Regioselectivity of the Claisen Rearrangement of Meta- and Para-Substituted Allyl Aryl Ethers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. General Procedure for the Synthesis of Substituted Allyl Aryl Ethers
- Via silica gel chromatography where the mobile phase was 9:1 hexane–ethyl acetate.
- By dissolving it in ethyl acetate and washing with a solution of KH2PO4, Na2CO3, and water. Then, it was dried over sodium sulfate, filtered, and solvent evaporated off.
- Recrystallization from ethyl acetate (EtOAc) for m = NHCOCH3.
2.4. Characterization of Meta-Substituted Allyl Aryl Ethers
- 1-(allyloxy)-3-chlorobenzene [19]
- 1-(allyloxy)-3-iodobenzene [21]
- 1-(allyloxy)-3-methylbenzene [19]
- 1-(allyloxy)-3-ethylbenzene [18]
- 1-(allyloxy)-3-(tert-butyl)benzene [22]
- 3-(allyloxy)-1,1’-biphenyl [23]
- N-(3-(allyloxy)phenyl)acetamide [26]
- 3-(allyloxy)-benzaldehyde [27]
- allyl 3-(allyloxy)benzoate [28]
- 1-(allyloxy)-3-nitrobenzene [9]
2.5. Characterization of Meta- and Para-Substituted Allyl Aryl Ethers
- 4-(allyloxy)-1-fluoro-2-methylbenzene
- 4.-(allyloxy)-1-chloro-2-methylbenzene [9]
- 4-(allyloxy)-1-bromo-2-methylbenzene [29]
- 4-(allyloxy)-1,2-dimethylbenzene [30]
- 4-(allyloxy)-1-isopropyl-2-methylbenzene [31]
- (4-(allyloxy)-2-methylphenyl)(methyl)sulfane [17]
- 4-(allyloxy)-2-chloro-1-fluorobenzene
- 4-(allyloxy)-1,2-dichlorobenzene [32]
- 4-(allyloxy)-2-chloro-1-methylbenzene
- 4-(allyloxy)-2-chlorobenzonitrile
2.6. General Protocol for the Claisen Rearrangement
2.7. NMR Analysis of the Claisen Rearrangement Products of Meta-Substituted Allyl Aryl Ethers
- Claisen rearrangement of 1-(allyloxy)-3-chlorobenzene [19]
- Claisen rearrangement of 1-(allyloxy)-3-iodobenzene
- Claisen rearrangement of 1-(allyloxy)-3-methylbenzene [9]
- Claisen rearrangement of 1-(allyloxy)-3-ethylbenzene [34]
- Claisen rearrangement of N-(3-(allyloxy)phenyl)acetamide [35]
- Claisen rearrangement of 3-(allyloxy)-benzaldehyde [36]
- Claisen rearrangement of allyl 3-(allyloxy)benzoate
- Claisen rearrangement of 4-(allyloxy)-1-fluoro-2-methylbenzene
- Claisen rearrangement of 4-(allyloxy)-1-chloro-2-methylbenzene [9]
- Claisen rearrangement of 4-(allyloxy)-1-bromo-2-methylbenzene
