An Iron-Dependent Alcohol Dehydrogenase Is Involved in Ethanol Metabolism of Aromatoleum aromaticum
Abstract
1. Introduction
2. Materials and Methods
3. Results
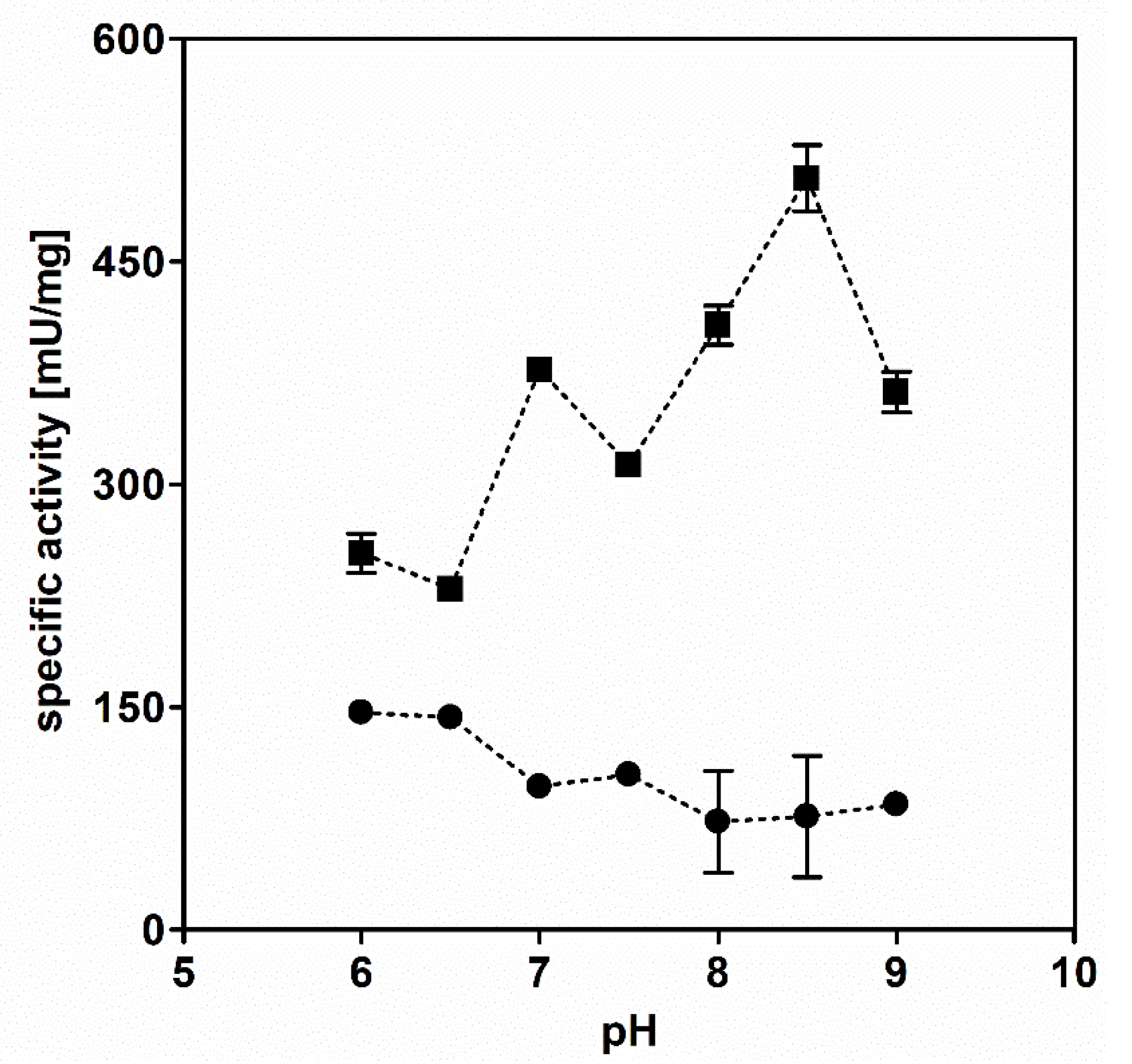
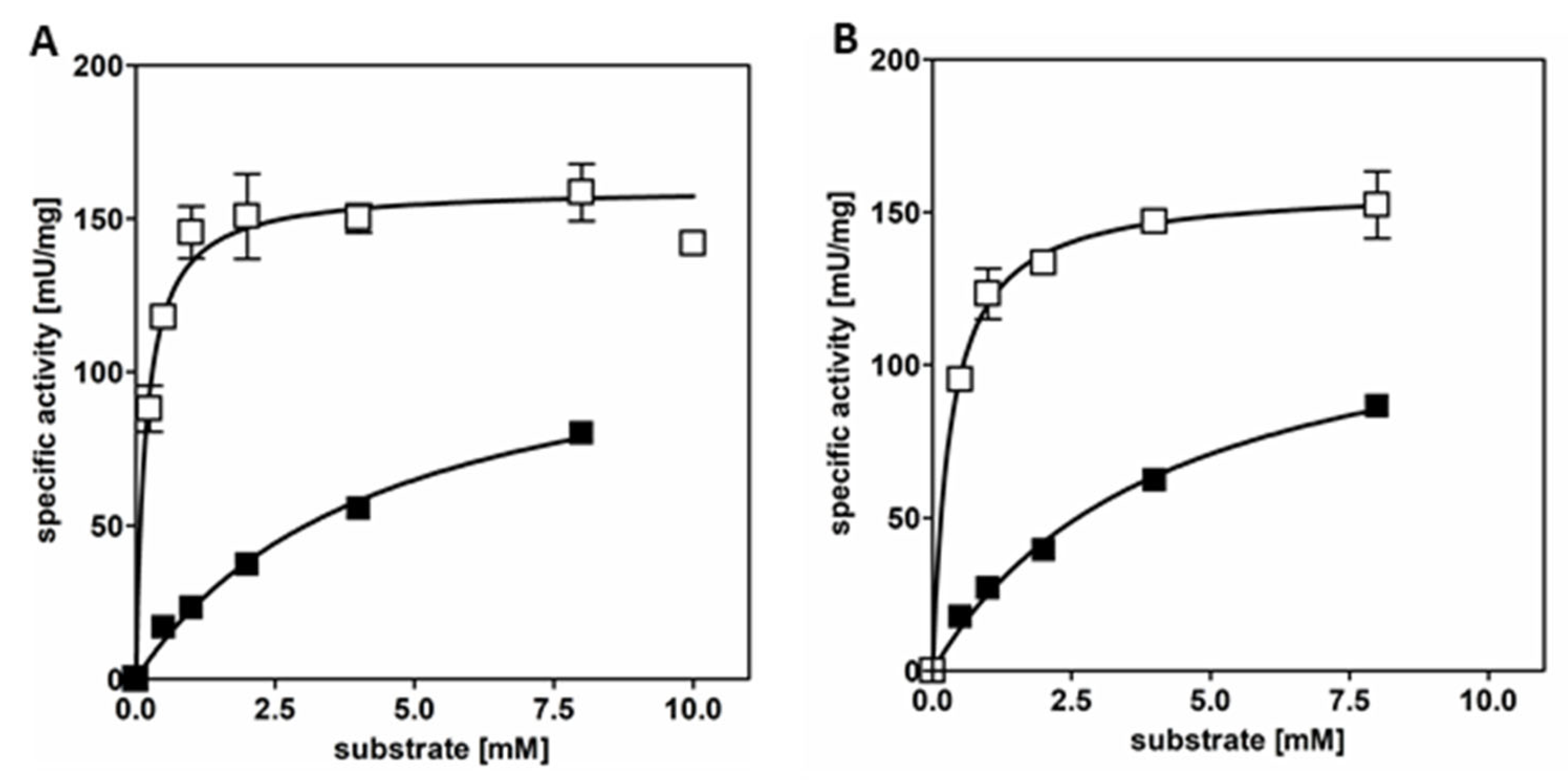
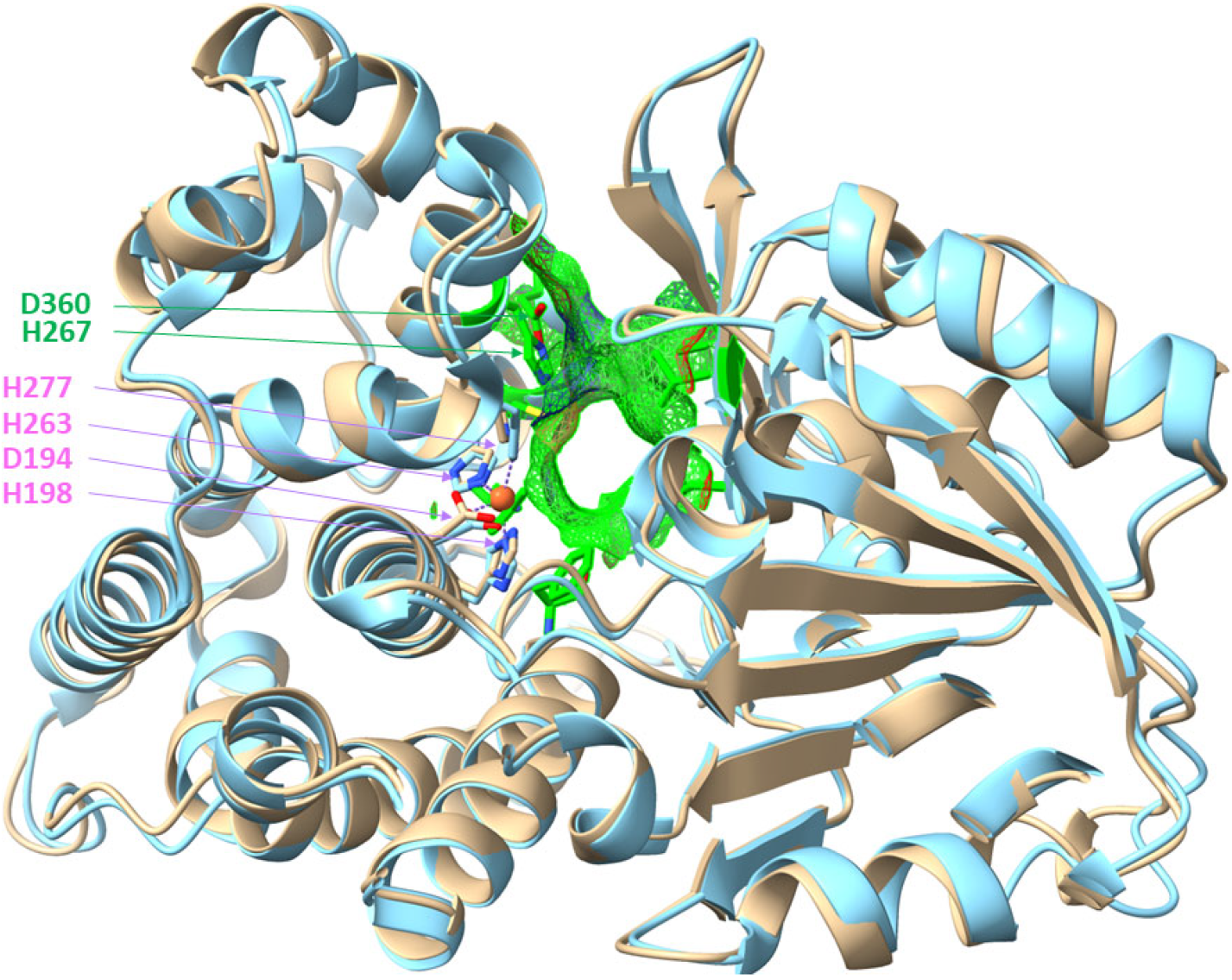
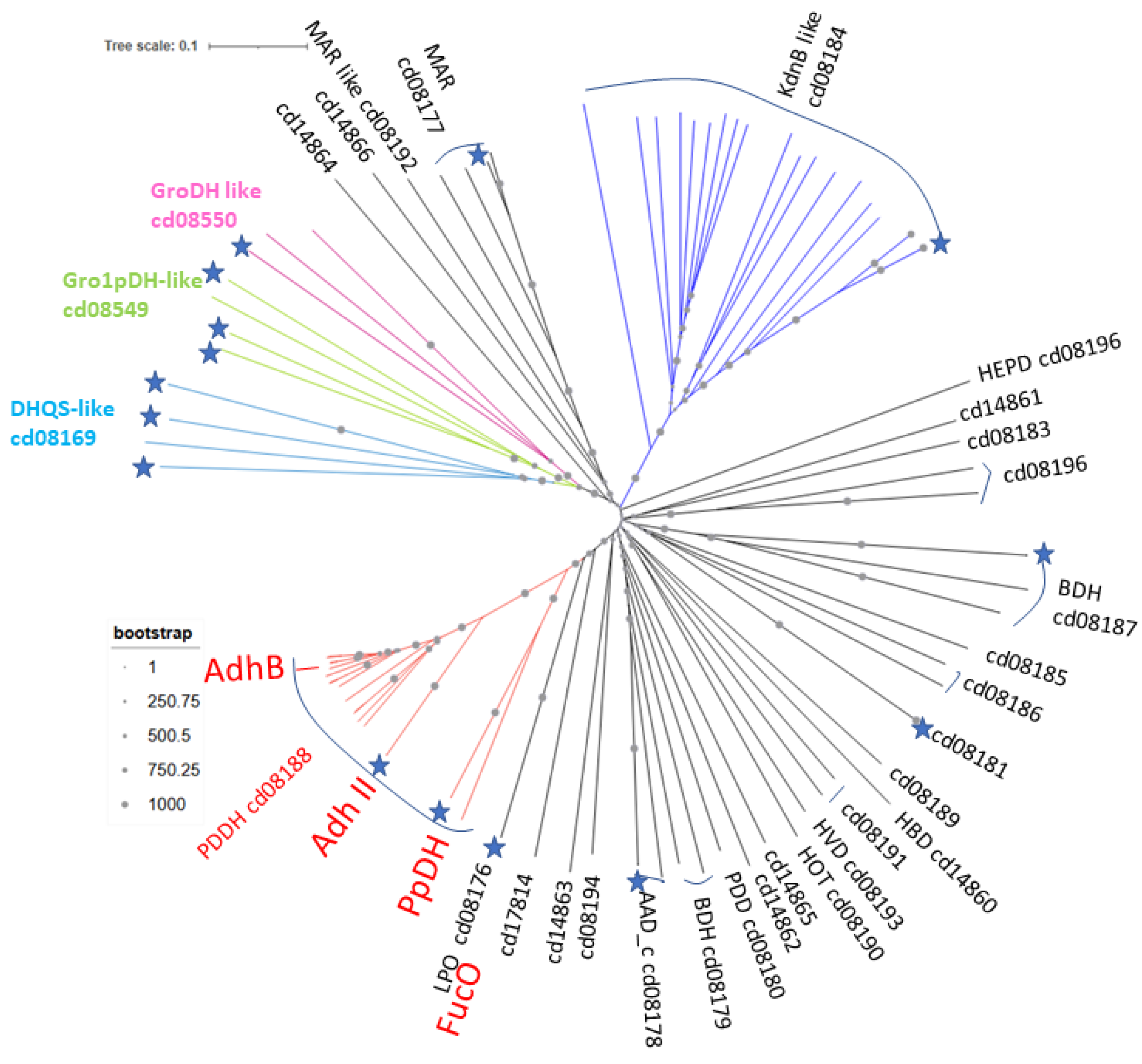
3.1. AdhB Is an Alcohol Dehydrogenase for Small Aliphatic Alcohols
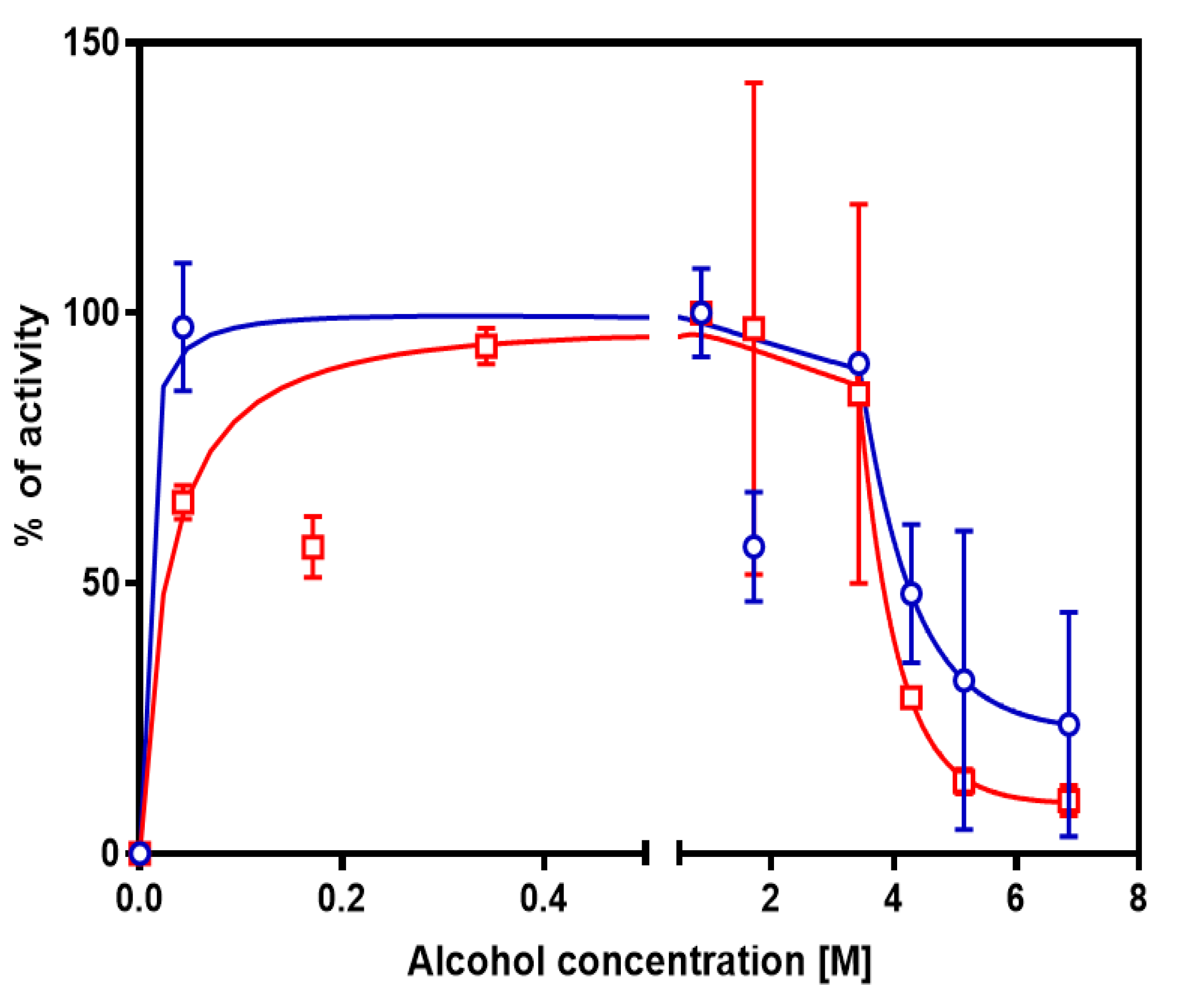
3.2. Tolerance to Alcohols
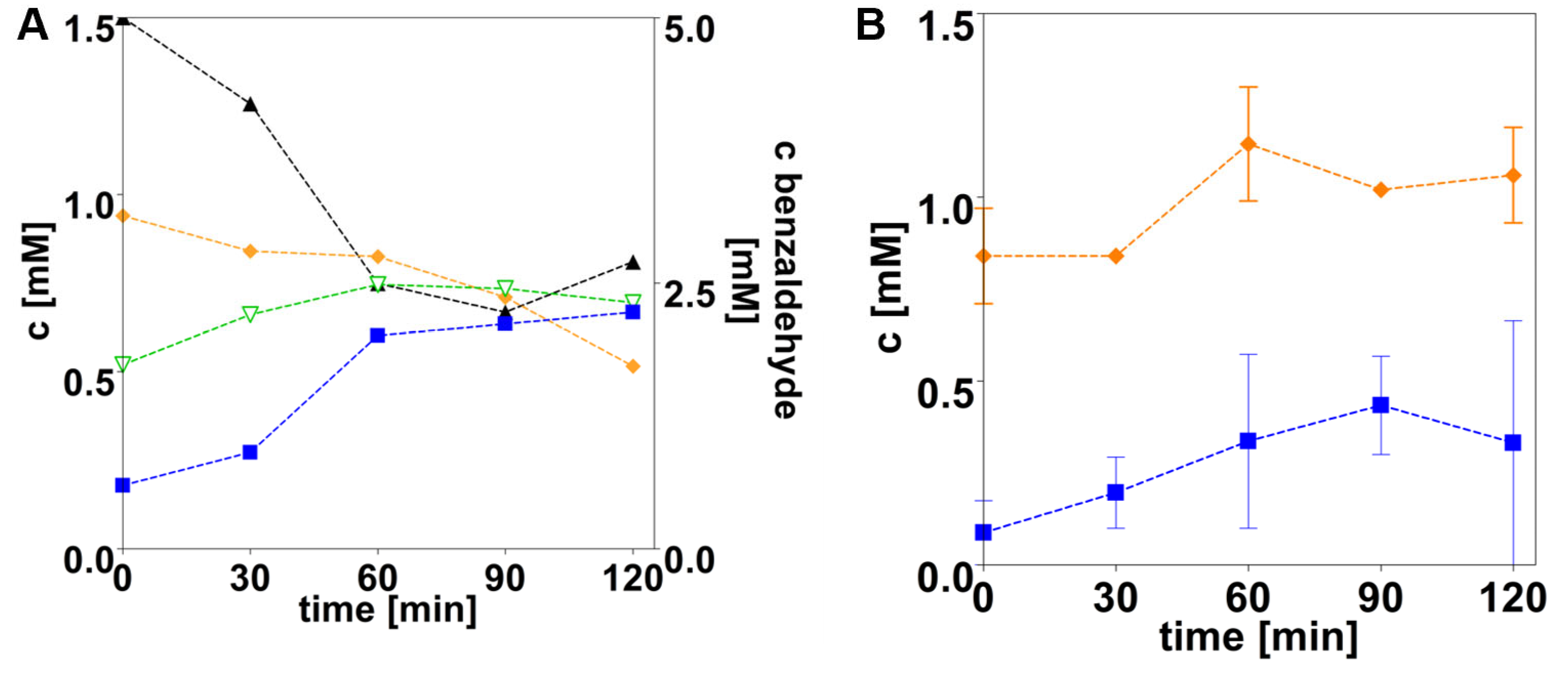
3.3. AdhB Enables Aromatoleum evansii to Grow on Ethanol as a Carbon Source
3.4. Application of AdhB in Coupled Enzyme Reactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AdhB | Fe-dependent alcohol dehydrogenase from A. aromaticum |
| NAD(P) | Nicotinamide adenine dinucleotide (phosphate) |
| Pdh | Phenylacetaldehyde dehydrogenase |
| ADH | Alcohol dehydrogenase |
| SDR | Short-chain dehydrogenase/reductase |
| UV–Vis | Ultraviolet–visible light |
| SDS-PAGE | Sodium dodecylsulfonate polyacrylamide gel electrophoresis |
| ICP-MS | Inductively coupled plasma-mass spectrometry |
| LC-MS/MS | Liquid chromatography–double mass spectrometry |
| Tris | Tris-(hydroxymethyl)aminomethan |
| HEPPS | 4-(Hydroxyethyl)-piperazine-1-propansulfonate |
| AOR | Aldehyde oxidoreductase |
| HPLC | High-performance liquid chromatography |
| RID | Refractive index detector |
| PCR | Polymerase chain reaction |
| TA | Thauera aromatica medium |
| Da | Dalton |
| BaDH | Benzyl alcohol dehydrogenase |
| Vmax | Michaelis–Menten maximum activity |
| Km | Michaelis–Menten constant |
| BV | Benzyl viologen |
| FAD | Flavin adenine dinucleotide |
| DHQS | Dehydroquinate synthase |
| Gro1pDH | Glycerol-1-phosphate dehydrogenase |
| GroDH | Glycerol-dehydrogenase |
References
- Rabus, R.; Kube, M.; Heider, J.; Beck, A.; Heitmann, K.; Widdel, F.; Reinhardt, R. The Genome Sequence of an Anaerobic Aromatic-Degrading Denitrifying Bacterium, Strain EbN1. Arch. Microbiol. 2005, 183, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.; Wünsch, D.; Wöhlbrand, L.; Neumann-Schaal, M.; Schomburg, D.; Rabus, R. The Catabolic Network of Aromatoleum aromaticum EbN1T. Microb. Physiol. 2024, 34, 1–77. [Google Scholar] [CrossRef] [PubMed]
