Electroreduction of Nitrogen on Pd, Rh, and PdRh Catalysts: An Online Mass Spectrometry Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Polyol-Based Synthesis
2.2. Physical Characterization
2.3. Working Electrode
2.4. OLEMS
2.5. Electrochemical Characterization
2.6. Determination of NH3
2.7. Measurement Protocol
3. Results and Discussion
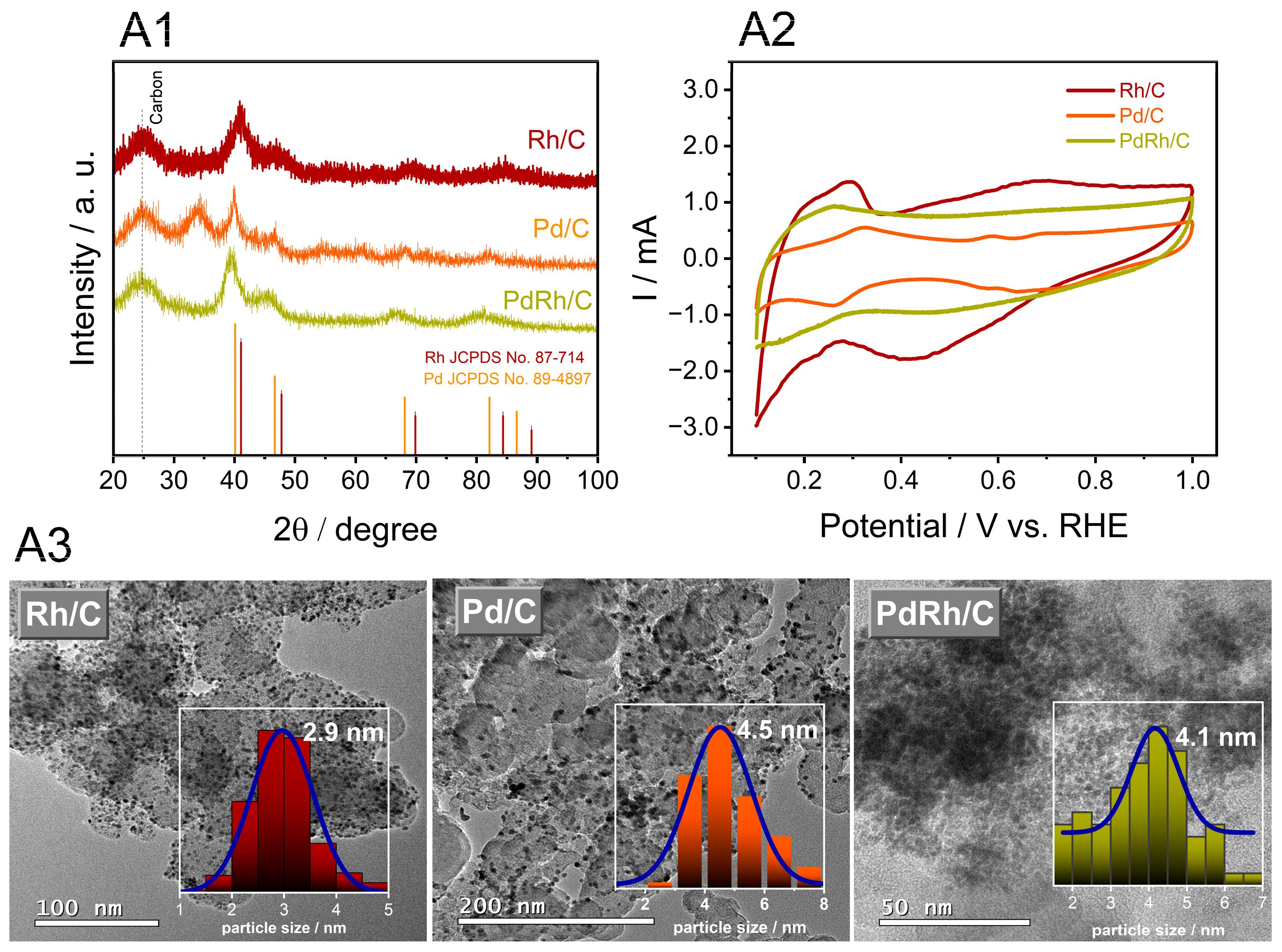
3.1. Characterization of the Physical Properties
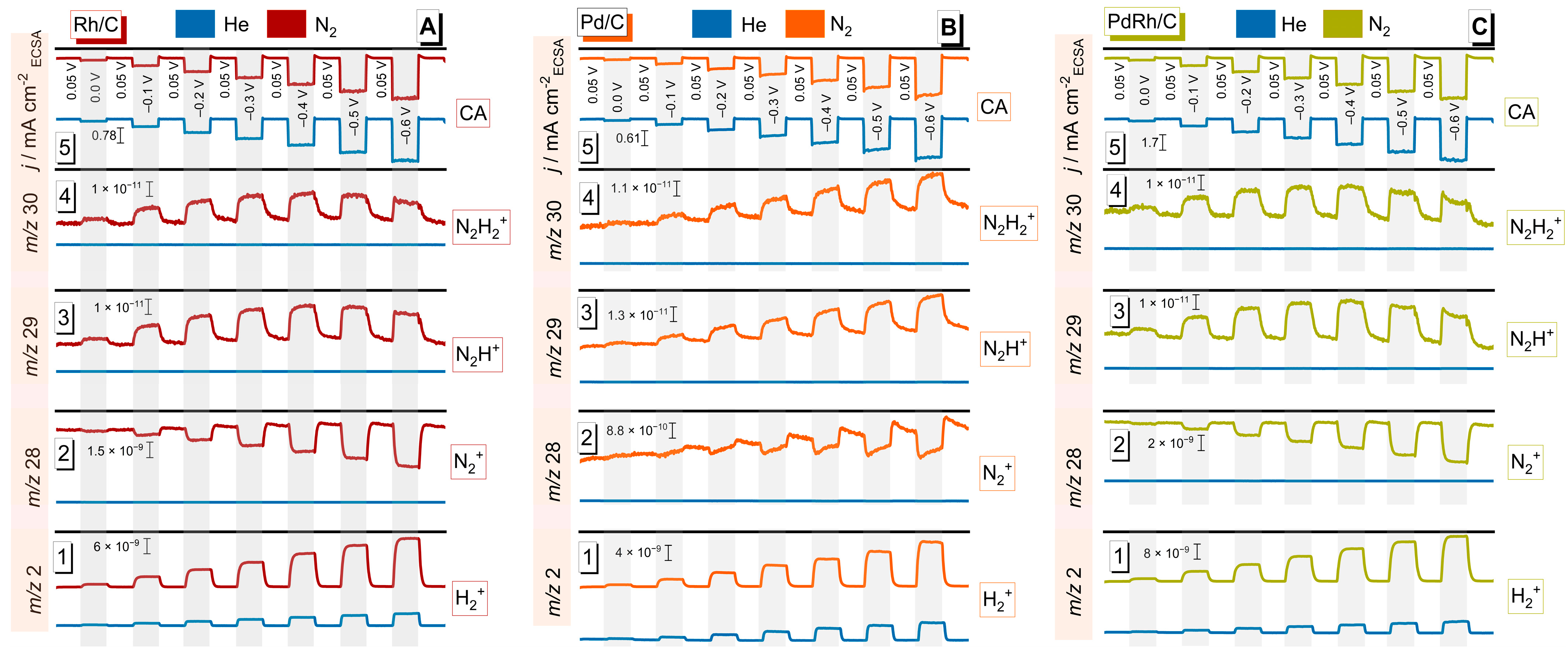
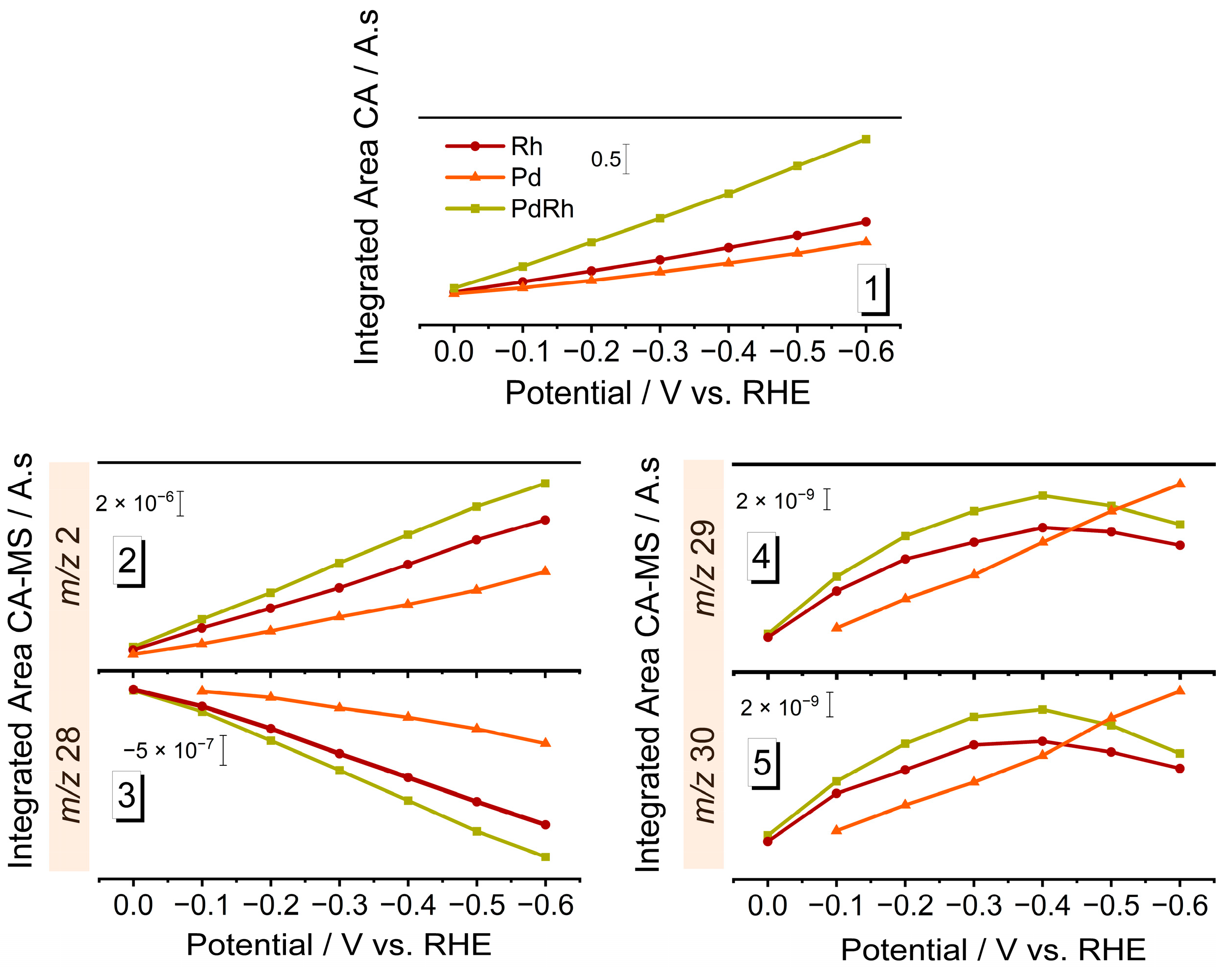
3.2. Electrocatalytic NRR Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qing, G.; Ghazfar, R.; Jackowski, S.T.; Habibzadeh, F.; Ashtiani, M.M.; Chen, C.-P.; Smith, M.R., III; Hamann, T.W. Recent Advances and Challenges of Electrocatalytic N2 Reduction to Ammonia. Chem. Rev. 2020, 120, 5437–5516. [Google Scholar] [CrossRef]
- Nayak-Luke, R.; Bañares-Alcántara, R.; Wilkinson, I. “Green” Ammonia: Impact of Renewable Energy Intermittency on Plant Sizing and Levelized Cost of Ammonia. Ind. Eng. Chem. Res. 2018, 57, 14607–14616. [Google Scholar] [CrossRef]
- Tang, C.; Qiao, S.-Z. How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully. Chem. Soc. Rev. 2019, 48, 3166–3180. [Google Scholar] [CrossRef]
- Bi, W.; Shaigan, N.; Malek, A.; Fatih, K.; Gyenge, E.; Wilkinson, D.P. Strategies in cell design and operation for the electrosynthesis of ammonia: Status and prospects. Energy Environ. Sci. 2022, 15, 2259–2287. [Google Scholar] [CrossRef]
- Guo, X.; Du, H.; Qu, F.; Li, J. Recent progress in electrocatalytic nitrogen reduction. J. Mater. Chem. A 2019, 7, 3531–3543. [Google Scholar] [CrossRef]
- Skúlason, E.; Bligaard, T.; Gudmundsdóttir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jónsson, H.; Nørskov, J.K. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245. [Google Scholar] [CrossRef]
