From Waste to Catalyst: The Properties of Mixed Oxides Derived from Layered Double Hydroxide Mg/Al Synthesized from Aluminum Residues and Their Use in Transesterification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Basic Leaching of Aluminum Waste
2.3. Synthesis of LDH Mg/Al: LDH-CP and LDH-H
2.4. Mixed Oxides Mg-Al-OLDH-CP and Mg-Al-OLDH-H—Preparation and Characterization
2.5. Catalytic Assays
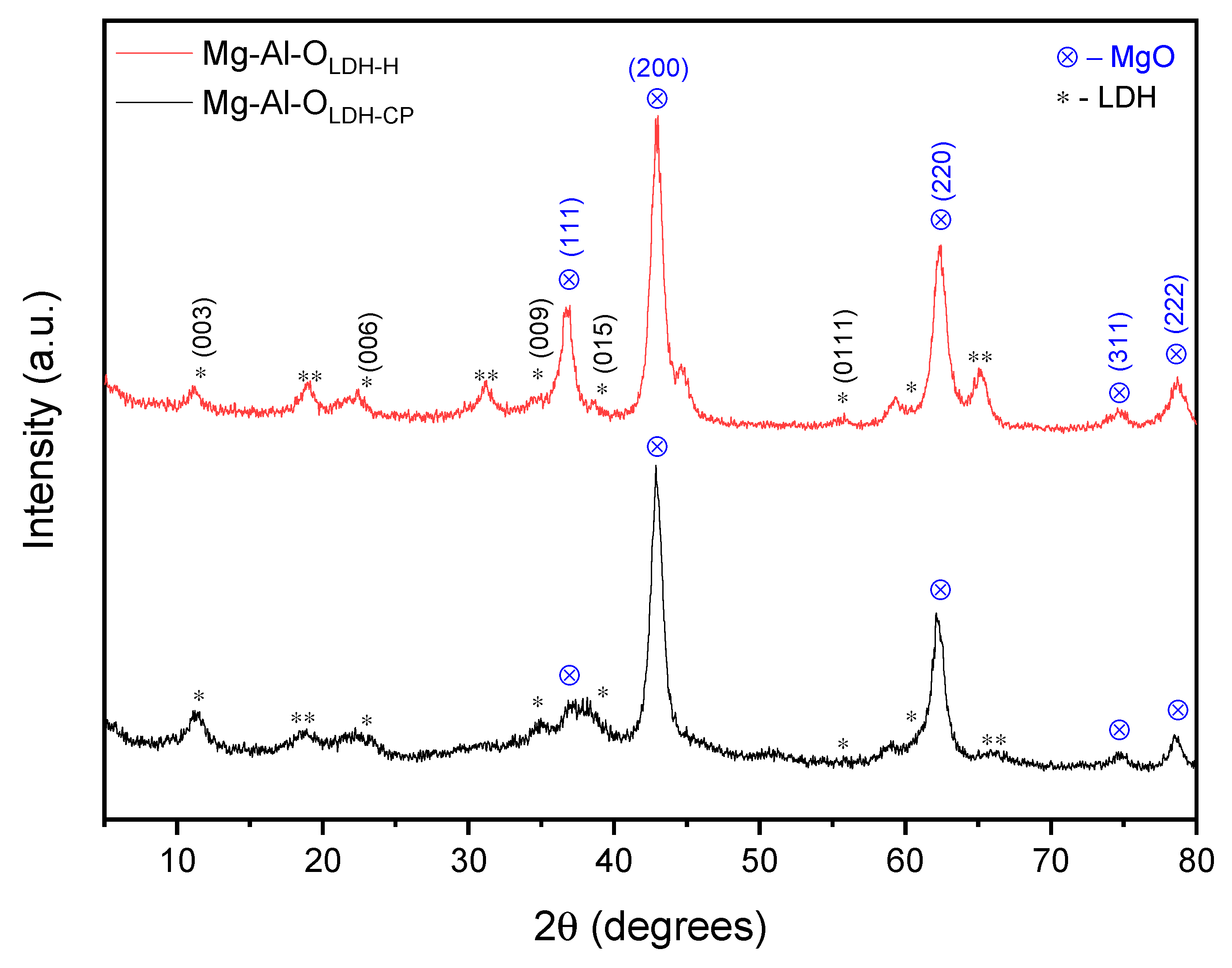
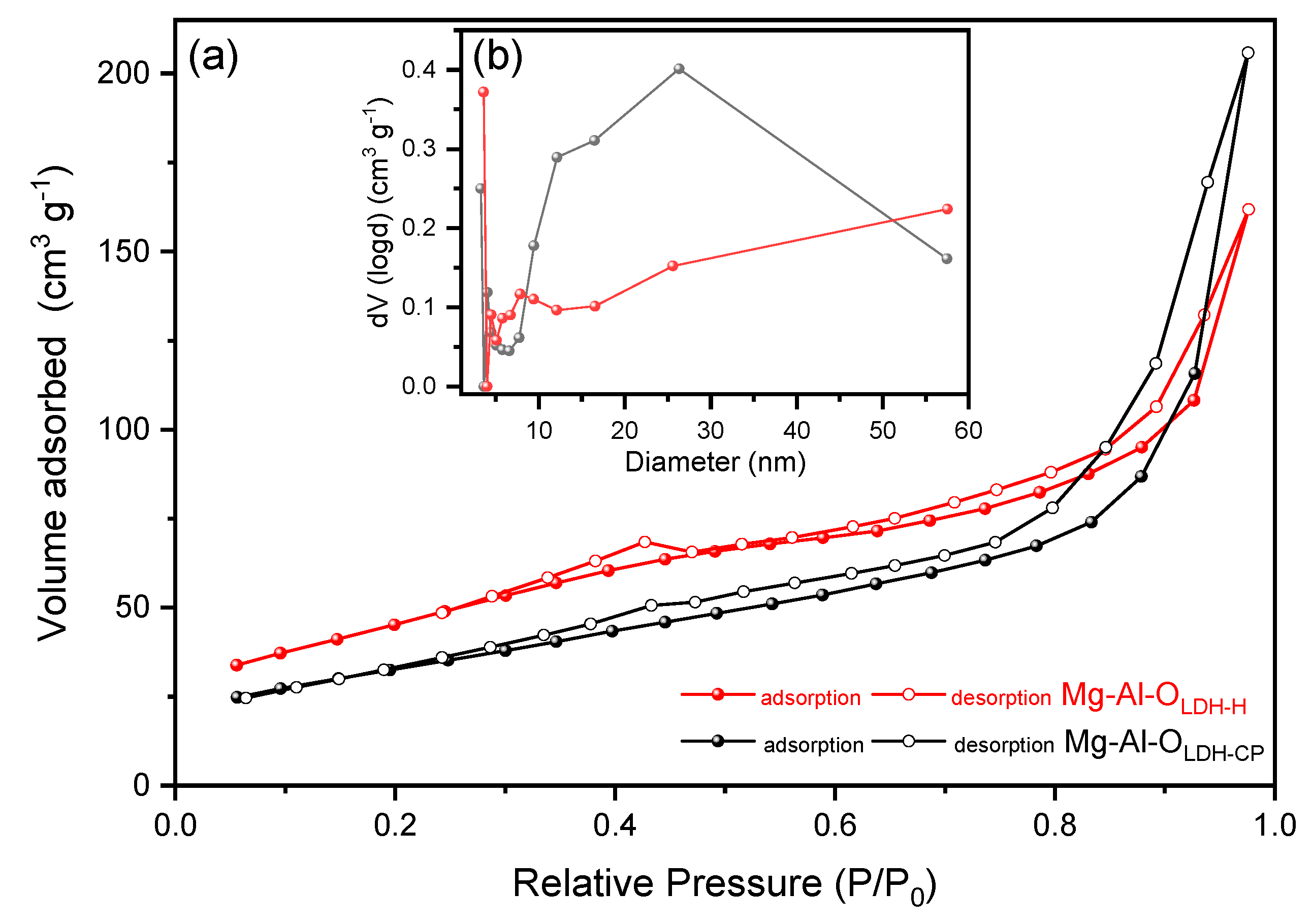
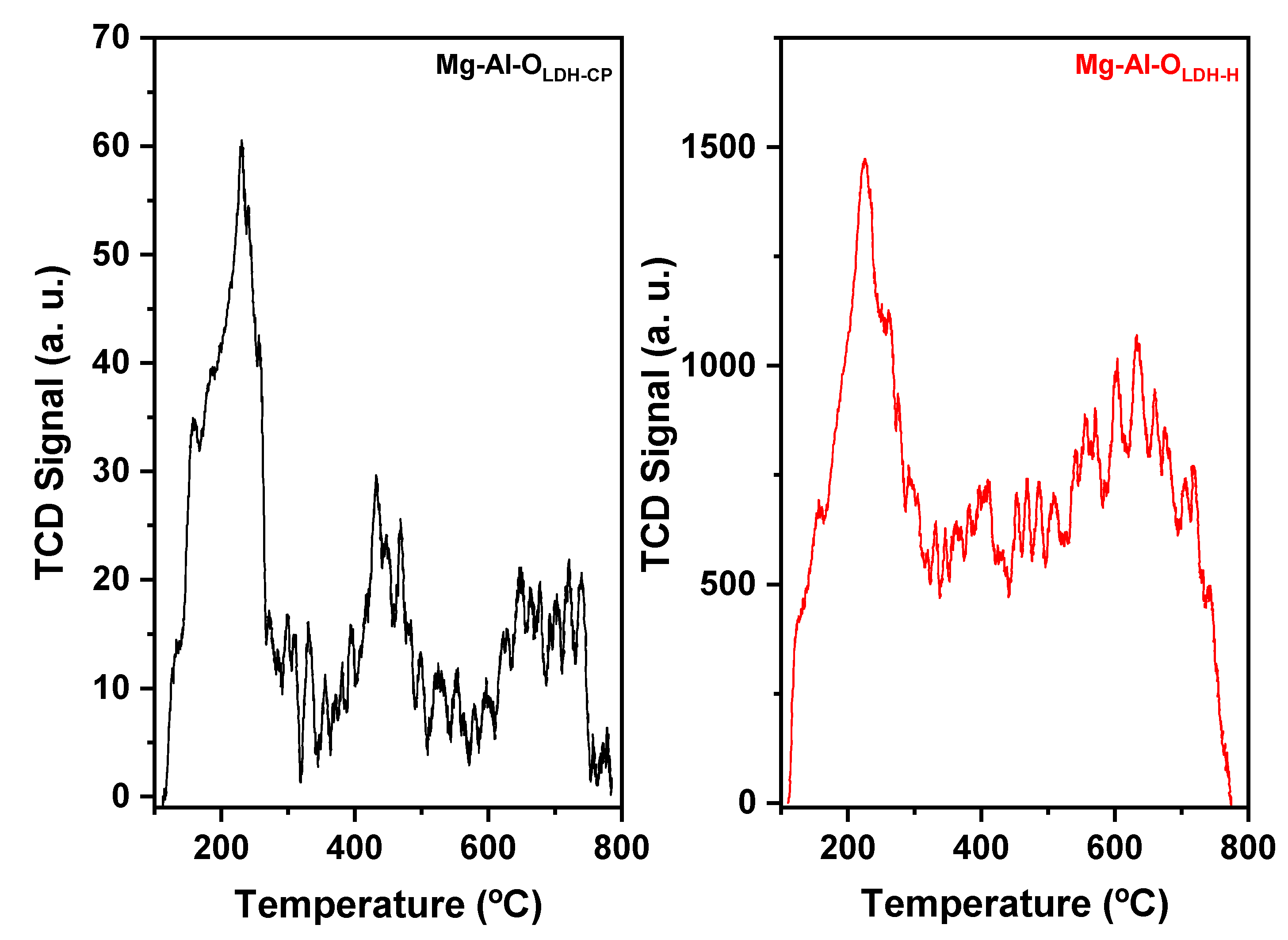
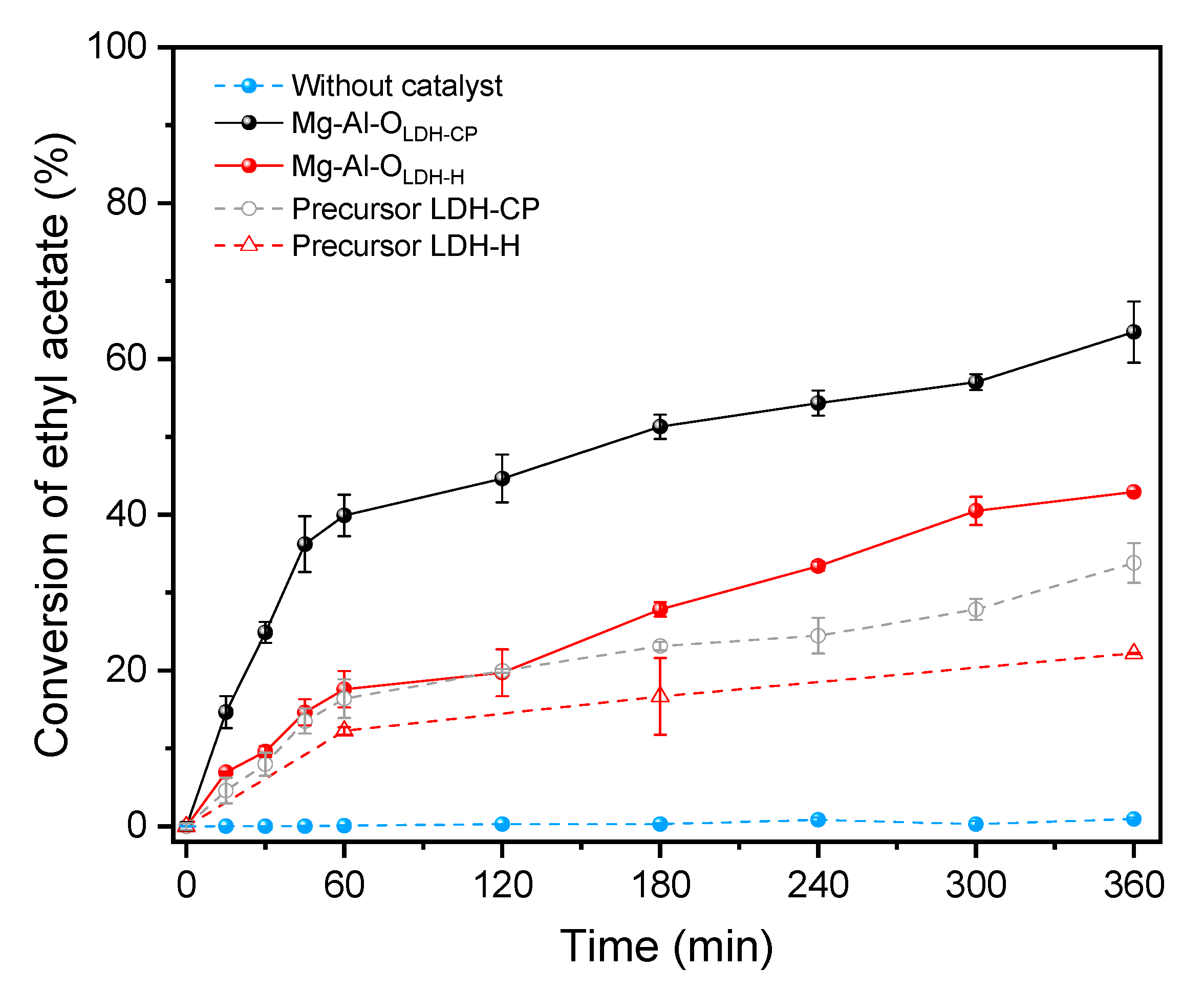
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araskevas, D.; Kellens, K.; Van de Voorde, A.; Dewulf, W.; Duflou, J.R. Environmental Impact Analysis of Primary Aluminium Production at Country Level. Procedia CIRP 2016, 40, 209–213. [Google Scholar] [CrossRef]
- International Aluminium Institute. Aluminium Recycling Saves 95% of the Energy Needed for Primary Aluminium Production. Available online: https://international-aluminium.org/landing/aluminium-recycling-saves-95-of-the-energy-needed-for-primary-aluminium-production/ (accessed on 15 April 2025).
- Al-Alimi, S.; Yusuf, N.K.; Ghaleb, A.M.; Lajis, M.A.; Shamsudin, S.; Zhou, W.; Altharan, Y.M.; Abdulwahab, H.S.; Saif, Y.; Didane, D.H.; et al. Recycling aluminium for sustainable development: A review of different processing technologies in green manufacturing. Results Eng. 2024, 23, 102566. [Google Scholar] [CrossRef]
- Associação Brasileira do Alumínio (ABAL). Reciclagem de Alumínio no Brasil. Available online: https://abal.org.br/reciclagem/ (accessed on 15 April 2025).