- Rabus, R. Functional Genomics of an Anaerobic Aromatic-Degrading Denitrifying Bacterium, Strain EbN1. Appl. Microbiol. Biotechnol. 2005, 68, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Wöhlbrand, L.; Kallerhoff, B.; Lange, D.; Hufnagef, P.; Thiermann, J.; Reinhardt, R.; Rabus, R. Functional Proteomic View of Metabolic Regulation in “Aromatoleum aromaticum” Strain EbN1. Proteomics 2007, 7, 2222–2239. [Google Scholar] [CrossRef]
- Schmitt, G.; Arndt, F.; Kahnt, J.; Heider, J. Adaptations to a Loss-of-Function Mutation in the Betaproteobacterium Aromatoleum aromaticum: Recruitment of Alternative Enzymes for Anaerobic Phenylalanine Degradation. J. Bacteriol. 2017, 199, e00383-17. [Google Scholar] [CrossRef]
- Hege, D.; Gemmecker, Y.; Schall, I.; Oppong-Nti, P.; Schmitt, G.; Heider, J. Single Amino Acid Exchanges Affect the Substrate Preference of an Acetaldehyde Dehydrogenase. Appl. Microbiol. Biotechnol. 2025, 109, 103. [Google Scholar] [CrossRef]
- Heider, J.; Hege, D. The Aldehyde Dehydrogenase Superfamilies: Correlations and Deviations in Structure and Function. Appl. Microbiol. Biotechnol. 2025, 109, 106. [Google Scholar] [CrossRef]
- Conway, T.; Sewell, G.W.; Osman, Y.A.; Ingram, L.O. Cloning and Sequencing of the Alcohol Dehydrogenase II Gene from Zymomonas mobilis. J. Bacteriol. 1987, 169, 2591–2597. [Google Scholar] [CrossRef]
- Ingram, L.O.; Conway, T.; Clark, D.P.; Sewell, G.W.; Preston, J.F. Genetic Engineering of Ethanol Production in Escherichia coli. Appl. Environ. Microbiol. 1987, 53, 2420–2425. [Google Scholar] [CrossRef]
- Reid, M.F.; Fewson, C.A. Molecular Characterization of Microbial Alcohol Dehydrogenases. Crit. Rev. Microbiol. 1994, 20, 13–56. [Google Scholar] [CrossRef]
- Hernández-Tobías, A.; Julián-Sánchez, A.; Piña, E.; Riveros-Rosas, H. Natural Alcohol Exposure: Is Ethanol the Main Substrate for Alcohol Dehydrogenases in Animals? Chem. Interact. 2011, 191, 14–25. [Google Scholar] [CrossRef]
- Gaona-López, C.; Julián-Sánchez, A.; Riveros-Rosas, H. Diversity and Evolutionary Analysis of Iron-Containing (Type-III) Alcohol Dehydrogenases in Eukaryotes. PLoS ONE 2016, 11, e0166851. [Google Scholar] [CrossRef]