- Liu, H.; Han, S.; Zhao, Y.; Zhu, Y.; Tian, X.; Zeng, J.; Jiang, J.; Xia, B.; Chen, Y. Surfactant-free atomically ultrathin rhodium nanosheet nanoassemblies for efficient nitrogen electroreduction. J. Mater. Chem. A 2018, 6, 3211–3217. [Google Scholar] [CrossRef]
- Wang, J.; Huang, B.; Ji, Y.; Sun, M.; Wu, T.; Yin, R.; Zhu, X.; Li, Y.; Shao, Q.; Huang, X. A General Strategy to Glassy M-Te (M = Ru, Rh, Ir) Porous Nanorods for Efficient Electrochemical N2 Fixation. Adv. Mater. 2020, 32, 1907112. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, S.; Wang, H.; Li, H.; Shao, M. A spectroscopic study of electrochemical nitrogen and nitrate reduction on rhodium surfaces. Angew. Chem. 2020, 132, 10565–10569. [Google Scholar] [CrossRef]
- Chen, T.; Liu, S.; Ying, H.; Li, Z.; Hao, J. Reactive Ionic Liquid Enables the Construction of 3D Rh Particles with Nanowire Subunits for Electrocatalytic Nitrogen Reduction. Chem.—Asian J. 2020, 15, 1081–1087. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Liu, D.-X.; Wen, Z.; Yao, J.-X.; Shi, M.-M.; Zhu, Y.-F.; Yan, J.-M.; Jiang, Q. High spin polarization ultrafine Rh nanoparticles on CNT for efficient electrochemical N2 fixation to ammonia. Appl. Catal. B Environ. 2021, 298, 120592. [Google Scholar] [CrossRef]
- Shen, P.; Li, X.; Luo, Y.; Zhang, N.; Zhao, X.; Chu, K. Ultra-efficient N2 electroreduction achieved over a rhodium single-atom catalyst (Rh1/MnO2) in water-in-salt electrolyte. Appl. Catal. B Environ. 2022, 316, 121651. [Google Scholar] [CrossRef]