- Claisen rearrangement of 4-(allyloxy)-1,2-dimethylbenzene [37]
- Claisen rearrangement of 4-(allyloxy)-1-isopropyl-2-methylbenzene
- Claisen rearrangement of (4-(allyloxy)-2-methylphenyl)(methyl)sulfane
- Claisen rearrangement of 4-(allyloxy)-2-chloro-1-fluorobenzene
- Claisen rearrangement of 4-(allyloxy)-1,2-dichlorobenzene [38]
- Claisen rearrangement of 4-(allyloxy)-2-chloro-1-methylbenzene
- Claisen rearrangement of 4-(allyloxy)-2-chloro-benzonitrile
3. Results and Discussion
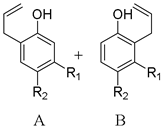
3.1. Meta-Substituted Allyl Aryl Ethers
3.2. Meta- and Para-Substited Allyl Aryl Ethers
3.3. Population Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EtOAc | Ethyl acetate |
| HPA | Hirshfeld population analysis |
| K2CO3 | Potassium carbonate |
| KI | Potassium Iodide |
| LPA | Löwdin population analysis |
| MPA | Mulliken population analysis |
| NMR | Nuclear Magnetic Resonance |
| NPA | Natural population analysis |
References
- Olah Vol, G.A. Mechanism of Electrophilic Aromatic Substitutions. Acc. Chem. Res. 1971, 4, 240–248. [Google Scholar] [CrossRef]
- Richard, J.P.; Jencks, W.P. A Simple Relationship between Carbocation Lifetime and Reactivity-Selectivity Relationships for the Solvolysis of Ring-Substituted 1-Phenylethyl Derivatives. J. Am. Chem. Soc. 1982, 104, 4689–4691. [Google Scholar] [CrossRef]
- Claisen, L. Über Umlagerung von Phenol-Allyl-Äthern in C-Allyl-Phenole. Ber. Dtsch. Chem. Ges. 1912, 45, 3157–3166. [Google Scholar] [CrossRef]
- Castro, A.M.M. Claisen Rearrangement over the Past Nine Decades. Chem. Rev. 2004, 104, 2939–3002. [Google Scholar] [CrossRef]
- Ganem, B. The Mechanism of the Claisen Rearrangement: Déja Vu All Over Again. Angew. Chem. Int. Ed. Engl. 1996, 35, 936–945. [Google Scholar] [CrossRef]
- Claisen, L.; Tietze, E. Über Den Mechanismus Der Umlagerung Der Phenol-Allyläther. Ber. Dtsch. Chem. Ges. 1925, 58, 275–281. [Google Scholar] [CrossRef]
- Jefferson, A.; Scheinmann, F. Molecular Rearrangements Related to the Claisen Rearrangement. Q. Rev. Chem. Soc. 1968, 22, 391–421. [Google Scholar] [CrossRef]
- Hiersemann, M.; Nubbemeyer, U. (Eds.) The Claisen Rearrangement; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; ISBN 9783527308255. [Google Scholar]