- Shanbhag, A.P.; Ghatak, A.; Rajagopal, S. Industrial Light at the End of the Iron-Containing (Group III) Alcohol Dehydrogenase Tunnel. Biotechnol. Appl. Biochem. 2023, 70, 537–552. [Google Scholar] [CrossRef]
- Maier, T.; Drapal, N.; Thanbichler, M.; Böck, A. Strep-Tag II Affinity Purification: An Approach to Study Intermediates of Metalloenzyme Biosynthesis. Anal. Biochem. 1998, 259, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, Y.; Nagatoishi, S.; Nakagawa, M.; Tsumoto, K.; Arakawa, T.; Akuta, T. Ferguson Plot Analysis of Multiple Intermediate Species of Thermally Unfolded Bovine Serum Albumin. Biophys. Chem. 2023, 301, 107095. [Google Scholar] [CrossRef] [PubMed]
- Gemmecker, Y.; Winiarska, A.; Hege, D.; Kahnt, J.; Seubert, A.; Szaleniec, M.; Heider, J. A PH-Dependent Shift of Redox Cofactor Specificity in a Benzyl Alcohol Dehydrogenase of Aromatoleum aromaticum EbN1. Appl. Microbiol. Biotechnol. 2024, 108, s00253-024. [Google Scholar] [CrossRef] [PubMed]
- Coligan, J.E.; Dunn, B.M.; Ploegh, H.L.; Speicher, D.W.; Wingfield, P.T. Current Protocols in Protein Science; Coligan, J.E., Dunn, B.M., Ploegh, H.L., Speicher, D.W., Wingfield, P.T., Eds.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Arndt, F.; Schmitt, G.; Winiarska, A.; Saft, M.; Seubert, A.; Kahnt, J.; Heider, J. Characterization of an Aldehyde Oxidoreductase from the Mesophilic Bacterium Aromatoleum aromaticum EbN1, a Member of a New Subfamily of Tungsten-Containing Enzymes. Front. Microbiol. 2019, 10, 71. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vaccaro, B.J.; Menon, A.L.; Lancaster, W.A.; Adams, M.W.W. Metallomics Using Inductively Coupled Plasma Mass Spectrometry. Curr. Protoc. Chem. Biol. 2012, 4, 249–274. [Google Scholar] [CrossRef]
- Gomes-Filho, J.V.; Breuer, R.; Morales-Filloy, H.G.; Pozhydaieva, N.; Borst, A.; Paczia, N.; Soppa, J.; Höfer, K.; Jäschke, A.; Randau, L. Identification of NAD-RNA Species and ADPR-RNA Decapping in Archaea. Nat. Commun. 2023, 14, 7597. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 14th ed.; Dean, J.A., Ed.; McGraw-Hill: New York, NY, USA, 1992; ISBN 0-07-016194-1. [Google Scholar]