- Xu, W.; Fan, G.; Chen, J.; Li, J.; Zhang, L.; Zhu, S.; Su, X.; Cheng, F.; Chen, J. Nanoporous Palladium Hydride for Electrocatalytic N2 Reduction under Ambient Conditions. Angew. Chem. Int. Ed. 2020, 59, 3511–3516. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.H.; Tsai, C.; Vojvodic, A.; Nørskov, J.K. The Challenge of Electrochemical Ammonia Synthesis: A New Perspective on the Role of Nitrogen Scaling Relations. ChemSusChem 2015, 8, 2180–2186. [Google Scholar] [CrossRef]
- Assad, S.; Tariq, T.; Zaeem Idrees, M.; Mannan Butt, A.; Bakhat, K.; Shamraiz, U. Recent progress in Pd based electrocatalysts for electrochemical nitrogen reduction to ammonia. J. Electroanal. Chem. 2023, 931, 117174. [Google Scholar] [CrossRef]
- Wang, J.; Yu, L.; Hu, L.; Chen, G.; Xin, H.; Feng, X. Ambient ammonia synthesis via palladium-catalyzed electrohydrogenation of dinitrogen at low overpotential. Nat. Commun. 2018, 9, 1795. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhang, H.; Wang, S.; Wang, P.; Xu, Y.; Li, X.; Wang, L.; Wang, H. Palladium Nanothorn Assembly Array for Efficient Electroreduction of Nitrogen to Ammonia. ACS Sustain. Chem. Eng. 2020, 8, 14228–14233. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, H.; Li, J.; Wang, Y.; Deng, K.; Yu, H.; Wang, H.; Wang, Z.; Wang, L. Boron-Doped PdRh Mesoporous Nanotubes for Electrocatalytic Nitrogen Reduction to Ammonia. ACS Appl. Nano Mater. 2024, 7, 10895–10901. [Google Scholar] [CrossRef]
- Shipman, M.; Symes, M. Recent progress towards the electrosynthesis of ammonia from sustainable resources. Catal. Today 2017, 286, 57–68. [Google Scholar] [CrossRef]
- Cui, X.; Tang, C.; Zhang, Q. A Review of Electrocatalytic Reduction of Dinitrogen to Ammonia under Ambient Conditions. Adv. Energy Mater. 2018, 8, 1800369. [Google Scholar] [CrossRef]