- Abdelkader, A.; Osman, A.I.; Halawy, S.A.; Rooney, D.W. Preparation and characterization of mesoporous γ-Al2O3 recovered from aluminum can waste and its use in the dehydration of methanol to dimethyl ether. J. Mater. Cycles Waste Manag. 2018, 20, 1428–1436. [Google Scholar] [CrossRef]
- Marafi, M.; Rana, M.S.; Navvamani, R.; Al-Sheeha, H. Utilization of waste spent hydroprocessing catalyst: Development of a process for full recovery of deposited metals and alumina support. Waste Manag. 2012, 163, 221. [Google Scholar] [CrossRef]
- Bin Mokaizh, A.A.; Alazaiza, M.Y.D.; Ramu, M.B.; Nassani, D.E. Synthesis of Sustainable γ-Alumina Catalyst/Catalyst Support from Aluminum Can Waste: Study of the Influence of Reaction Temperature. Catalysts 2025, 15, 215. [Google Scholar] [CrossRef]
- Silva, T.S.; Fernandes, E.P.; Vithanage, M.; Meneghetti, S.M.P.; Meili, L. A Facile Synthesis of MgAl/Layered Double Hydroxides from Aluminum Wastes. Mater. Lett. 2022, 324, 132624. [Google Scholar] [CrossRef]
- Zawrah, M.F.; El Defrawy, S.A.; Ali, O.A.M.; Sadek, H.E.H.; Ghanaym, E.E. Recycling of LCW Produced Form Water Plants for Synthesizing of Nano FeO(OH), Al(OH)3, and Layered Double Hydroxide: Effect of Heat-Treatment. Ceram. Int. 2018, 44, 9950–9957. [Google Scholar] [CrossRef]
- Raheem, S.A.; Mohammed, A.A. Synthesis, characterization, and applications of layered double hydroxides nanocomposites for the adsorption of organic and inorganic contaminants from an aqueous solution: An overview. Results Surf. Interfaces 2025, 18, 100386. [Google Scholar] [CrossRef]
- Xu, M.; Wei, M. Layered Double Hydroxide-Based Catalysts: Recent Advances in Preparation, Structure, and Applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Wang, J.; Liu, Y.; Chen, Y. Layered double hydroxide-based nanomaterials for biomedical applications: A review. Adv. Sci. 2023, 10, 2306035. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Maestro-Madurga, B.; Pesquera-Rodríguez, A.; Ramírez-López, C.; Belsué, M. Synthesis of glycerol carbonate from glycerol and dialkyl carbonates using hydrotalcite as a reusable heterogeneous base catalyst. Green Chem. 2010, 12, 578–581. [Google Scholar] [CrossRef]
- Zheng, L.; Xia, S.; Hou, Z.; Zhang, M.; Hou, Z. Transesterification of glycerol with dimethyl carbonate over Mg–Al hydrotalcites. Chin. J. Catal. 2014, 35, 310–318. [Google Scholar] [CrossRef]
- Kondawar, S.; Rode, C. Solvent-Free Glycerol Transesterification with Propylene Carbonate to Glycerol Carbonate over a Solid Base Catalyst. Energy Fuels 2017, 31, 4361–4371. [Google Scholar] [CrossRef]
- Szabados, M.; Ádám, A.A.; Traj, P.; Muráth, S.; Baán, K.; Bélteky, P.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Mechanochemical and wet chemical syntheses of CaIn-layered double hydroxide and its performance in a transesterification reaction compared to those of other Ca2M(III) hydrocalumites (M: Al, Sc, V, Cr, Fe, Ga) and Mg(II)-, Ni(II)-, Co(II)- or Zn(II)-based hydrotalcites. J. Catal. 2020, 391, 282–297. [Google Scholar] [CrossRef]