- Cornish-Bowden, A. Fundamentals of Enzyme Kinetics, 3rd ed.; Portland Press: London, UK, 2004. [Google Scholar]
- Winiarska, A.; Hege, D.; Gemmecker, Y.; Kryściak-Czerwenka, J.; Seubert, A.; Heider, J.; Szaleniec, M. Tungsten Enzyme Using Hydrogen as an Electron Donor to Reduce Carboxylic Acids and NAD+. ACS Catal. 2022, 12, 8707–8717. [Google Scholar] [CrossRef]
- Hege, D.; Gemmecker, Y.; Clermont, L.; Aleksic, I.; Olesky, G.; Szaleniec, M.; Heider, J. Genetic Manipulation of the Betaproteobacterial Genera Thauera and Aromatoleum. Methods Enzymol. 2025, 714, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Kaetzke, A.; Kampfer, P.; Ludwig, W.; Fuchs, G. Taxonomic Position of Aromatic-Degrading Denitrifying Pseudomonad Strains K 172 and KB 740 and Their Description as New Members of the Genera Thauera, as Thauera aromatica sp. nov., and Azoarcus, as Azoarcus evansii sp. nov., Respectively, Members of the Beta Suclass of Proteobacteria. Int. J. Syst. Bacteriol. 1995, 45, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Sun, S.; Zhou, Y.; Peng, T.; Xu, X.; Li, B.; Tan, G. Escherichia coli Alcohol Dehydrogenase YahK Is a Protein That Binds Both Iron and Zinc. PeerJ 2024, 12, e18040. [Google Scholar] [CrossRef] [PubMed]
- Rabus, R.; Wöhlbrand, L.; Thies, D.; Meyer, M.; Reinhold-Hurek, B.; Kampfer, P. Aromatoleum gen. nov., a Novel Genus Accommodating the Phylogenetic Lineage Including Azoarcus evansii and Related Species, and Proposal of Aromatoleum aromaticum sp. nov., Aromatoleum petrolei sp. nov., Aromatoleum bremense sp. nov., Aromatoleum toluolicum sp. nov. and Aromatoleum diolicum sp. nov. Int. J. Syst. Evol. Microbiol. 2019, 69, 982–997. [Google Scholar] [CrossRef]
- Rabus, R.; Widdel, F. Anaerobic Degradation of Ethylbenzene and Other Aromatic Hydrocarbons by New Denitrifying Bacteria. Arch. Microbiol. 1995, 163, 96–103. [Google Scholar] [CrossRef]
- Rabus, R.; Widdel, F. Utilization of Alkylbenzenes during Anaerobic Growth of Pure Cultures of Denitrifying Bacteria on Crude Oil. Appl. Environ. Microbiol. 1996, 62, 1238–1241. [Google Scholar] [CrossRef]
