -Pollutant concentration: 10 ppm (10 mL); -Mass of NPs: 10 mg | 96% | [70] |
| TiO2 | Lemon peel | RhB | -Calcination temperature: T = 500 °C (2 h); -Irradiation source: UV light -Time of photocatalysis: 120 min | >70% | [76] |
| TiO2 | Citrus limetta | -Calcination temperature: 550 °C (2 h); -Irradiation source: UV light; -Time of photocatalysis: 80 min; -Pollutant concentration: 10 mg/L (50 mL); -Mass of NPs: 0.7 g. | >90% | [77] | |
| TiO2 | Syzygium cumini | Pb | -Calcination temperature: 570 °C (3 h); -Irradiation source: UV light; -Time of photocatalysis: 17 h; -pollutant concentration: 8.6 ppm (500 mL); -Mass of NPs: 0.3 g. | 75.5% | [78] |
| COD | -Calcination temperature: 570 °C (3 h); -Irradiation source: UV light; -Time of photocatalysis: 17 h; -Pollutant concentration: 8450 mg/L (500 mL); -Mass of NPs: 0.3 g. | 82.53% | |||
| TiO2 | Piper betel | Malachite green dye. | -Calcination temperature: T = 400 °C (3 h); -Irradiation source: Solar light; -Time of photocatalysis: 30 min; -pollutant concentration: 100 ppm (50 mL); -Mass of NPs: 100 mg | ~50% | [79] |
| Ocimum tenuiflorum | ~70% | ||||
| Moringa oleifera | ~100% | ||||
| Coriandrum sativum | ~60% | ||||
| TiO2 | Mentha spicata | Reactive Black 5 | -Calcination temperature: T = 400 °C; -Irradiation source: UV light -Time of photocatalysis: 90 min; -Pollutant concentration: 5 ppm (75 mL) -Mass of NPs: 0.075 g | 96% | [80] |
| TiO2 | Monsonia burkeana | MB | -Calcination temperature: T = 500 °C (1 h); -Irradiation source: UV light -Time of photocatalysis: 120 min; -Pollutant concentration: 20 mg/L -Mass of NPs: 60 mg; -pH = 10 | 85.5% | [83] |
| TiO2 | Jatropha curcas | COD | -Calcination temperature: T = 450 °C (3 h); -Irradiation source: Solar light. -Time of photocatalysis: 5 h; -Pollutant concentration: 1428 mg/L. | 82.26% | [84] |
| Plant Extract | Metal Precursor | Size (nm) | BG (eV) | Ref. |
|---|---|---|---|---|
| Beetroot | Ca-ZnO | 20–50 | - | [66] |
| Vitex negundo | ZnO | 19 | 3.16 | [87] |
| Myrtus communis | Acetate-ZnO | 100 | 3.21 | [88] |
| Nitrate-ZnO | 100 | 3.24 | ||
| Spinacia oleracea | ZnO | 35–40 | 3.12 | [91] |
| Camellia sinensis | ZnO | 12.2 | 3.15 | [92] |
| Lawsonia inermis | ZnO | 22 | 3.37 | [93] |
| ZnO/Fe2O3 | 39 | 2.8 |
| Photocatalyst | Plant Used | Pollutant | Experimental Conditions | Degradation (%) | Ref. |
|---|---|---|---|---|---|
| ZnO | Beetroot | MB | -Calcination temperature: T = 450 °C (15 min); -Time of photocatalysis: 40 min; -Irradiation source: sunlight; -Pollutant concentration: 10 mg/L (100 mL); -Masse of NPs: 0.01 g | 100% | [66] |
| Ca-ZnO | -Calcination temperature: T = 450 °C (15 min); -Time of photocatalysis: 60 min; -Irradiation source: sunlight; -Pollutant concentration: 10 mg/L (100 mL); -Masse of NPs: 0.01 g | 90% | |||
| ZnO-acetate | Myrtus communis | MB | -Calcination temperature: T = 400 °C; -Irradiation source: UV light.; Time photocatalysis: 50 min; -Pollutant concentration: 10 ppm (100 mL); -Masse of NPs: 0.1 g. | 98% | [88] |