- Gozzo, F.C.; Fernandes, S.A.; Rodrigues, D.C.; Eberlin, M.N.; Marsaioli, A.J. Regioselectivity in Aromatic Claisen Rearrangements. J. Org. Chem. 2003, 68, 5493–5499. [Google Scholar] [CrossRef] [PubMed]
- Krenske, E.H.; Burns, J.M.; McGeary, R.P. Claisen Rearrangements of Benzyl Vinyl Ethers: Theoretical Investigation of Mechanism, Substituent Effects, and Regioselectivity. Org. Biomol. Chem. 2017, 15, 7887–7893. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Fusegi, K.; Kumamoto, T.; Ishikawa, T. Boron Trichloride Mediated Regioselective Claisen Rearrangement of Resorcinol Derivatives: Application to Resorcinol Carvonyl Ethers. Synthesis 2007, 2007, 1785–1796. [Google Scholar] [CrossRef]
- Meyer, M.P.; DelMonte, A.J.; Singleton, D.A. Reinvestigation of the Isotope Effects for the Claisen and Aromatic Claisen Rearrangements: The Nature of the Claisen Transition States. J. Am. Chem. Soc. 1999, 121, 10865–10874. [Google Scholar] [CrossRef]
- Lto, H.; Sato, A.; Taguchi, T. Enantioselective Aromatic Claisen Rearrangement. Tetrahedron Lett. 1997, 38, 4815–4818. [Google Scholar] [CrossRef]
- Pincock, A.L.; Pincock, J.A.; Stefanova, R. Substituent Effects on the Rate Constants for the Photo-Claisen Rearrangement of Allyl Aryl Ethers. J. Am. Chem. Soc. 2002, 124, 9768–9778. [Google Scholar] [CrossRef]
- White, W.N.; Gwynn, D.; Schlitt, R.; Girard, C.; Fife, W. The Ortho-Claisen Rearrangement. I. The Effect of Substituents on the Rearrangement of Allyl p-X-Phenyl Ethers. J. Am. Chem. Soc. 1958, 80, 3271–3277. [Google Scholar] [CrossRef]
- Kirsten, M.; Rehbein, J.; Hiersemann, M.; Strassner, T. Organocatalytic Claisen Rearrangement: Theory and Experiment. J. Org. Chem. 2007, 72, 4001–4011. [Google Scholar] [CrossRef]
- Pan, W.; Li, C.; Zhu, H.; Li, F.; Li, T.; Zhao, W. A Mild and Practical Method for Deprotection of Aryl Methyl/Benzyl/Allyl Ethers with HPPh 2 and t BuOK. Org. Biomol. Chem. 2021, 19, 7633–7640. [Google Scholar] [CrossRef]
- Kathe, P.M.; Berkefeld, A.; Fleischer, I. Nickel Hydride Catalyzed Cleavage of Allyl Ethers Induced by Isomerization. Synlett 2021, 32, 1629–1632. [Google Scholar] [CrossRef]