- Rabus, R.; Strittmatter, A. Functional Genomics of Sulphate-Reducing Prokaryotes. In Sulphate-Reducing Bacteria: Environmental and Engineered Systems; Cambridge University Press: Cambridge, UK, 2007; pp. 117–140. ISBN 9780511541490. [Google Scholar]
- Szaleniec, M.; Heider, J. Obligately Tungsten-Dependent Enzymes—Catalytic Mechanisms, Models and Applications. Biochemistry 2025, 64, 2154–2174. [Google Scholar] [CrossRef]
- Wills, C.; Kratofil, P.; Londo, D.; Martin, T. Characterization of the Two Alcohol Dehydrogenases of Zymomonas mobilis. Arch. Biochem. Biophys. 1981, 210, 775–785. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L. Biochemical and Functional Characterization of an Iron-Containing Alcohol Dehydrogenase from Thermococcus barophilus Ch5. Appl. Biochem. Biotechnol. 2022, 194, 5537–5555. [Google Scholar] [CrossRef]
- Ma, K.; Adams, M.W.W. An Unusual Oxygen-Sensitive, Iron- and Zinc-Containing Alcohol Dehydrogenase from the Hyperthermophilic Archaeon Pyrococcus furiosus. J. Bacteriol. 1999, 181, 1163–1170. [Google Scholar] [CrossRef]
- Lin, Y.; Yin, Y.; Oger, P.; Gong, Y.; Zhou, X.; Bai, Y.; Zhang, L. New Insights into Thermostable Iron-Containing/Activated Alcohol Dehydrogenases from Hyperthermophiles. Int. J. Biol. Macromol. 2024, 275, 033707. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Lee, H.J.; Park, S.Y.; Song, J.M.; Park, M.Y.; Park, H.M.; Sun, J.; Park, J.H.; Kim, B.Y.; Kim, J.S. Structures of Iron-Dependent Alcohol Dehydrogenase 2 from Zymomonas mobilis ZM4 with and without NAD+ Cofactor. J. Mol. Biol. 2011, 407, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, K.F.; Eddy, C.K.; Ingram, L.O. Modulation of Alcohol Dehydrogenase Isoenzyme Levels in Zymomonas mobilis by Iron and Zinc. J. Bacteriol. 1989, 171, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Montella, C.; Bellsolell, L.; Pérez-Luque, R.; Badía, J.; Baldoma, L.; Coll, M.; Aguilar, J. Crystal Structure of an Iron-Dependent Group III Dehydrogenase That Interconverts L-Lactaldehyde and L-1,2-Propanediol in Escherichia coli. J. Bacteriol. 2005, 187, 4957–4966. [Google Scholar] [CrossRef]
- Marçal, D.; Rêgo, A.T.; Carrondo, M.A.; Enguita, F.J. 1,3-Propanediol Dehydrogenase from Klebsiella pneumoniae: Decameric Quaternary Structure and Possible Subunit Cooperativity. J. Bacteriol. 2009, 191, 1143–1151. [Google Scholar] [CrossRef]
