-
 Engineering O-Glycosylation in an RSV G Vaccine Antigen
Engineering O-Glycosylation in an RSV G Vaccine Antigen -
 Microbiome–Immune Interaction and Harnessing for Next-Generation Vaccines Against Highly Pathogenic Avian Influenza in Poultry
Microbiome–Immune Interaction and Harnessing for Next-Generation Vaccines Against Highly Pathogenic Avian Influenza in Poultry -
 Toxoplasma gondii at the Host Interface: Immune Modulation and Translational Strategies for Infection Control
Toxoplasma gondii at the Host Interface: Immune Modulation and Translational Strategies for Infection Control -
 Efficacy and Safety of Anti-Respiratory Syncytial Virus Monoclonal Antibody Nirsevimab in Neonates: A Real-World Monocentric Study
Efficacy and Safety of Anti-Respiratory Syncytial Virus Monoclonal Antibody Nirsevimab in Neonates: A Real-World Monocentric Study
Journal Description
Vaccines
Vaccines
is an international, peer-reviewed, open access journal published monthly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q2 (Medicine, Research and Experimental) / CiteScore - Q1 (Pharmacology (medical))
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 19.6 days after submission; acceptance to publication is undertaken in 2.8 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
3.4 (2024);
5-Year Impact Factor:
3.7 (2024)
Latest Articles
Assessing Vaccine Confidence Using the Vaccine Hesitancy Scale Among Adolescent Girls and Young Women at Risk of HIV Acquisition Living in Uganda, Zambia, and South Africa
Vaccines 2025, 13(11), 1083; https://doi.org/10.3390/vaccines13111083 (registering DOI) - 22 Oct 2025
Abstract
Background: Vaccine hesitancy (VH) remains a major threat to global health that can reverse the progress in tackling vaccine-preventable diseases. Vaccine uptake among adolescent Girls and young women (AGYW) is often low. We assessed VH using a validated scale among AGYW in Uganda,
[...] Read more.
Background: Vaccine hesitancy (VH) remains a major threat to global health that can reverse the progress in tackling vaccine-preventable diseases. Vaccine uptake among adolescent Girls and young women (AGYW) is often low. We assessed VH using a validated scale among AGYW in Uganda, Zambia, and South Africa. Methods: From June 2023 to February 2024, we recruited AGYW from fishing communities in Uganda, urban and peri-urban locations in Lusaka and Ndola, Zambia, and mining communities in Rustenburg, South Africa. Eligible participants were aged 15–24 years, sexually active, and HIV-negative but at risk for HIV acquisition. We collected demographic, HIV-related behavioral data, and vaccine hesitancy data using a structured questionnaire. Vaccine confidence was assessed using the 10-question Vaccine Hesitancy Scale that describes two factors, i.e., “vaccine confidence” and “risk tolerance”. Exploratory and Confirmatory Factor Analyses were performed to assess scale validity and internal consistency. Logistic regression was used to determine associations between demographics and vaccine confidence. Results: A total of 1213 AGYW participated in the study, with a mean age of 19.4 (SD ± 2.6) years. More than half (54%) were aged between 15 and 19 years. The majority of AGYW (94%) strongly believed that vaccines were important for their health and the community and that vaccination is a good way to protect them from diseases. About two-thirds of the AGYW (66%) indicated that they were concerned about the adverse effects of vaccines, while 30% responded that they did not need vaccines for diseases that were not common. We observed that 951 (78%) of the AGYW reported high vaccine confidence, while 494 (41%) reported low concerns over risks. Vaccine confidence varied across countries, with Zambia and Uganda showing lower vaccine confidence (adjusted odds ratios of 0.28 and 0.45, respectively, p < 0.005) in comparison to South Africa. Conclusions: A high level of vaccine confidence was observed among AGYW. Vaccine confidence among AGYW was driven more by the trust in vaccine safety and the need to protect communities against diseases. These findings suggest the potential for acceptance of vaccines, including future HIV vaccines, among AGYW. Despite high levels of vaccine confidence, concerns over vaccine risks remain substantial and must be addressed.
Full article
(This article belongs to the Special Issue Acceptance and Hesitancy in Vaccine Uptake: 2nd Edition)
►
Show Figures
Open AccessCase Report
Observations of Wart Clearance Following COVID-19 Vaccination: Coincidence or Missed Immunologic Signals?
by
Qiwei Wilton Sun, Caroline A. Nelson and Howard P. Forman
Vaccines 2025, 13(11), 1081; https://doi.org/10.3390/vaccines13111081 (registering DOI) - 22 Oct 2025
Abstract
Background/Objectives: The COVID-19 vaccines have been extensively studied for their potential adverse side effects. However, reports of unexpected but potentially beneficial immune responses have received comparatively less attention. Methods: In this case series, a PubMed search was conducted using the terms “warts”, “verruca”,
[...] Read more.
Background/Objectives: The COVID-19 vaccines have been extensively studied for their potential adverse side effects. However, reports of unexpected but potentially beneficial immune responses have received comparatively less attention. Methods: In this case series, a PubMed search was conducted using the terms “warts”, “verruca”, “HPV”, “COVID-19”, “SARS-CoV-2”, “immunization”, and “vaccination.” All reported cases of wart clearance temporally linked to COVID-19 vaccination were identified and summarized, including patient demographics, vaccine type, number of doses, timing of clearance, and follow-up duration. Results: Five cases were identified. Patients varied in age, sex, comorbidity, and immunologic status. Warts were long-standing and treatment-resistant in all cases. Clearance occurred within approximately 2–4 weeks following the second or third vaccine dose (either mRNA-based [BNT162b2, mRNA-1273] or adenoviral vector [ChAdOx1-S]) and was sustained for 2–8 months of follow-up with no recurrences reported. Conclusions: While causality cannot be determined, the convergence of reports across diverse patients, consistent timing of clearance, and plausible immunologic pathways suggest that COVID-19 vaccination may, in rare instances, trigger beneficial immune activation against HPV-infected keratinocytes. Recognition of such unexpected outcomes underscores the need for broader vaccine safety and efficacy surveillance that includes both adverse and beneficial immune effects.