- Lin, Y.L.; Cheng, J.Y.; Chu, Y.H. Microwave-Accelerated Claisen Rearrangement in Bicyclic Imidazolium [b-3C-Im][NTf2] Ionic Liquid. Tetrahedron 2007, 63, 10949–10957. [Google Scholar] [CrossRef]
- Minutolo, F.; Bellini, R.; Bertini, S.; Carboni, I.; Lapucci, A.; Pistolesi, L.; Prota, G.; Rapposelli, S.; Solati, F.; Tuccinardi, T.; et al. Monoaryl-Substituted Salicylaldoximes as Ligands for Estrogen Receptor β. J. Med. Chem. 2008, 51, 1344–1351. [Google Scholar] [CrossRef]
- Verhasselt, S.; Roman, B.I.; De Wever, O.; Van Hecke, K.; Van Deun, R.; Bracke, M.E.; Stevens, C.V. Discovery of (S)-3′-Hydroxyblebbistatin and (S)-3′-Aminoblebbistatin: Polar Myosin II Inhibitors with Superior Research Tool Properties. Org. Biomol. Chem. 2017, 15, 2104–2118. [Google Scholar] [CrossRef]
- Maity, S.; Kancherla, R.; Dhawa, U.; Hoque, E.; Pimparkar, S.; Maiti, D. Switch to Allylic Selectivity in Cobalt Catalyzed Dehydrogenative Heck Reaction with Unbiased Aliphatic Olefins Supporting Information. ACS Catal. 2016, 6, 5493–5499. [Google Scholar] [CrossRef]
- Sánchez-Peris, M.; Murga, J.; Falomir, E.; Carda, M.; Marco, J.A. Synthesis of Honokiol Analogues and Evaluation of Their Modulating Action on VEGF Protein Secretion and Telomerase-Related Gene Expressions. Chem. Biol. Drug Des. 2017, 89, 577–584. [Google Scholar] [CrossRef]
- Khan, A.; Komejan, S.; Patel, A.; Lombardi, C.; Lough, A.J.; Foucher, D.A. Reduction of C,O-Chelated Organotin(IV) Dichlorides and Dihydrides Leading to Protected Polystannanes. J. Organomet. Chem. 2015, 776, 180–191. [Google Scholar] [CrossRef]
- Han, X.; Armstrong, D.W. Using Geminal Dicationic Ionic Liquids as Solvents for High-Temperature Organic Reactions. Org. Lett. 2005, 7, 4205–4208. [Google Scholar] [CrossRef]
- Kisiel-Nawrot, E.; Pindjakova, D.; Latocha, M.; Bak, A.; Kozik, V.; Suwinska, K.; Sochanik, A.; Cizek, A.; Jampilek, J.; Zięba, A. Design, Synthesis and Antimicrobial Properties of New Tetracyclic Quinobenzothiazine Derivatives. Int. J. Mol. Sci. 2022, 23, 15078. [Google Scholar] [CrossRef]
- Thiruvengetam, P.; Chakravarthy, R.D.; Chand, D.K. A Molybdenum Based Metallomicellar Catalyst for Controlled and Chemoselective Oxidation of Activated Alcohols in Aqueous Medium. J. Catal. 2019, 376, 123–133. [Google Scholar] [CrossRef]