- O’Mullan, P.J.; Buchholz, S.E.; Chase, T.; Eveleigh, D.E. Roles of Alcohol Dehydrogenases of Zymomonas mobilis (ZADH): Characterization of a ZADH-2-Negative Mutant. Appl. Microbiol. Biotechnol. 1995, 43, 675–678. [Google Scholar] [CrossRef]
- Weiten, A.; Kalvelage, K.; Becker, P.; Reinhardt, R.; Hurek, T.; Reinhold-Hurek, B.; Rabus, R. Complete Genomes of the Anaerobic Degradation Specialists Aromatoleum petrolei ToN1Tand Aromatoleum bremense PbN1T. Microb. Physiol. 2021, 31, 16–35. [Google Scholar] [CrossRef]
- Luo, S.; Adam, D.; Giaveri, S.; Barthel, S.; Cestellos-Blanco, S.; Hege, D.; Paczia, N.; Castañeda-Losada, L.; Klose, M.; Arndt, F.; et al. ATP Production from Electricity with a New-to-Nature Electrobiological Module. Joule 2023, 7, 1745–1758. [Google Scholar] [CrossRef]
- White, H.; Strobl, G.; Feicht, R.; Simon, H. Carboxylic Acid Reductase: A New Tungsten Enzyme Catalyses the Reduction of Non-activated Carboxylic Acids to Aldehydes. Eur. J. Biochem. 1989, 184, 89–96. [Google Scholar] [CrossRef]
- Strobl, G.; White, H.; Feicht, R.; Simon, H. The Tungsten-Containing Aldehyde Oxidoreductase from Clostridium thermoaceticum and Its Complex with a Viologen-Accepting NADPH Oxidoreductase. Biol. Chem. Hoppe-Seyler 1992, 373, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.; Skopan, H.; Feicht, R.; White, H.; Simon, H. Pterin Cofactor, Substrate Specificity, and Observations on the Kinetics of the Reversible Tungsten-Containing Aldehyde Oxidoreductase from Clostridium thermoaceticum. Arch. Microbiol. 1995, 164, 110–118. [Google Scholar] [CrossRef]
- Winiarska, A.; Ramírez-Amador, F.; Hege, D.; Gemmecker, Y.; Prinz, S.; Hochberg, G.; Heider, J.; Szaleniec, M.; Schuller, J.M. A Bacterial Tungsten-Containing Aldehyde Oxidoreductase Forms an Enzymatic Decorated Protein Nanowire. Sci. Adv. 2023, 9, eadg668. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W.; Thauer, R.K. Energy Conservation via Electron Bifurcating Ferredoxin Reduction and Proton/Na+ Translocating Ferredoxin Oxidation. Biochim. Biophys. Acta-Bioenerg. 2013, 1827, 94–113. [Google Scholar] [CrossRef]