Full article
(This article belongs to the Section COVID-19 Vaccines and Vaccination)
Open AccessArticle
Norovirus in Pediatric Gastroenteritis: A Study in Argentine Hospitals Before and After the Introduction of Universal Rotavirus Vaccination
by
Karina A. Gomes, Karina A. Rivero, Christian Barrios Mathieur, Juan I. Degiuseppe, Paulo R. Cortes, Patricia A. Gonzalez, Abel Zurschmitten, María P. Castro, Viviana Parreño, Marina V. Mozgovoj and Juan A. Stupka
Vaccines 2025, 13(11), 1080; https://doi.org/10.3390/vaccines13111080 (registering DOI) - 22 Oct 2025
Abstract
Norovirus (NoV) is a leading cause of acute gastroenteritis (AGE) in young children worldwide. Following the introduction of universal rotavirus (RVA) vaccination in Argentina in 2015, the role of NoV in pediatric AGE warrants evaluation. This study aimed to assess the prevalence, clinical
[...] Read more.
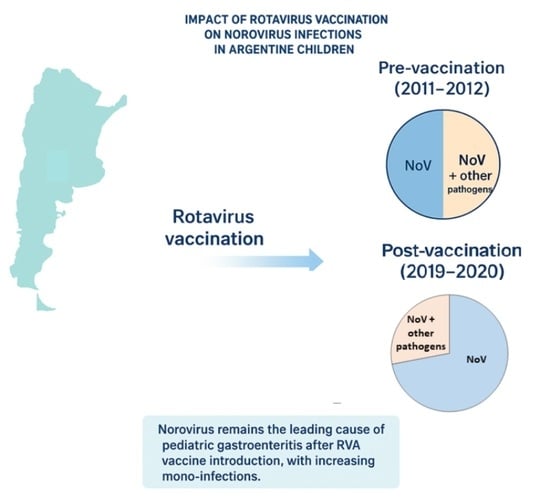
Norovirus (NoV) is a leading cause of acute gastroenteritis (AGE) in young children worldwide. Following the introduction of universal rotavirus (RVA) vaccination in Argentina in 2015, the role of NoV in pediatric AGE warrants evaluation. This study aimed to assess the prevalence, clinical characteristics, and molecular diversity of NoV in children under five years of age, comparing the periods before and after RVA vaccine implementation. Methods: A descriptive observational study was conducted in two pediatric hospitals in Argentina. Stool samples were obtained from both outpatient and hospitalized children presenting with acute gastroenteritis (AGE) during two distinct one-year periods: 285 samples from the pre-vaccination period (2011–2012) and 212 samples from the post-vaccination period (2019–2020). NoV, RVA and other viral enteropathogens were detected by RT-qPCR or immunoassay. Positive NoV samples were genotyped by Sanger sequencing of the ORF1/ORF2 junction. Results: NoV was detected in 30.1% (86/285) and 23.5% (50/212) of cases in the pre- and post-vaccination periods, respectively. Children under two years of age and inpatients had significantly higher NoV detection in both periods. NoV mono-infections were more frequent in post-vaccination period (72% vs. 50%). NoV GII predominated in both periods, with increased genotype diversity observed post-vaccination, including GII.3[P12], GII.4 Sydney[P16], GII.6[P7], and GII.2[P16]. Conclusions: NoV remains a major cause of pediatric AGE in Argentina, particularly in children under two years old. Although NoV prevalence did not increase after RVA vaccine introduction, its clinical relevance persists. Continued molecular surveillance is essential to monitor genotype dynamics and implement prevention strategies.
Full article
(This article belongs to the Special Issue Epidemiology, Immunology, and Therapeutic Vaccines of Enteric Pathogens)
►▼
Show Figures
Graphical abstract
Open AccessArticle
Invasive Meningococcal Disease in the Post–COVID-19 Era in South America
by
Marco A. P. Sáfadi, Juan Francisco Falconi, Maria Gabriela Abalos, Lidia Serra, Angela Gentile, Alejandro Diaz, Claudia P. Cortes and Rodolfo Villena
Vaccines 2025, 13(11), 1079; https://doi.org/10.3390/vaccines13111079 - 22 Oct 2025
Abstract
Background: During the COVID-19 pandemic, reductions in cases of bacterial diseases transmitted via the respiratory route were reported by the Invasive Respiratory Infection Surveillance Consortium. Here, we evaluate the epidemiology of invasive meningococcal disease (IMD) in Argentina, Brazil, Chile, and Colombia during and
[...] Read more.
Background: During the COVID-19 pandemic, reductions in cases of bacterial diseases transmitted via the respiratory route were reported by the Invasive Respiratory Infection Surveillance Consortium. Here, we evaluate the epidemiology of invasive meningococcal disease (IMD) in Argentina, Brazil, Chile, and Colombia during and after the COVID-19 pandemic. Methods: The epidemiology of meningococcal disease was reviewed in selected South American countries through 2023 from publicly available national surveillance system databases. Results: The incidence of IMD decreased substantially in 2020 in Argentina, Brazil, Chile, and Colombia and was followed by a trend of increased disease. Similarly to observations in several European countries, the post-pandemic rebounds in cases of IMD in the four South American countries included in this analysis were mainly caused by serogroup B, that became one of the predominant serogroups causing IMD in all four countries. Conclusions: Enhanced surveillance of IMD, including genomic characterization of strains, is needed to inform public health policymakers and guide future vaccination strategies in the region.