- de Araujo, R.G.; Perez, J. Nitrogen Electrochemical Reduction Reaction Pathways Evidenced by Online Electrochemical Mass Spectrometry and Isotope Labeling on the MoS2 Surface. ACS Electrochem. 2025, 1, 294–302. [Google Scholar] [CrossRef]
- Santiago, E.I.; Varanda, L.C.; Villullas, H.M. Carbon-Supported Pt−Co Catalysts Prepared by a Modified Polyol Process as Cathodes for PEM Fuel Cells. J. Phys. Chem. C 2007, 111, 3146–3151. [Google Scholar] [CrossRef]
- Fievet, F.; Lagier, J.P.; Figlarz, M. Preparing Monodisperse Metal Powders in Micrometer and Submicrometer Sizes by the Polyol Process. MRS Bull. 1989, 14, 29–34. [Google Scholar] [CrossRef]
- Aminot, A.; Kirkwood, D.S.; Kérouel, R. Determination of ammonia in seawater by the indophenol-blue method: Evaluation of the ICES NUTS I/C 5 questionnaire. Mar. Chem. 1997, 56, 59–75. [Google Scholar] [CrossRef]
- Biswas, A.; Ghosh, B.; Dey, R.S. Refining the Spectroscopic Detection Technique: A Pivot in the Electrochemical Ammonia Synthesis. Langmuir 2023, 39, 3810–3820. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Du, H.-L.; Wang, D.; Chen, J.; Simonov, A.N.; Macfarlane, D.R. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2019, 2, 290–296. [Google Scholar] [CrossRef]
- Wang, H.; Abruña, H.D. Rh and Rh Alloy Nanoparticles as Highly Active H2 Oxidation Catalysts for Alkaline Fuel Cells. ACS Catal. 2019, 9, 5057–5062. [Google Scholar] [CrossRef]
- Dector, A.; Cuevas-Muñiz, F.M.; Guerra-Balcázar, M.; Godínez, L.A.; Ledesma-García, J.; Arriaga, L.G. Glycerol oxidation in a microfluidic fuel cell using Pd/C and Pd/MWCNT anodes electrodes. Int. J. Hydrogen Energy 2013, 38, 12617–12622. [Google Scholar] [CrossRef]