- Golubev, G.S.; Borisov, I.L.; Volkov, V.V. Thermopervaporative Removal of Isopropanol and Butanol from Aqueous Media Using Membranes Based on Hydrophobic Polysiloxanes. Pet. Chem. 2018, 58, 975–982. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane Distillation: A Comprehensive Review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Shalygin, M.G.; Kozlova, A.A.; Heider, J.; Sapegin, D.A.; Netrusov, A.A.; Teplyakov, V.V. Polymeric Membranes for Vapor-Phase Concentrating Volatile Organic Products from Biomass Processing. Membr. Membr. Technol. 2023, 5, 55–67. [Google Scholar] [CrossRef]
- Zavarise, A.; Sridhar, S.; Kiema, T.R.; Wierenga, R.K.; Widersten, M. Structures of Lactaldehyde Reductase, FucO, Link Enzyme Activity to Hydrogen Bond Networks and Conformational Dynamics. FEBS J. 2023, 290, 465–481. [Google Scholar] [CrossRef]
- Extance, J.; Crennell, S.J.; Eley, K.; Cripps, R.; Hough, D.W.; Danson, M.J. Structure of a Bifunctional Alcohol Dehydrogenase Involved in Bioethanol Generation in Geobacillus thermoglucosidasius. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 2104–2115. [Google Scholar] [CrossRef]
- Kinoshita, S.; Kakizono, T.; Kadota, K.; Das, K.; Taguchi, H. Purification of Two Alcohol Dehydrogenases from Zymomonas mobilis and Their Properties. Appl. Microbiol. Biotechnol. 1985, 22, 249–254. [Google Scholar] [CrossRef]
- Sun, S.; Shu, L.; Lu, X.; Wang, Q.; Tišma, M.; Zhu, C.; Shi, J.; Baganz, F.; Lye, G.J.; Hao, J. 1,2-Propanediol Production from Glycerol via an Endogenous Pathway of Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2021, 105, 9003–9016. [Google Scholar] [CrossRef]
- Li, D.; Stevenson, K.J. Purification and Sequence Analysis of a Novel NADP(H) Dependent Type III Alcohol Dehydrogenase from Thermococcus Strain AN1. J. Bacteriol. 1997, 179, 4433–4437. [Google Scholar] [CrossRef]
- Ma, K.; Robb, F.T.; Adams, M.W.W. Purification and Characterization of NADP-Specific Alcohol Dehydrogenase and Glutamate Dehydrogenase from the Hyperthermophilic Archaeon Thermococcus litoralis. Appl. Environ. Microbiol. 1994, 60, 562–568. [Google Scholar] [CrossRef]
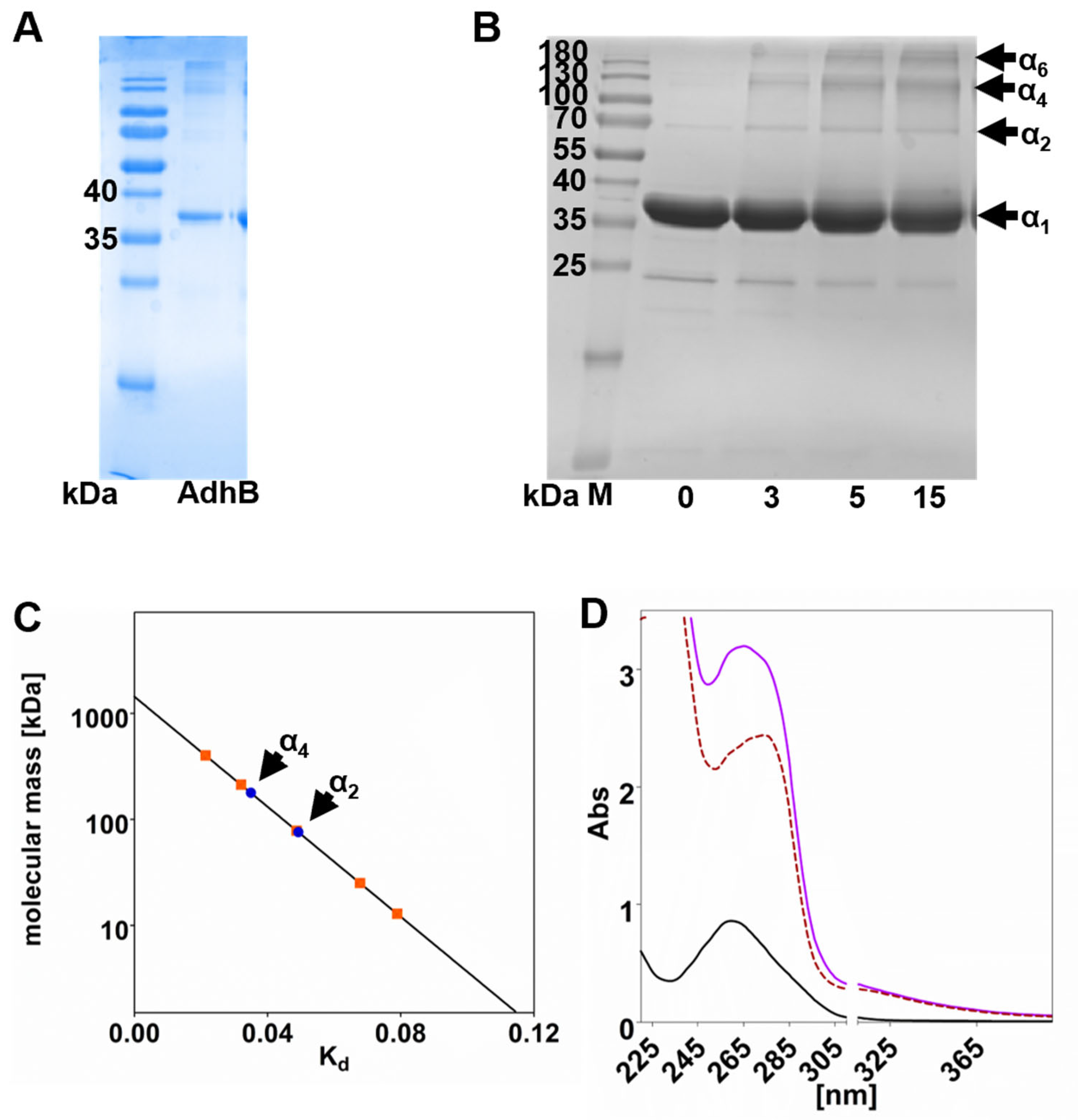

| Desalted AdhB | |
|---|---|
| Mg | 0.01 |
| P | 1.88 |