Full article
(This article belongs to the Section Epidemiology and Vaccination)
►▼
Show Figures
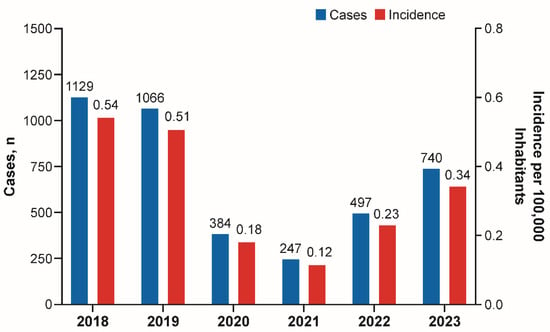
Figure 1
Open AccessPerspective
Balancing Innovation and Equity: A Successful Dynamic Between Private and Public Sectors Is Essential to Ensure True Pandemic Influenza Preparedness
by
Lyn Morgan Marsden and Marie Mazur
Vaccines 2025, 13(11), 1078; https://doi.org/10.3390/vaccines13111078 - 22 Oct 2025
Abstract
The COVID-19 pandemic demonstrated both the transformative capacity of vaccine innovation and the persistent inequities that accompany emergency access, underscoring the critical need for stronger collaboration between global health governance and the vaccine industry. Influenza pandemics remain inevitable threats. The continued emergence of
[...] Read more.
The COVID-19 pandemic demonstrated both the transformative capacity of vaccine innovation and the persistent inequities that accompany emergency access, underscoring the critical need for stronger collaboration between global health governance and the vaccine industry. Influenza pandemics remain inevitable threats. The continued emergence of avian influenza strains such as H5N1 reinforces the necessity of robust preparedness. This perspective examines the underutilization of private sector vaccine manufacturers in current pandemic influenza frameworks and identifies three central areas where industry participation is indispensable: predictable vaccine demand through robust seasonal influenza programs, economic incentives that de-risk investments in research and development, and diversification of vaccine platforms to expand response capacity. In addition, regionalizing manufacturing, advancing collaborative regulatory models, and negotiating export waivers are presented as potential mechanisms to strengthen equity and supply security. The review highlights demand-based tiered pricing and Advance Purchase Agreements as practical tools to align commercial incentives with public health priorities. Furthermore, it makes the case for embedding private sector representation and knowledge into top-level decision-making and preparedness planning, ensuring investment in innovation is aligned with global health objectives. Ultimately, true pandemic influenza readiness depends on building a sustained seasonal influenza market, embedding private sector engagement into governance structures, and fostering mutual trust to ensure timely access and equitable protection for populations worldwide.
Full article
(This article belongs to the Special Issue Pandemic Influenza Vaccination)
►▼
Show Figures
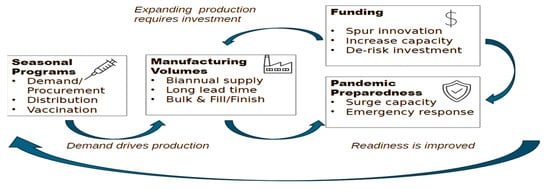
Figure 1
Open AccessReview
Construction and Research Progress of Animal Models and Mouse Adapted Strains of Seasonal Influenza Virus
by
Haijun Zhu, Siyu Pu, Peiqing He, Junhao Luo and Rongbao Gao
Vaccines 2025, 13(10), 1077; https://doi.org/10.3390/vaccines13101077 - 21 Oct 2025
Abstract
Influenza viruses, featured by high variability, pose a persistent public health threat because of an annual seasonal epidemic in the world and irregular global pandemic, requiring animal models to elucidate their pathogenic mechanisms and advance preventive strategies. Mice have been selected as the
[...] Read more.
Influenza viruses, featured by high variability, pose a persistent public health threat because of an annual seasonal epidemic in the world and irregular global pandemic, requiring animal models to elucidate their pathogenic mechanisms and advance preventive strategies. Mice have been selected as the primary animal model, although several experimental animals have been used in studies of the influenza virus. However, the limited susceptibility of wild-type influenza viruses to mice poses significant challenges for studying pathogenesis and intervention strategies. Here, to help understand the construction of mouse-adapted influenza viruses, we reviewed the recent research progress in constructing mouse-adapted influenza virus strains to overcome species-specific barriers.
Full article
(This article belongs to the Special Issue Immunity to Influenza Viruses and Vaccines)
►▼
Show Figures
Figure 1
Open AccessArticle
Efficacy of a Novel PCV2d and Mycoplasma hyopneumoniae Combined Vaccine in Piglets with High and Low Levels of PCV2 Maternally Derived Antibodies at Vaccination
by
Mònica Sagrera, Laura Garza-Moreno, Àlex Cobos, Anna Maria Llorens, Eva Huerta, Mónica Pérez, Diego Pérez, David Espigares, Joaquim Segalés and Marina Sibila
Vaccines 2025, 13(10), 1076; https://doi.org/10.3390/vaccines13101076 (registering DOI) - 21 Oct 2025
Abstract
Background/Objectives: Maternally derived antibody (MDA) levels of porcine circovirus 2 (PCV2) may eventually interfere with humoral response and vaccination efficacy. This study aimed to evaluate the efficacy of a ready-to-use PCV2d and Mycoplasma hyopneumoniae combined vaccine in piglets with different PCV2 MDA levels
[...] Read more.
Background/Objectives: Maternally derived antibody (MDA) levels of porcine circovirus 2 (PCV2) may eventually interfere with humoral response and vaccination efficacy. This study aimed to evaluate the efficacy of a ready-to-use PCV2d and Mycoplasma hyopneumoniae combined vaccine in piglets with different PCV2 MDA levels at vaccination in an experimental inoculation with a heterologous viral genotype. Methods: Forty-eight piglets were allocated into vaccinated (V) and non-vaccinated (NV) groups with high (H) and low (L) PCV2 MDA subgroups (H-V, H-NV, L-V, L-NV). At 3 weeks of age, the piglets received either one dose of vaccine or placebo. Five weeks later, all animals were intranasally challenged with a PCV2b inoculum. Body weight was registered at different time points. Blood samples, peripheral blood mononuclear cells and tracheobronchial lymph nodes (TBLN) were collected and used to assess viraemia, viral load, humoral and cellular responses and histological lesions. Results: The V group showed higher PCV2 antibody levels from challenge onwards, along with a lower percentage of viraemic pigs and reduced viral load in serum at 2 and 3 weeks post-challenge (wpc) and in TBLN tissues compared to the NV group. The H-V group had the highest antibody levels post-challenge, showed no detectable viraemia and had a lower overall amount of virus in tissues. The NV group (especially H-NV) exhibited increased levels of IFN-γ, IFN-α and TNF-α post-challenge. Conclusions: The tested vaccine elicited humoral and cellular immune responses and reduced viral presence in serum and tissues, demonstrating efficacy in a PCV2 subclinical infection model despite high MDA levels at the time of vaccination. Understanding both humoral and cellular immune responses according to different MDA levels can help design more effective vaccination strategies against PCV2.