- Jurzinsky, T.; Bär, R.; Cremers, C.; Tübke, J.; Elsner, P. Highly active carbon supported palladium-rhodium PdXRh/C catalysts for methanol electrooxidation in alkaline media and their performance in anion exchange direct methanol fuel cells (AEM-DMFCs). Electrochim. Acta 2015, 176, 1191–1201. [Google Scholar] [CrossRef]
- Sharma, R.; Gyergyek, S.; Andersen, S.M. Critical thinking on baseline corrections for electrochemical surface area (ECSA) determination of Pt/C through H-adsorption/H-desorption regions of a cyclic voltammogram. Appl. Catal. B Environ. 2022, 311, 121351. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, T.; Hao, J.; Zhuang, Z.; Gao, G.; Lai, F.; Lu, S.; Wang, X.; Kang, Q.; Wu, G.; et al. A Coherent Pd–Pd16B3 Core–Shell Electrocatalyst for Controlled Hydrogenation in Nitrogen Reduction Reaction. Adv. Funct. Mater. 2024, 34, 2400849. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araujo, R.G.; de França, C.E.C.; Perez, J. Electroreduction of Nitrogen on Pd, Rh, and PdRh Catalysts: An Online Mass Spectrometry Study. Reactions 2025, 6, 44. https://doi.org/10.3390/reactions6030044
de Araujo RG, de França CEC, Perez J. Electroreduction of Nitrogen on Pd, Rh, and PdRh Catalysts: An Online Mass Spectrometry Study. Reactions. 2025; 6(3):44. https://doi.org/10.3390/reactions6030044
Chicago/Turabian Stylede Araujo, Rodrigo Gomes, Caio Eduardo Canin de França, and Joelma Perez. 2025. "Electroreduction of Nitrogen on Pd, Rh, and PdRh Catalysts: An Online Mass Spectrometry Study" Reactions 6, no. 3: 44. https://doi.org/10.3390/reactions6030044
APA Stylede Araujo, R. G., de França, C. E. C., & Perez, J. (2025). Electroreduction of Nitrogen on Pd, Rh, and PdRh Catalysts: An Online Mass Spectrometry Study. Reactions, 6(3), 44. https://doi.org/10.3390/reactions6030044