- Mahdavi, R.; Ghasemi, M.; Gharibi, A. Synthesis of advanced MgAl-LDH based geopolymer as a potential catalyst in the conversion of waste sunflower oil into biodiesel: Response surface studies. Fuel 2021, 282, 118865. [Google Scholar] [CrossRef]
- Altalhi, A.A.; Mohamed, E.A.; Negm, N.A. Recent advances in layered double hydroxide (LDH)-based materials: Fabrication, modification strategies, characterization, promising environmental catalytic applications, and prospective aspects. Energy Adv. 2024, 3, 2136–2151. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Wang, J.; Zhang, Y.; Chen, Y. Lewis Acid-Base Site-Assisted In Situ Transesterification Catalysis to Produce Biodiesel: A Review. Catalysts 2024, 14, 731. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Zhang, Y.; Chen, Y. Solid Acid–Base Catalysts Based on Layered Double Hydroxides for Green Catalytic Transformations. Catalysts 2024, 14, 28. [Google Scholar] [CrossRef]
- Xu, C.; Enache, D.I.; Lloyd, R.; Knight, D.W.; Bartley, J.K.; Hutchings, G.J. MgO Catalysed Triglyceride Transesterification for Biodiesel Synthesis. Catal. Lett. 2010, 138, 1–7. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Zhang, C.; Zeng, G.M.; Tan, X.F.; Huang, D.L.; Zhou, J.W.; Fang, Q.Z.; Yang, K.H.; Wang, H.; Wei, J.; et al. State-of-the-Art Progress in the Rational Design of Layered Double Hydroxide Based Photocatalysts for Photocatalytic and Photoelectrochemical H2/O2 Production. Coord. Chem. Rev. 2021, 446, 214103. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Duan, X.; Evans, G. Structure and Bonding—Layered Double Hydroxides; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Daniel, S.; Thomas, S. Layered Double Hydroxides: Fundamentals to Applications. Woodhead Publ. Ser. Compos. Sci. Eng. 2020, 1–76. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered Double Hydroxides: A Brief Review from Fundamentals to Application as Evolving Biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Gabriel, R.; de Carvalho, S.H.V.; Duarte, J.L.d.S.; Oliveira, L.M.T.M.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; Soletti, J.I.; Meili, L. Mixed Metal Oxides Derived from Layered Double Hydroxide as Catalysts for Biodiesel Production. Appl. Catal. A Gen. 2022, 630, 118470. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Ghanaym, E.E.; Sadek, H.E.H.; El Defrawy, S.A.; Ali, O.A.M. Synthesis, Characterization and Sinterability of Pure and Ni-Doped Nano Layered Double Hydroxides from Aluminum Dross. Ceram. Int. 2019, 45, 17598–17610. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Primo, J.; Rey, F. One-Step Synthesis of Citronitril on Hydrotalcite Derived Base Catalysts. Appl. Catal. A Gen. 1994, 114, 215–225. [Google Scholar] [CrossRef]