Full article
(This article belongs to the Section Veterinary Vaccines)
►▼
Show Figures
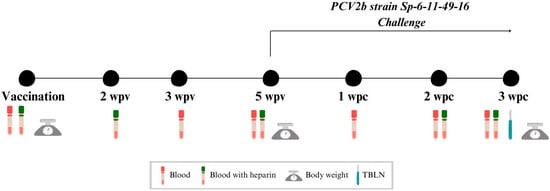
Figure 1
Open AccessTechnical Note
Improving the Suitability of Vaccine Design for Immunisation Programmes and Enhancing Vaccine Policy Quality Through User Research
by
Stefano Malvolti, Melissa Malhame, Adam Soble, Carsten Mantel, Melissa Ko, Lorena Perrin, Tiziana Scarna, Marion Menozzi-Arnaud and Jean-Pierre Amorij
Vaccines 2025, 13(10), 1075; https://doi.org/10.3390/vaccines13101075 - 21 Oct 2025
Abstract
Background: The achievement of the goals of the Immunization Agenda 2030 (IA2030) requires vaccines to be developed and implemented that meet the needs and requirements of the final users: vaccinators and vaccinees. A detailed and shared understanding of these needs should inform policy
[...] Read more.
Background: The achievement of the goals of the Immunization Agenda 2030 (IA2030) requires vaccines to be developed and implemented that meet the needs and requirements of the final users: vaccinators and vaccinees. A detailed and shared understanding of these needs should inform policy and programme guidance directing stakeholders’ efforts and investments. Currently, relevant guidance documents only partially capture vaccine users’ perspectives. Method: To help overcome this gap, we propose an operational research method grounded in the principles of the design approach that systematically maps and integrates user perspectives in vaccine development, policy, and implementation decisions. Results: The method, named the seven Ws, guides researchers through a three-step process. First, it clarifies the contribution of a vaccine to solving a public health problem—the solution–problem fit. Second, it maps potential implementation strategies for the vaccine in different settings. Lastly, it describes the relevant vaccine’s use cases across the implementation strategies, elucidating the user requirements for the vaccine to be successfully implemented—the solution–provider and solution–user fits. Conclusions: By explicitly pursuing these three fits, policymakers, vaccine developers, and programme managers will be able to better contribute towards the achievement of the IA2030 goals. This framework is intended as a conceptual contribution rather than an empirical validation study.
Full article
(This article belongs to the Special Issue Promoting Research, Development and Access to Vaccines to Address Global Inequities)
►▼
Show Figures
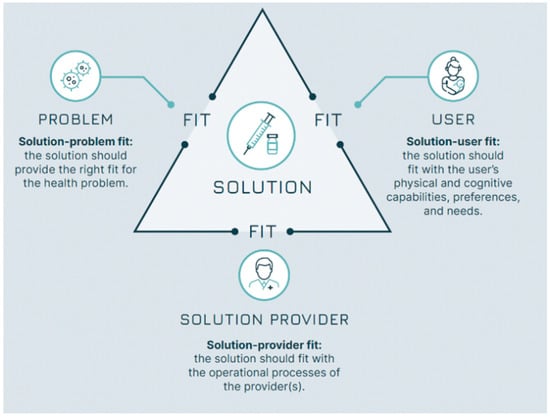
Figure 1
Open AccessArticle
Impact of SARS-CoV-2 Vaccination on Disease Activity and Severity of COVID-19 Infection in Patients with Systemic Lupus Erythematosus: A Multicenter Cohort Study
by
Natália Sarzi Sartori, Ketty Lysie Libardi Lira Machado, Samira Tatiyama Miyamoto, Flavia Zon Pretti, Maria da Penha Gomes Gouveia, Yasmin Gurtler Pinheiro de Oliveira, Vanezia Gonçalves da Silva, Filipe Faé, Ana Paula Neves Burian, Karina Rosemarie Lallemand Tapia, Anna Carolina Simões Moulin, Luiza Lorenzoni Grillo, Paula dos Santos Athayde, Helena da Silva Corona, Sabrina de Souza Ramos, Flávia Maria Matos Melo Campos Peixoto, Priscila Dias Cardoso Ribeiro, Vanessa de Oliveira Magalhães, Mariana Freitas de Aguiar, Erika Biegelmeyer, Cristiane Kayser, Alexandre Wagner Silva De Souza, Charlles Heldan de Moura Castro, Juliana Bühring, Sandra Lúcia Euzébio Ribeiro, Sérgio Henrique Oliveira dos Santos, Clara Pinheiro Martins, Jonathan Willian da Silva Rodrigues, Marcos Mavignier Sousa Dias, Bruna Guimarães Dutra, Camila Maria Paiva França Telles, Samuel Elias Basualto Dias, Rodrigo Poubel Vieira de Rezende, Katia Lino Baptista, Rodrigo Cutrim Gaudio, Ana Karla Guedes de Melo, Valéria Bezerra da Silva, Vitor Alves Cruz, Jozelia Rêgo, Rejane Maria Rodrigues de Abreu Vieira, Adah Sophia Rodrigues Vieira, Adriana Maria Kakehasi, Anna Carolina Faria Moreira Gomes Tavares, Artur José Azevedo Pereira, Pollyana Vitoria Thomaz da Costa, Valderilio Feijó Azevedo, Nicole Pamplona Bueno de Andrade, Guilherme Levi Tres, Olindo Assis Martins-Filho, Vanessa Peruhype-Magalhães, Valéria Valim, Gilda Aparecida Ferreira, Andréa Teixeira-Carvalho, Edgard Torres dos Reis-Neto, Emilia Inoue Sato, Marcelo de Medeiros Pinheiro, Viviane Angelina de Souza, Ricardo Machado Xavier, Gecilmara Salviato Pileggi and Odirlei André Monticieloadd
Show full author list
remove
Hide full author list
Vaccines 2025, 13(10), 1074; https://doi.org/10.3390/vaccines13101074 - 21 Oct 2025
Abstract
Background: To prospectively evaluate the safety and clinical impact of SARS-CoV-2 vaccines in patients with systemic lupus erythematosus (SLE). Methods: Subanalysis of the Brazilian multicenter observational study “Safety, Effectiveness and Duration of Immunity after Vaccination against SARS-CoV-2 in Patients with Immune-Mediated Inflammatory Diseases
[...] Read more.