| ZnO-Nitrate | -Calcination temperature: T = 400 °C; -Irradiation source: UV light.; -Time of photocatalysis: 60 min; -Pollutant concentration: 10 ppm (100 mL); -Masse of NPs: 0.1 g | 99% | |||
| ZnO | Spinach leaves | Toluene | -Calcination temperature: T = 300 °C (120 min); -Irradiation source: sunlight; Time photocatalysis: 240 min; Pollutant concentration: 0.5 g/L (150 mL); -Masse of NPs: 0.05 g; pH = 8 | 84.26% | [91] |
| Xylene | -Calcination temperature: T = 300 °C (120 min); -Irradiation source: sunlight; Time photocatalysis: 240 min; Pollutant concentration: 0.5 g/L (150 mL); -Masse of NPs: 0.05 g; pH = 8 | 90.36% | |||
| COD | -Calcination temperature: T = 300 °C (120 min); -Irradiation source: sunlight; -Time photocatalysis: 240 min; Pollutant concentration: 0.5 g/L (150 mL); -Masse of NPs: 0.05 g; pH = 8 | 81.24% | |||
| ZnO | Camellia sinensis | chlortetracycline | -Calcination temperature: T 400 °C (3 h); -Irradiation source: UV-light; -Time photocatalysis: 60 min; -Pollutant concentration: 10 mg/L (50 mL); -Masse of NPs: 50 mg; pH = 3.5 | 94.70% | [92] |
| ZnO/H2O2 | Lawsonia inermis | Effluent dye mixture | -Irradiation source: Solar light; -Time photocatalysis: 3 h; -Pollutant (50 mL); -Masse of NPs: 1 mg; pH = 7 | 70.50% | [93] |
| ZnO/Fe2O3/H2O2 | -Irradiation source: Solar light; -Time photocatalysis: 3 h; -Pollutant (50 mL); -Masse of NPs: 1 mg; pH = 7 | 90.40% | |||
| ZnO | Solanum trilobatum | MB | -Irradiation source: Sunlight; -Time photocatalysis: 90 min; -Pollutant concentration: 10 μm; -Catalyst Amount: 0.6 g/L | 94.07% | [94] |
| ZnO | Lepidagathis ananthapuramensis | MB | -Irradiation source: Sunlight; -Time photocatalysis: 120 min; -Pollutant concentration: 10 ppm; -Catalyst Amount: 5 mg | 98.50% | [95] |
| ZnO | Euphorbia milii | MB | -Irradiation source: Sunlight; -Time photocatalysis: 50 min; -Pollutant concentration: 10 μm -Catalyst Amount: 0.6 g/L | 98.17% | [96] |
| ZnO | Phoenix roebelenii | MB | -Irradiation source: UV lamp; -Time photocatalysis: 105 min; -Pollutant concentration: 10 ppm; -Masse of NPs: 0.2 g | 98% | [97] |
| 2θ | 25.01° | 28.91° | 32.21° | 33.21° | 33.85° | 35.5° | 37.7° | 44.96° | 65.11° |
| Crystal plane | (202) | (400) | (110) | (200) | (002) | (111) | (−142) | (112) | (113) |
| Photocatalyst | Plant Used | Pollutant | Experimental Conditions | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|
| CuO | Ephedra Alata | MB | -Calcination temperature: T = 400 °C (4 h); -Irradiation source: Sunlight -Time of photocatalysis 3 h; -Pollutant concentration: 10 mg/L; -Mass of NPs: 20 mg | 93.4% | [67] |
| CuO | Green tea extract | MB | -Calcination temperature: T = 380 °C (10 min) -Irradiation source: Sunlight (850 Wm−2); -Time of photocatalysis: 3 h -Pollutant concentration: (5–20 ppm); -Mass of NPs: 50–200 mg | 92% | [103] |
| -Calcination temperature: T = 380 °C (10 min) -Irradiation source: UV-light (125 Wm−2); -Time of photocatalysis: 3 h -Pollutant concentration: (5–20 ppm); -Mass of NPs: 50–200 mg | 99% | ||||