- Stanimirova, T.; Piperov, N.B.; Petrova, N. Thermal Evolution of Mg–Al–CO3 Hydrotalcites. Clays Clay Miner. 2004, 39, 121–132. [Google Scholar] [CrossRef]
- Forano, C.; Hibino, T.; Leroux, F.; Taviot-Guého, C. Some Other Materials Related to Clays. In Handbook of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1021–1096. [Google Scholar]
- de Melo Costa Serge, N.; Gonçalves, R.G.L.; Lima, K.V.L.; Correales, Y.E.S.; de Aquino, J.M.B.T.; Gonçalves, R.R.; Hammer, P.; Nogueira, R.F.P. Consequences of the Calcination on the Morphological, Structural, Optical, and Catalytic Properties of CuMgFe-Layered Double Hydroxide for Photo-Fenton Degradation. Appl. Clay Sci. 2025, 268, 107740. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Shipko, M.; Kalazhokov, Z.; Kalazhokov, H.; Stepovich, M.; Savchenko, E.; Agafonov, A. Plasma Chemical Synthesis of Magnetic Fe2O3-Ni-Cr Layered Double Hydroxide Composites for Environmental Applications. Solid State Commun. 2025, 399, 115886. [Google Scholar] [CrossRef]
- Almerindo, G.I.; Probst, L.F.D.; Campos, C.E.M.; De Almeida, R.M.; Meneghetti, S.M.P.; Meneghetti, M.R.; Clacens, J.M.; Fajardo, H.V. Magnesium Oxide Prepared via Metal-Chitosan Complexation Method: Application as Catalyst for Transesterification of Soybean Oil and Catalyst Deactivation Studies. J. Power Sources 2011, 196, 8057–8063. [Google Scholar] [CrossRef]
- Miyata, S. Physico-chemical properties of synthetic hydrotalcites in relation to composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Evans, D.G.; Duan, X. Thermal decomposition of layered double hydroxides. Thermochim. Acta 2007, 454, 79–87. [Google Scholar] [CrossRef]
- Rocha, J.; del Arco, M.; Rives, V.; Ulibarri, M.A. Reconstruction of layered double hydroxides from calcined precursors: A powder XRD and 27Al MAS NMR study. J. Mater. Chem. 1999, 9, 2499–2503. [Google Scholar] [CrossRef]
- Hadia, N.M.A.; Mohamed, H.A.H. Characteristics and Optical Properties of MgO Nanowires Synthesized by Solvothermal Method. Mater. Sci. Semicond. Process 2015, 29, 238–244. [Google Scholar] [CrossRef]
- Almeida, A.A.; Santos, R.M.M.; Alves Rosa, M.A.; Pulcinelli, S.H.; John, V.M.; Santilli, C.V. MgAl-Layered Double Hydroxide Nanoparticles as Smart Nanofillers to Control the Rheological Properties and the Residual Porosity of Cement-Based Materials. ACS Appl. Nano. Mater. 2022, 5, 7896–7907. [Google Scholar] [CrossRef]
- Hibino, T.; Yamashita, Y.; Kosuge, K.; Tsunashima, A. Decarbonation Behavior of Mg-Al-CO3 Hydrotalcite-like Compounds during Heat Treatment. Clays Clay Min. 1995, 43, 427–432. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Baraka, S.; Bouearan, K.; Caner, L.; Fontaine, C.; Epron, F.; Brahmi, R.; Bion, N. Catalytic Performances of Natural Ni-Bearing Clay Minerals for Production of Syngas from Dry Reforming of Methane. J. CO2 Util. 2021, 52, 101696. [Google Scholar] [CrossRef]