Background: To prospectively evaluate the safety and clinical impact of SARS-CoV-2 vaccines in patients with systemic lupus erythematosus (SLE). Methods: Subanalysis of the Brazilian multicenter observational study “Safety, Effectiveness and Duration of Immunity after Vaccination against SARS-CoV-2 in Patients with Immune-Mediated Inflammatory Diseases (SAFER)”, which included SLE patients vaccinated with CoronaVac, ChAdOx1, or BNT162b2. Patients with HIV infection, pregnant women, or those with immunosuppression not related to SLE were excluded. Safety data related to adverse events and underlying disease activity were assessed. Additionally, COVID-19 cases were monitored throughout the follow-up period. Results: The study included 373 patients with systemic lupus erythematosus (SLE), with a mean age of 36 years, the majority being women (89.8%). The most common adverse events after SARS-CoV-2 vaccination were injection site reactions and headache, observed both after the first and subsequent doses. The ChAdOx-1 vaccine was associated with a higher frequency of adverse events compared to CoronaVac. At baseline, 38.3% of patients were in remission, 32.8% had low disease activity, and 28.9% had moderate to high activity. Following CoronaVac vaccination, there was an increase in remission rates (from 34.6% to 51.1%) and a significant reduction in moderate to high activity (from 37.6% to 15.0%) after the first dose, with this reduction partially maintained after the second dose. In contrast, patients vaccinated with ChAdOx-1 showed an increase in moderate to high activity (from 14.5% to 38.2% after the first dose), a trend that persisted after the second dose. No statistically significant changes in disease activity were observed among those who received BNT162b2. During follow-up, 44 cases of COVID-19 were reported, all mild, with no deaths or need for intensive care unit admission. Conclusions: Vaccination against SARS-CoV-2 demonstrated a favorable safety profile in patients with SLE, with a low frequency of serious adverse events. While analysis of disease activity revealed variations across vaccine platforms, most notably an increased proportion of moderate to high disease activity among those receiving ChAdOx-1 compared with CoronaVac and BNT162b2, the overall occurrence of COVID-19 during follow-up was limited to mild cases, with no severe outcomes. These findings highlight that, despite potential risks of disease exacerbation, the clear protection against severe COVID-19 supports vaccination as a beneficial strategy for this immunocompromised population.
Full article
(This article belongs to the Section COVID-19 Vaccines and Vaccination)
►▼
Show Figures
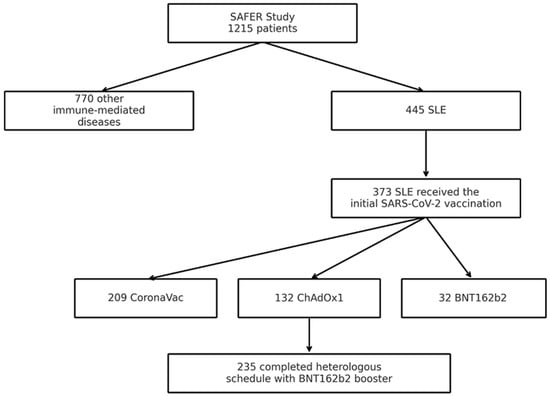
Figure 1
Open AccessReview
Vaccination Against Herpes Zoster in Adults: Current Strategies in European Union Countries
by
Manuela Chiavarini, Angela Bechini, Sara Boccalini, Alisa Barash, Enrica Castellana, Alessandro Senape and Paolo Bonanni
Vaccines 2025, 13(10), 1073; https://doi.org/10.3390/vaccines13101073 - 20 Oct 2025
Abstract
Background/Objectives: Herpes zoster (HZ), caused by varicella zoster virus (VZV) reactivation, significantly affects the functional status and quality of life of older adults and immunocompromised individuals. Vaccination represents an effective strategy to reduce the incidence of HZ. Methods: This review offers a cross-sectional
[...] Read more.
Background/Objectives: Herpes zoster (HZ), caused by varicella zoster virus (VZV) reactivation, significantly affects the functional status and quality of life of older adults and immunocompromised individuals. Vaccination represents an effective strategy to reduce the incidence of HZ. Methods: This review offers a cross-sectional assessment of the current landscape of adult herpes zoster vaccination strategies across the 27 EU member states, drawing on data available up to July 2025 from official sources such as the ECDC, the WHO, and national health authorities. Results: HZ vaccination is recommended in 17 EU countries (63%) according to the National Immunization Programs (NIPs) or by other institutional national health documents; in only 7 countries, vaccination is fully covered by the national healthcare system. HZ vaccination is recommended for healthy adults aged ≥50 years in 23.5% of countries (4/17), ≥60 years in 29.4% (5/17), and ≥65 years in 41.2% (7/17). At-risk groups are targeted in 94.1% of countries (16/17), predominantly from age 18 years (14 countries). Conclusions: An overall tendency toward broader HZ vaccination strategies, targeting both older adults and risk groups, is emerging. However, differences among national policies, together with the European Commission’s withdrawal of the live-attenuated Zostavax vaccine effective 1 June 2025, highlight the urgent need for comprehensive, harmonized immunization strategies to ensure adequate coverage of adult HZ vaccination across Europe.