| CuO | Lantana camara | MB | -Calcination temperature: T = 500 °C (4 h); -Irradiation source: Sunlight; -Time of photocatalysis: 90 min; -Molarity of NPs: 0.2 M | 94% | [104] |
| CuO | Psidium guajava | MB | -Irradiation source: sunlight; -Time of photocatalysis: 2 h; -Pollutant concentration: 30 mg/L; -Mass of NPs: 20 mg | 89% | [109] |
| Congo Red | -Irradiation source: sunlight; -Time of photocatalysis: 2 h; -Pollutant concentration: 30 mg/L; -Mass of NPs: 20 mg | 81% | |||
| Cu2O | Curcumin | MO | -Irradiation source: UV-light; -Time of photocatalysis: 2 h -Pollutant concentration: 150 μm; -Mass of NPs: 50 mg; pH = 3 | 94.5% | [116] |
| Sample | Plant Extract | Pollutant/Dye | Volume | Pollutant Dose | Catalyst Dose | Light Irradiation | Contact Time | BG (eV) | Crystallite Size (nm) | Removal (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Fe2O3 | Syzygium cumini | Norfloxacin | 50 mL | 50 mg/L | 50 mg | UV | 90 min | 1.9–2.0 | 28.51–37.64 | 96 | [118] |
| Fe2O3 | Trachyspermum ammi | MB | 100 mL | 10 ppm | 10 mg | Visible | 120 min | - | 26 | 91.0 | [127] |
| α-Fe2O3 | Carica papaya | Remazol Yellow | 100 mL | 50 ppm | 0.8 g/L | Sunlight | 250 min | - | 21.59 | 75 | [128] |
| Ag/Fe2O3 | Carica papaya | MB | - | 20 mg/L | 0.2 g/L | Visible | 90 min | 2.91 | 29.17 | 92.97 | [129] |
| Fe2O3 | Pomegranate seeds | Reactive Blue | 100 mL | 20 mg/L | 15 mg | UV | 56 min | - | 25–55 | 95.08 | [130] |
| Fe2O3 | Cynometra ramiflora | MB | 50 mL | 20 ppm | 30 mg | Sunlight | 4 h | - | 68.17 | 94.0 | [131] |
| Fe2O3 | Cassia auriculata | Malachite Green | 100 mL | - | 20 mg | Visible | 150 min | - | - | 91.2 | [132] |
| Fe2O3 | Withania coagulans | Safranin | 100 mL | 10 ppm | 0.5 mg | Sunlight | 180 min | 4.20 | 16 ± 2 | 68.8 | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallah, S.; El Mchaouri, M.; El Meziani, S.; Agnaou, H.; El Haddaj, H.; Boumya, W.; Barka, N.; Elhalil, A. Green Photocatalysis: A Comprehensive Review of Plant-Based Materials for Sustainable Water Purification. Reactions 2025, 6, 55. https://doi.org/10.3390/reactions6040055
Mallah S, El Mchaouri M, El Meziani S, Agnaou H, El Haddaj H, Boumya W, Barka N, Elhalil A. Green Photocatalysis: A Comprehensive Review of Plant-Based Materials for Sustainable Water Purification. Reactions. 2025; 6(4):55. https://doi.org/10.3390/reactions6040055
Chicago/Turabian StyleMallah, Safiya, Mariam El Mchaouri, Salma El Meziani, Hafida Agnaou, Hajar El Haddaj, Wafaa Boumya, Noureddine Barka, and Alaâeddine Elhalil. 2025. "Green Photocatalysis: A Comprehensive Review of Plant-Based Materials for Sustainable Water Purification" Reactions 6, no. 4: 55. https://doi.org/10.3390/reactions6040055
APA StyleMallah, S., El Mchaouri, M., El Meziani, S., Agnaou, H., El Haddaj, H., Boumya, W., Barka, N., & Elhalil, A. (2025). Green Photocatalysis: A Comprehensive Review of Plant-Based Materials for Sustainable Water Purification. Reactions, 6(4), 55. https://doi.org/10.3390/reactions6040055