- Noda, L.K.; De Almeida, R.M.; Probst, L.F.D.; Gonçalves, N.S. Characterization of Sulfated TiO2 Prepared by the Sol-Gel Method and Its Catalytic Activity in the n-Hexane Isomerization Reaction. J. Mol. Catal. A Chem. 2005, 225, 39–46. [Google Scholar] [CrossRef]
- Mulyatun, M.; Prameswari, J.; Istadi, I.; Widayat, W. Synthesis Method Effect on the Catalytic Performance of Acid–Base Bifunctional Catalysts for Converting Low-Quality Waste Cooking Oil to Biodiesel. Catal. Lett. 2024, 154, 4837–4855. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.; Zhu, M.; Yue, C.; Dong, Y.; Leng, Y.; Fan, M.; Jiang, P. Acidic–Basic Bifunctional Magnetic Mesoporous CoFe2O4@(CaO–ZnO) for the Synthesis of Glycerol Carbonate. Catal. Lett. 2020, 150, 2863–2872. [Google Scholar] [CrossRef]
- Jiang, W.; Niu, X.; Yuan, F.; Zhu, Y.; Fu, H. Preparation of KF-La2O2CO3 Solid Base Catalysts and Their Excellent Catalytic Activities for Transesterification of Tributyrin with Methanol. Catal. Sci. Technol. 2014, 4, 2957–2968. [Google Scholar] [CrossRef]





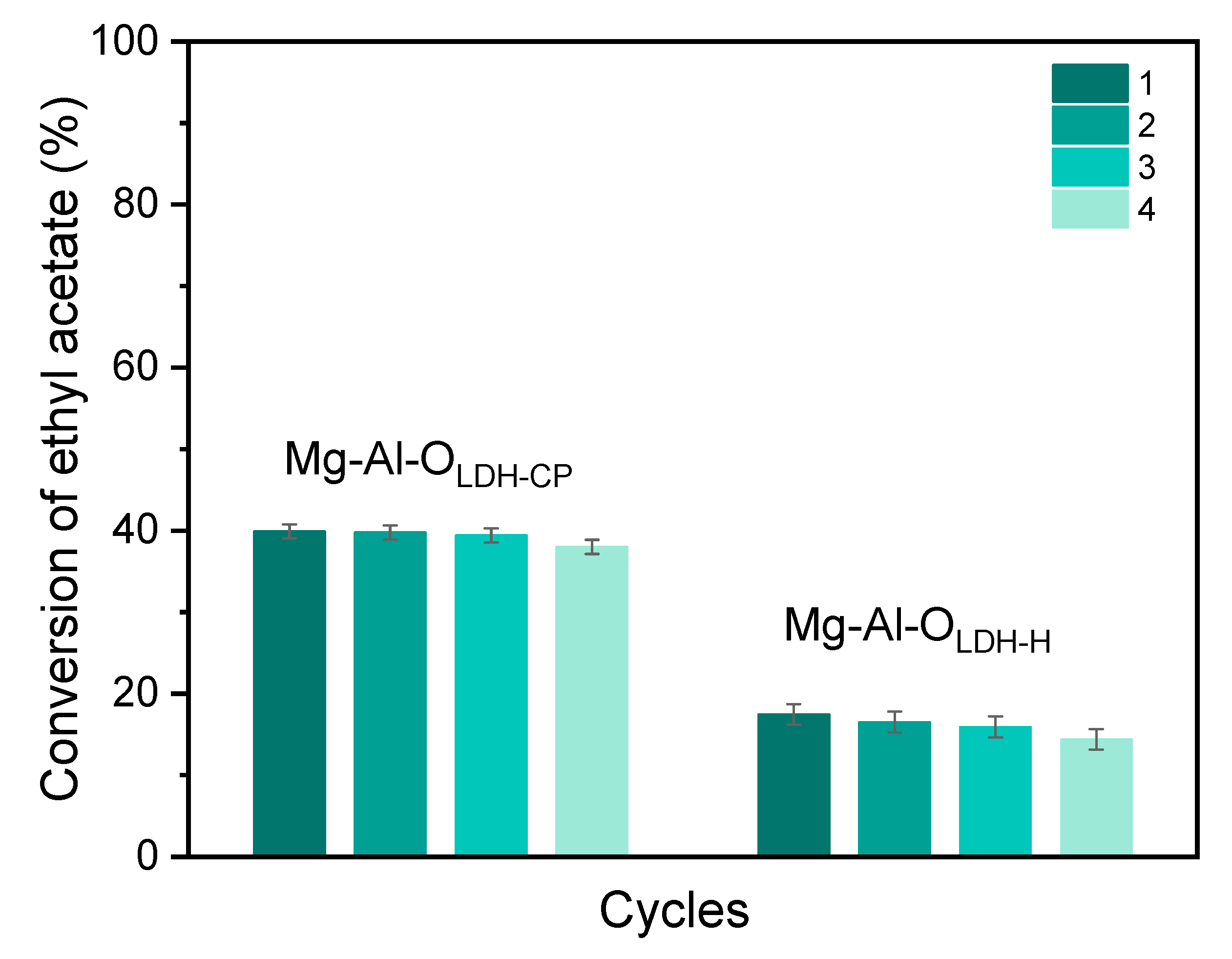
| Sample | SBET a (m2.g−1) | V b (cm3.g−1) | DBJH c (nm) |
|---|---|---|---|
| Mg-Al-OLDH-CP | 118.5 | 0.290 | 32.7 |
| Mg-Al-OLDH-H | 167.4 | 0.192 | 32.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, T.S.; da Silva, L.L.F.F.; da Silva, E.P.S.; Motta, R.J.B.; da Silva, B.J.B.; Meneghetti, M.R.; Meili, L.; Meneghetti, S.M.P. From Waste to Catalyst: The Properties of Mixed Oxides Derived from Layered Double Hydroxide Mg/Al Synthesized from Aluminum Residues and Their Use in Transesterification. Reactions 2025, 6, 33. https://doi.org/10.3390/reactions6020033
da Silva TS, da Silva LLFF, da Silva EPS, Motta RJB, da Silva BJB, Meneghetti MR, Meili L, Meneghetti SMP. From Waste to Catalyst: The Properties of Mixed Oxides Derived from Layered Double Hydroxide Mg/Al Synthesized from Aluminum Residues and Their Use in Transesterification. Reactions. 2025; 6(2):33. https://doi.org/10.3390/reactions6020033
Chicago/Turabian Styleda Silva, Tarsila Santos, Laura Leticia Freitas Ferreira da Silva, Evellyn Patricia Santos da Silva, Rayssa Jossanea Brasileiro Motta, Bruno José Barros da Silva, Mario Roberto Meneghetti, Lucas Meili, and Simoni Margareti Plentz Meneghetti. 2025. "From Waste to Catalyst: The Properties of Mixed Oxides Derived from Layered Double Hydroxide Mg/Al Synthesized from Aluminum Residues and Their Use in Transesterification" Reactions 6, no. 2: 33. https://doi.org/10.3390/reactions6020033
APA Styleda Silva, T. S., da Silva, L. L. F. F., da Silva, E. P. S., Motta, R. J. B., da Silva, B. J. B., Meneghetti, M. R., Meili, L., & Meneghetti, S. M. P. (2025). From Waste to Catalyst: The Properties of Mixed Oxides Derived from Layered Double Hydroxide Mg/Al Synthesized from Aluminum Residues and Their Use in Transesterification. Reactions, 6(2), 33. https://doi.org/10.3390/reactions6020033