Full article
(This article belongs to the Special Issue From Knowledge to Action: Advances in Herpes Zoster Vaccine and Vaccination)
Open AccessArticle
Efficacy Evaluation of an E2 Subunit Vaccine Against Highly Virulent Classical Swine Fever Virus Strain
by
Yu-Chieh Chen, Chi-Chih Chen, Wen-Bin Chung, Yen-Li Huang, Guan-Ming Ke and Hso-Chi Chaung
Vaccines 2025, 13(10), 1072; https://doi.org/10.3390/vaccines13101072 - 20 Oct 2025
Abstract
Background/Objectives: Classical swine fever (CSF) is listed by the World Organisation for Animal Health as a highly devastating and contagious pig disease, causing severe economic losses to the swine industry. In spite of the successful elimination of CSF in Taiwan, preparedness against
[...] Read more.
Background/Objectives: Classical swine fever (CSF) is listed by the World Organisation for Animal Health as a highly devastating and contagious pig disease, causing severe economic losses to the swine industry. In spite of the successful elimination of CSF in Taiwan, preparedness against potential reintroduction remains essential. The live attenuated vaccines have been effective in disease control, but are not capable of a viable strategy that differentiates infected from vaccinated animals (DIVA). Subunit vaccines are recognized for their safety and ability to induce protective immunity against infectious diseases. Methods: In this study, the recombinant CSF virus (CSFV) E2 proteins were formulated with a CpG motif as an adjuvant to produce the E2-CpG subunit vaccine. Its efficiency in specific-pathogen-free (SPF) pigs was compared with a commercially available E2 subunit vaccine (Bayovac® CSF-E2; Bayer Taiwan Co., Ltd., Taipei City, Taiwan). Results: Significantly higher titers of anti-E2 antibodies were induced in pigs immunized with a single dose of the E2-CpG vaccine, particularly the reduced E-0.5A formulation, than those immunized with a dose of the commercialized E2 subunit vaccine adjusted to double dosage. This designed subunit vaccine showed high efficacy in protection against clinical symptoms and significant pathological alterations in pigs after a highly virulent CSFV (genotype 1.1) challenge. Viral shedding was not detected in vaccinated pigs before completion of the challenge study, and the viral load in their spleens remained undetectable. Conclusions: These results could support the potential of the E2-CpG vaccine as a cost-effective, single-dose subunit vaccine capable of inducing robust CSFV-specific immunity and providing 100% protection against lethal CSFV challenges.
Full article
(This article belongs to the Special Issue Swine Vaccines and Vaccination)
►▼
Show Figures
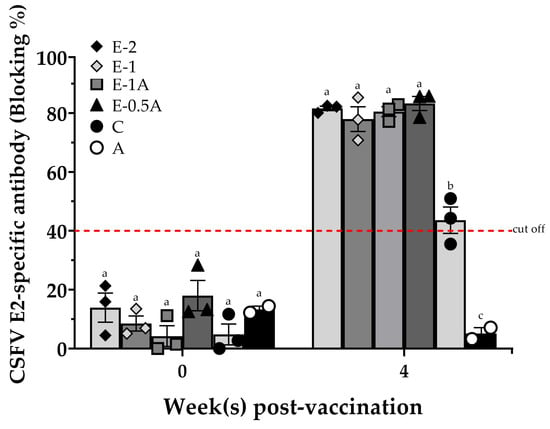
Figure 1
Open AccessReview
Global Transmission, Prevention, Control, and Treatment of Mpox Virus in 2025: A Comprehensive Review from Infection Mechanisms to Vaccine Development
by
Quan Quan, Nan Wu, Ying-Hua Luo, Yan-Jun Tang, Yan-Zhi Liu, Xi-Chun Huang, Jun-Hao Li, Wan-Xia Ren and Cheng-Hao Jin
Vaccines 2025, 13(10), 1071; https://doi.org/10.3390/vaccines13101071 - 20 Oct 2025
Abstract
The World Health Organization (WHO) declared the mpox (MPX) outbreak a public health emergency of international concern (PHEIC) on 23 July 2022, and 14 August 2024, respectively, underscoring the confirmed and concerning global spread of the disease. A gap exists in our fundamental
[...] Read more.
The World Health Organization (WHO) declared the mpox (MPX) outbreak a public health emergency of international concern (PHEIC) on 23 July 2022, and 14 August 2024, respectively, underscoring the confirmed and concerning global spread of the disease. A gap exists in our fundamental understanding of the mpox virus (MPXV), despite its genetic relatedness to the variola virus (VARV). This knowledge deficit is evident in the performance of current medical countermeasures; vaccines and antiviral therapies adapted from smallpox programs demonstrate only partial efficacy and are constrained by issues of safety and suboptimal effectiveness against MPXV. In this context, the development of MPX-specific vaccines and antiviral drugs has become a critical priority in the global effort to combat MPX. However, MPXV employs multiple strategies to evade host immune responses, such as producing specific and poxvirus homologous proteins that suppress both innate immunity (including the six principal innate immune signaling pathways and antiviral strategies, notably the interferon [IFN] pathway) and adaptive immunity, thereby complicating vaccine and drug development. Insights from research on vaccinia virus (VACV) and VARV may inform the investigation of MPXV pathogenesis and immune evasion mechanisms. Drawing on relevant scientific literature, this review systematically examines key aspects of MPX infection, pathogenicity, and immune evasion, as well as the coordination between innate and adaptive immune responses. Furthermore, this review elucidates the current application and deployment landscape of the three principal therapeutics and three major vaccines for MPX, aiming to provide a theoretical foundation for future research and development of vaccines and targeted antiviral agents.