| Ca | 0.03 |
| Mn | 0.02 |
| Fe | 0.10 |
| Co | 0.00 |
| Ni | 0.06 |
| Cu | 0.01 |
| Zn | 0.13 |
| Se | 0.00 |
| Mo | 0.00 |
| W | 0.00 |
| Substrate | Ethanol | Acetaldehyde | n-Propanol | Propionaldehyde |
|---|---|---|---|---|
| App. Vmax [U/mg] | 121.3 | 160.1 | 129.8 | 158.1 |
| app. kcat [s−1] | 79.7 | 105.1 | 85.2 | 103.8 |
| app. Km [mM] | 4.4 | 0.2 | 4.2 | 0.3 |
| kcat/Km [mM−1 s−1] | 18.1 | 525.5 | 20.3 | 346.1 |
| Organism | Growth on Ethanol | adhB Gene Present (% Protein Identity) |
|---|---|---|
| A. aromaticum EbN1 | + | + (100) |
| A. aromaticum pCyN1 | + | + (100) |
| A. bremense PbN1 | − | − |
| A. petrolei ToN1 | + | − |
| Aromatoleum sp. strain EB1 | ND | − |
| A. toluolicum T | + | − |
| A. diolicum 22Lin | − | − |
| A. evansii KB740 | − | − |
| A. buckelii U120 | + | + (99) |
| A. anaerobium LuFRes1 | + | − |
| A. tolulyticum Tol-4 | + | − |
| A. toluvorans Td21 | + | − |
| A. toluclasticum MF63 | ND | − |
| Azoarcus indigens VB32 | ND | − |
| Az. communis SWub3 | + | − |
| Az. olearius BH72 | + | − |
| Thauera aromatica K172/AR-1 | + | + (90) |
| T. chlorobenzoica 3CB1 | ND | + (90) |
| T. aromatica SP/LG356 | ND | + (89) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gemmecker, Y.; Schall, I.; Seubert, A.; Paczia, N.; Heider, J. An Iron-Dependent Alcohol Dehydrogenase Is Involved in Ethanol Metabolism of Aromatoleum aromaticum. Reactions 2025, 6, 46. https://doi.org/10.3390/reactions6030046
Gemmecker Y, Schall I, Seubert A, Paczia N, Heider J. An Iron-Dependent Alcohol Dehydrogenase Is Involved in Ethanol Metabolism of Aromatoleum aromaticum. Reactions. 2025; 6(3):46. https://doi.org/10.3390/reactions6030046
Chicago/Turabian StyleGemmecker, Yvonne, Iris Schall, Andreas Seubert, Nicole Paczia, and Johann Heider. 2025. "An Iron-Dependent Alcohol Dehydrogenase Is Involved in Ethanol Metabolism of Aromatoleum aromaticum" Reactions 6, no. 3: 46. https://doi.org/10.3390/reactions6030046
APA StyleGemmecker, Y., Schall, I., Seubert, A., Paczia, N., & Heider, J. (2025). An Iron-Dependent Alcohol Dehydrogenase Is Involved in Ethanol Metabolism of Aromatoleum aromaticum. Reactions, 6(3), 46. https://doi.org/10.3390/reactions6030046