- Brown, D.P.; Duong, H.Q. Synthesis of Novel Aromatic Macrolactones via Ring Closing Metathesis of Substituted Phenylalkanoic Acid Allylic Esters. J. Heterocycl. Chem. 2008, 45, 435–443. [Google Scholar] [CrossRef]
- Kölbel, M.; Beyersdorff, T.; Tschierske, C.; Diele, S.; Kain, J. Thermotropic and Lyotropic Liquid Crystalline Phases of Rigid Aromatic Amphiphiles. Chem. Eur. J. 2000, 6, 3821–3837. [Google Scholar] [CrossRef]
- Bredikhina, Z.A.; Kurenkov, A.V.; Bredikhin, A.A. Nonracemic Dimethylphenyl Glycerol Ethers in the Synthesis of Physiologically Active Aminopropanols. Russ. J. Org. Chem. 2019, 55, 837–844. [Google Scholar] [CrossRef]
- Hsieh, C.E.; Jiang, Y.M.; Chou, C.M. Functionalized Allyl Aryl Ether Synthesis from Benzoic Acids Using a Dearomatization and Decarboxylative Allylation Approach. J. Org. Chem. 2019, 84, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, B.; Ranjith Kumar, T.; Narsimha, S.; Vasudeva Reddy, N. Synthesis, Characterization and in Vitro Microbial Evaluation of Regioisomers of Allyl Phenyl Ethers Derived 1,2,4-Triazoles. Int. J. Pharm. Pharm. Sci. 2014, 6, 572–575. [Google Scholar]
- Chen, P.; Chen, C.; Zheng, Y.; Chen, F.; Liu, Z.; Ren, S.; Song, H.; Liu, T.; Lu, Z.; Sun, H.; et al. Discovery of 2,3-Dihydro[1,4]Dioxino[2,3-g]Benzofuran Derivatives as Protease Activated Receptor 4 (PAR4) Antagonists with Potent Antiplatelet Aggregation Activity and Low Bleeding Tendency. J. Med. Chem. 2024, 67, 5502–5537. [Google Scholar] [CrossRef]
- Geresh, S.; Markovits, Y.; Shani, A. On the Mechanism of Intramolecular Photocycloaddition of Substituted O-Allylphenols to Cyclic Ethers. Tetrahedron 1975, 31, 2803–2807. [Google Scholar] [CrossRef]
- Sakamuri, S.; Kozikowski, A.P. Synthesis of 7-Methoxybenzolactam-V8 Using a Diastereoselective Strecker Synthesis. Chem. Commun. 2001, 32, 475–476. [Google Scholar] [CrossRef]
- Vargas, D.F.; Larghi, E.L.; Kaufman, T.S. Synthesis of Polysubstituted 3-Methylisoquinolines through the 6π-Electron Cyclization/Elimination of 1-Azatrienes Derived from 1,1-Dimethylhydrazine. Eur. J. Org. Chem. 2018, 2018, 5605–5614. [Google Scholar] [CrossRef]