Full article
(This article belongs to the Section Vaccines and Public Health)
►▼
Show Figures
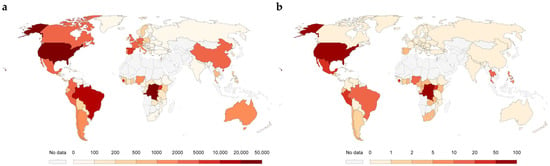
Figure 1
Open AccessSystematic Review
Efficacy, Safety and Predictive Biomarkers of Oncolytic Virus Therapy in Solid Tumors: A Systematic Review and Meta-Analysis
by
Mohamed El-Tanani, Syed Arman Rabbani, Mohamed Anas Patni, Rasha Babiker, Shakta Mani Satyam, Imran Rashid Rangraze, Adil Farooq Wali, Yahia El-Tanani and Thantrira Porntaveetus
Vaccines 2025, 13(10), 1070; https://doi.org/10.3390/vaccines13101070 - 20 Oct 2025
Abstract
Background: Oncolytic virus (OV) therapy couples direct tumor lysis with systemic immune priming, yet clinical benefit remains heterogeneous and the predictive biomarker landscape is poorly defined. We undertook a systematic review and meta-analysis to quantify the efficacy and safety of OV therapy in
[...] Read more.
Background: Oncolytic virus (OV) therapy couples direct tumor lysis with systemic immune priming, yet clinical benefit remains heterogeneous and the predictive biomarker landscape is poorly defined. We undertook a systematic review and meta-analysis to quantify the efficacy and safety of OV therapy in solid tumors and to synthesize current evidence on response-modulating biomarkers. Methods: Following PRISMA 2020 guidelines, MEDLINE, Embase, Cochrane CENTRAL, ProQuest and Scopus were searched from inception to May 2025. Phase II–III randomized trials of genetically engineered or naturally occurring OV reporting objective response rate (ORR), progression-free survival (PFS), overall survival (OS) or biomarker data were eligible. Hazard ratios (HRs) or odds ratios (OR) were pooled with random-effects models; heterogeneity was assessed with I2 statistics. Qualitative synthesis integrated genomic, immunologic and microbiome biomarkers. Results: Thirty-six trials encompassing around 4190 patients across different tumor types met inclusion criteria. Compared with standard therapy, OV-based regimens significantly improved ORR nearly three-fold (pooled OR = 2.77, 95% CI 1.85–4.16), prolonged PFS by 11% (HR = 0.89, 95% CI 0.80–0.99) and reduced mortality by 16% (OS HR = 0.84, 95% CI 0.72–0.97; I2 = 59%). Benefits were most pronounced in melanoma (ORR 26–49%; OS HR 0.57–0.79) and in high-dose vaccinia virus for hepatocellular carcinoma (HR = 0.39). Grade ≥ 3 adverse events were not increased versus control (risk ratio 1.05, 95% CI 0.89–1.24); common toxicities were transient flu-like symptoms and injection-site reactions. Biomarker synthesis revealed that high tumor mutational burden, interferon-pathway loss-of-function mutations, baseline CD8+ T-cell infiltration, post-OV upregulation of IFN-γ/PD-L1, and favorable gut microbial signatures correlated with response, whereas intact antiviral signaling, immune-excluded microenvironments and myeloid dominance predicted resistance. Conclusions: OV therapy confers clinically meaningful improvements in tumor response, PFS and OS with a favorable safety profile. Integrating composite genomic–immune–microbiome biomarkers into trial design is critical to refine patient selection and realize precision viro-immunotherapy. Future research should prioritize biomarker-enriched, rational combination strategies to overcome resistance and extend benefit beyond melanoma.
Full article
(This article belongs to the Special Issue Cancer Vaccines and Immunotherapy: Latest Advances, Limitations and Prospects)
►▼
Show Figures

Figure 1
Open AccessArticle
Demographic and Clinical Characteristics of Patients Vaccinated Against Herpes Zoster in a Primary Care Setting in Spain: A Retrospective Study
by
José María Blanc-Rodriguez-Arias, Isabel Jimeno-Sanz, Valentín Hernández-Barrera, Alejandro Álvaro-Meca, Manuel Torres-Ramos and Ángel Gil-de-Miguel
Vaccines 2025, 13(10), 1069; https://doi.org/10.3390/vaccines13101069 - 20 Oct 2025
Abstract
Background: Herpes zoster (HZ), commonly known as shingles, is a reactivation of the varicella-zoster virus that predominantly affects older adults and immunocompromised individuals. Vaccination with the recombinant zoster vaccine (RZV) has proven effective in preventing HZ and its complications in real-world scenarios, yet
[...] Read more.
Background: Herpes zoster (HZ), commonly known as shingles, is a reactivation of the varicella-zoster virus that predominantly affects older adults and immunocompromised individuals. Vaccination with the recombinant zoster vaccine (RZV) has proven effective in preventing HZ and its complications in real-world scenarios, yet coverage remains suboptimal in primary care settings. This study aims to analyse the demographic and clinical characteristics of HZ-vaccinated patients in a primary care centre in Madrid, Spain, in 2023 and to assess adherence to the vaccination schedule. Methods: A retrospective descriptive analysis was conducted, including 1146 patients from a single primary care centre who received at least one dose of RZV during the study period. Data were extracted from medical records. Patient characteristics, including age, sex, and the presence of comorbidities (e.g., diabetes, chronic obstructive pulmonary disease, hypertension, asthma), were examined. Vaccine coverage was calculated for the new age cohorts introduced in the year 2023. Comorbidity prevalence was also analysed by age and sex to determine patterns in vaccination uptake. Results: The analysis revealed that 66% of vaccinated individuals presented one or more of the analysed comorbidities. Adherence to the two-dose schedule was 91.97%, and vaccine coverages were 46.56% in those born between 1943–1948 and 39.32% in the 1958 cohort. The findings underscore the need for targeted strategies to raise disease awareness and improve HZ vaccination rates and adherence, particularly among elderly individuals and those with comorbid conditions. Conclusions: This study specifies the clinical characteristics of HZ-vaccinated patients in a primary care setting and highlights the importance of enhanced vaccination awareness and adherence strategies to reduce the burden of HZ among at-risk populations.