- Wang, B.; Ren, M.; Iqbal, N.; Mu, X.; Yang, B. Environmentally Friendly Synthesis of Highly Substituted Phenols Using Enallenoates and Grignard Reagents. Org. Lett. 2024, 26, 3361–3365. [Google Scholar] [CrossRef] [PubMed]
- Marín-Ramos, N.I.; Piñar, C.; Vázquez-Villa, H.; Martín-Fontecha, M.; González, Á.; Canales, Á.; Algar, S.; Mayo, P.P.; Jiménez-Barbero, J.; Gajate, C.; et al. Development of a Nucleotide Exchange Inhibitor That Impairs Ras Oncogenic Signaling. Chem. A Eur. J. 2017, 23, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- White, W.N.; Slater, C.D. The Ortho-Claisen Rearrangement. V. The Products of Rearrangement of Allyl m-X-Phenyl Ethers. J. Org. Chem. 1961, 26, 3631–3638. [Google Scholar] [CrossRef]
- White, W.N.; Slater, C.D. The Ortho-Claisen Rearrangement. VI. The Rates of Rearrangement of Allyl m-X-Phenyl Ethers to 2-Allyl-5-X-Phenols. J. Org. Chem. 1962, 27, 2908–2914. [Google Scholar] [CrossRef]
- White, W.N.; Slater, C.D.; Fife, W. The Electronic Nature of the Transition State of the Claisen Rearrangement. J. Org. Chem. 1961, 26, 627–628. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 2nd ed.; Oxford University Press Inc.: New York, NY, USA, 2012; ISBN 978-0-19-927029-3. [Google Scholar]
- Contreras, R.; Domingo, L.R.; Silvi, B. Electron Densities: Population Analysis and Beyond. In Encyclopedia of Physical Organic Chemistry; Wang, Z., Wille, U., Juaristi, E., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; ISBN 9781118468586. [Google Scholar]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Nikolaienko, T.Y.; Bulavin, L.A.; Hovorun, D.M. JANPA: An Open Source Cross-Platform Implementation of the Natural Population Analysis on the Java Platform. Comput. Theor. Chem. 2014, 1050, 15–22. [Google Scholar] [CrossRef]
- Nikolaienko, T.Y.; Bulavin, L.A. Localized Orbitals for Optimal Decomposition of Molecular Properties. Int. J. Quantum Chem. 2019, 119, e25798. [Google Scholar] [CrossRef]
- Shusterman, A.J.; Hoistad, L.M. Teaching Chemistry with Electron Density Models. 2. Can Atomic Charges Adequately Explain Electrostatic Potential Maps? Chem. Educ. 2001, 6, 36–40. [Google Scholar] [CrossRef]

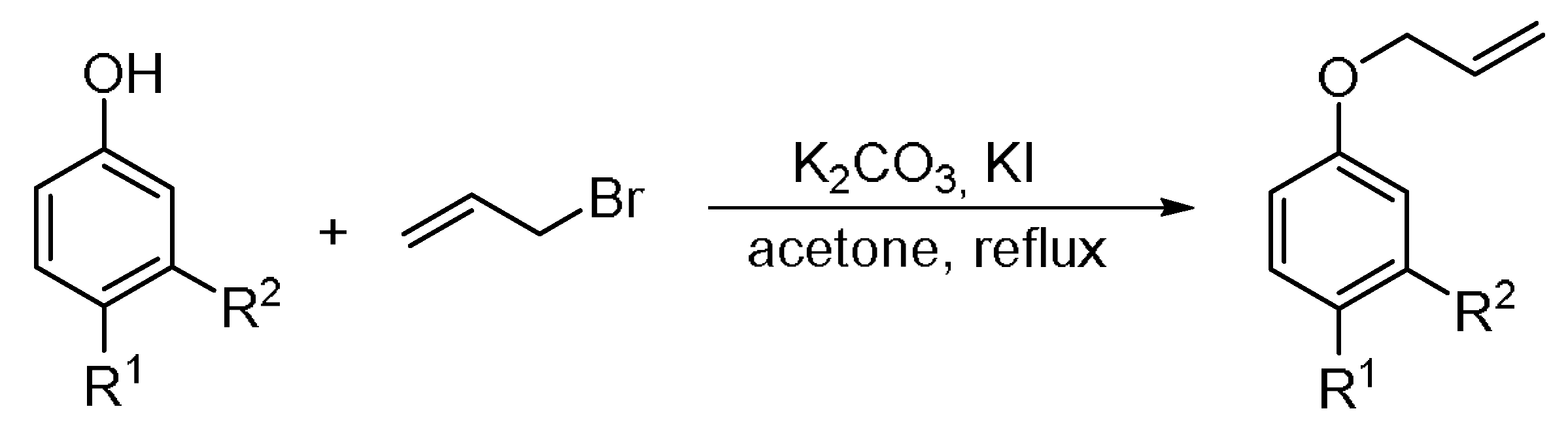
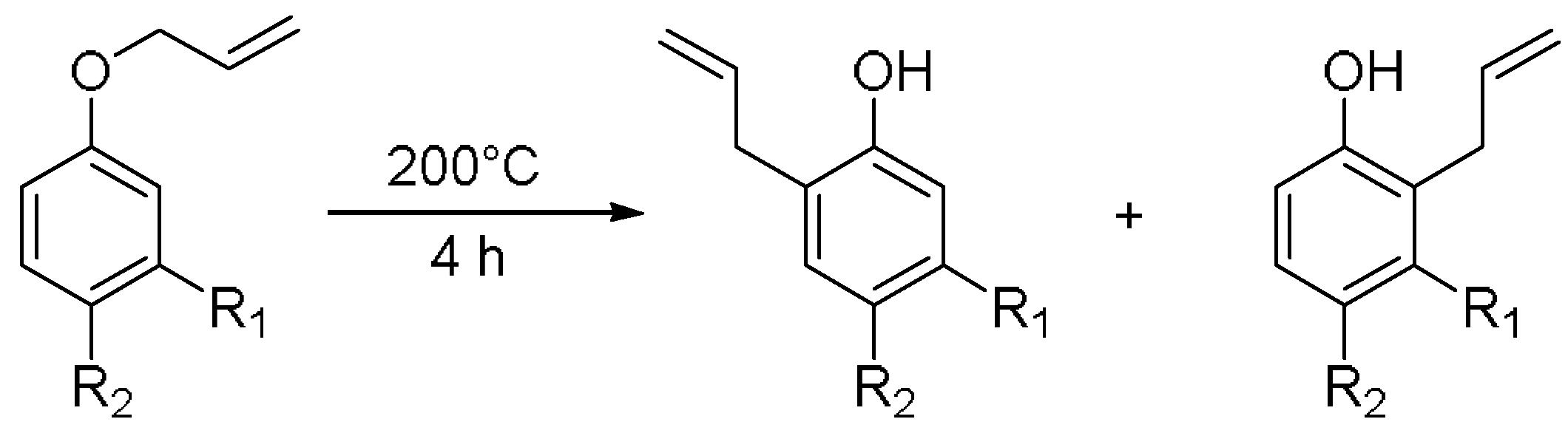
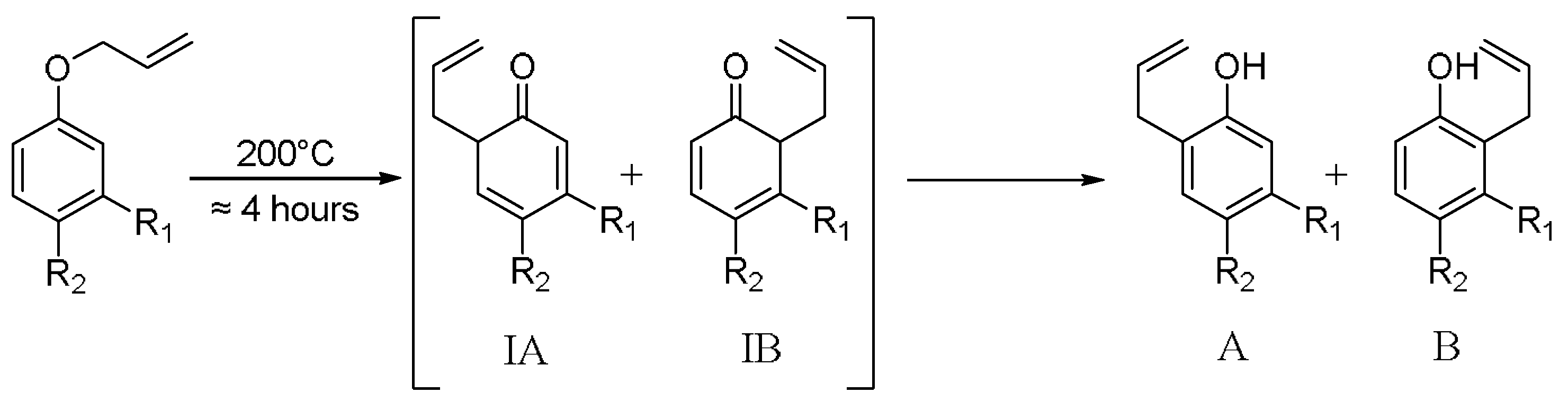
| R1 (Meta) | A:B |
|---|---|
| F | 1:0.6 |
| Cl | 1:1.4 |
| Br | 1:1.6 |
| I | 1:1.7 |
| Me | 1:1.2 |
| Et | 1:1.0 |
| tBu | 1:0.5 |
| Ph | 1:0.8 |
| NHCOCH3 | 1:0.8 |
| CHO | 1:3.0 |
| CO2CH2CH=CH2 | 1:1.8 |
| CF3 | 1:1.3 |
| NO2 | 1:1.6 |
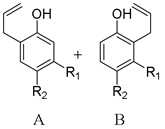 | ||
|---|---|---|
| R1 (Meta) | R2 (Para) | A:B |
| Me | H | 1:1.2 |
| F | 1:1.2 | |
| Cl | 1:2.3 | |
| Br | 1:1.7 | |
| Me | 1:1.2 | |
| iPr | 1:1.3 | |
| SMe | 1:1.1 | |
| Cl | H | 1:1.4 |
| F | 1:1.7 | |
| Cl | 1:2.4 | |
| Me | 1:2.1 | |
| CN | 1:1.6 | |
 | |||||
|---|---|---|---|---|---|
| R1 (Meta) | R2 (Para) | A | B | B/A (HPA) | B/A (Experiment) |
| F | H | −0.132672 | −0.099927 | 0.75 | 0.6 |
| Cl | −0.048462 | −0.069299 | 1.43 | 1.4 | |
| Br | −0.045385 | −0.066108 | 1.46 | 1.6 | |
| Me | −0.055549 | −0.074839 | 1.35 | 1.2 | |
| Et | −0.070555 | −0.05793 | 0.82 | 1.0 | |
| CF3 | −0.035212 | −0.057743 | 1.64 | 1.3 | |
| Me | F | −0.042287 | −0.059952 | 1.42 | 1.2 |
| Cl | −0.045936 | −0.067538 | 1.47 | 2.3 | |
| Me | −0.055732 | −0.072469 | 1.30 | 1.2 | |
| Cl | F | −0.035107 | −0.058832 | 1.68 | 1.7 |
| Cl | −0.039825 | −0.064215 | 1.61 | 2.4 | |
| Me | −0.047678 | −0.067995 | 1.43 | 2.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, W.T.; Hreinsdóttir, S.D.; Arana, L.A.; Sveinbjörnsson, B.R. Regioselectivity of the Claisen Rearrangement of Meta- and Para-Substituted Allyl Aryl Ethers. Reactions 2025, 6, 54. https://doi.org/10.3390/reactions6040054
Möller WT, Hreinsdóttir SD, Arana LA, Sveinbjörnsson BR. Regioselectivity of the Claisen Rearrangement of Meta- and Para-Substituted Allyl Aryl Ethers. Reactions. 2025; 6(4):54. https://doi.org/10.3390/reactions6040054
Chicago/Turabian StyleMöller, William Thomas, Svava Dögg Hreinsdóttir, Luis Antonio Arana, and Benjamín Ragnar Sveinbjörnsson. 2025. "Regioselectivity of the Claisen Rearrangement of Meta- and Para-Substituted Allyl Aryl Ethers" Reactions 6, no. 4: 54. https://doi.org/10.3390/reactions6040054
APA StyleMöller, W. T., Hreinsdóttir, S. D., Arana, L. A., & Sveinbjörnsson, B. R. (2025). Regioselectivity of the Claisen Rearrangement of Meta- and Para-Substituted Allyl Aryl Ethers. Reactions, 6(4), 54. https://doi.org/10.3390/reactions6040054