Full article
(This article belongs to the Section Vaccines and Public Health)
►▼
Show Figures
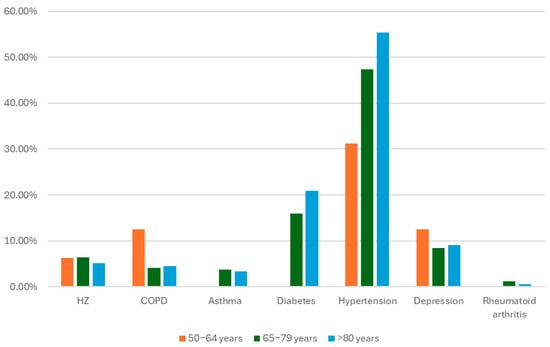
Figure 1
Open AccessEditorial
Vaccine Development for Influenza Virus
by
Ganesh Yadagiri and Mingtao Zeng
Vaccines 2025, 13(10), 1068; https://doi.org/10.3390/vaccines13101068 - 19 Oct 2025
Abstract
Influenza is a contagious respiratory viral infection that remains a persistent global public-health threat responsible for three to five million severe illnesses and 290,000 to 650,000 respiratory deaths annually [...]
Full article
(This article belongs to the Special Issue Vaccine Development for Influenza Virus)
Open AccessArticle
Seroprevalence of Poliovirus Types 1, 2, and 3 Among Children Aged 6–11 Months: Variations Across Survey Rounds in High-Risk Areas of Pakistan
by
Imtiaz Hussain, Ahmad Khan, Muhammad Umer, Muhammad Sajid, Haider Abbas, Muhammad Masroor Alam, Altaf Bosan, Jeffrey Partridge, Rehan Hafiz, Anwar-ul Haq and Sajid Soofi
Vaccines 2025, 13(10), 1067; https://doi.org/10.3390/vaccines13101067 - 19 Oct 2025
Abstract
Background: The current polio epidemiology in Pakistan poses a unique challenge for global eradication, with polio transmission dynamics influenced by regional variations in immunity and disparities in immunization coverage. This study assesses the immunity level for all three poliovirus types among children
[...] Read more.
Background: The current polio epidemiology in Pakistan poses a unique challenge for global eradication, with polio transmission dynamics influenced by regional variations in immunity and disparities in immunization coverage. This study assesses the immunity level for all three poliovirus types among children aged 6–11 months in polio high-risk regions of Pakistan. Methods: Four consecutive rounds of cross-sectional serological surveys were conducted in polio high-risk areas of Pakistan between November 2016 and October 2023. Twelve high-risk areas were covered in the first three rounds of the survey, while 44 high-risk areas were covered in the fourth round. 25 clusters from each geographical stratum were selected utilizing probability proportional to size. Results: Across the four rounds of the survey, 32,907 children aged 6–11 months from 2084 clusters and 32,371 households were covered. Comparative analysis across the survey rounds showed that seroprevalence of poliovirus type 1 was high in provinces (>95%), albeit consistently lower in Balochistan (going down to 89.7% in Round 4). Type 2 seroprevalence was significantly lower and more heterogeneous, from 34.6% in Sindh to 83.4% in Punjab, with sharp declines by round 4, particularly in Balochistan (40.4%). Type 3 seroprevalence was overall high (>94% in Punjab, Sindh, and KPK) but dropped in the last round, while Balochistan exhibited continually lower immunity (81.1%). Conclusions: The findings reflect the variations in population immunity to poliovirus in the country, with notable fluctuations over the years. The gaps in type 2 immunity over time and consistently lowest in Balochistan highlight the need for continued monitoring of immunity levels and adaptable vaccination strategies.
Full article
(This article belongs to the Section Epidemiology and Vaccination)
►▼
Show Figures

Figure 1
Open AccessArticle
Epidemiological Survey of Porcine Circovirus Type 2 (PCV2) in Large-Scale Pig Farms in Hubei Province and Comprehensive Evaluation of Commercial Vaccine Efficacy
by
Wenjun Liao, Zhaofang Xi, Rui Fang, Bang Shen and Junlong Zhao
Vaccines 2025, 13(10), 1066; https://doi.org/10.3390/vaccines13101066 - 18 Oct 2025
Abstract
Background: Porcine circovirus type 2 (PCV2) is the primary pathogen responsible for postweaning multisystemic wasting syndrome (PMWS) and related diseases, leading to significant economic losses in the global pig industry. Methods: This study conducted a thorough epidemiological survey between 2022 and 2024, gathering
[...] Read more.
Background: Porcine circovirus type 2 (PCV2) is the primary pathogen responsible for postweaning multisystemic wasting syndrome (PMWS) and related diseases, leading to significant economic losses in the global pig industry. Methods: This study conducted a thorough epidemiological survey between 2022 and 2024, gathering 6600 samples from 24 large-scale pig farms in Hubei Province. On the basis of these findings, the immune response and economic benefits of two representative commercial PCV2 subunit vaccines, recombinant baculovirus CP08 and Ingelvac CircoFLEX®, were assessed in a modern fattening farm in Xiangyang city. Results: The results indicated no detection of viral antigens in sows; however, weaned piglets and fattening pigs presented high positivity rates, with 8-week-old nursery pigs identified as the peak period for infection. Both vaccines significantly improved average weight gain and reduced antigen positivity, with Ingelvac CircoFLEX® demonstrating superior viral control and economic returns. Conclusions: This study offers valuable scientific and practical guidance for PCV2 control strategies and vaccine selection in Hubei and comparable regions.
Full article
(This article belongs to the Section Veterinary Vaccines)
►▼
Show Figures

Figure 1
Open AccessArticle
The Potential Public Health Impact of the mRNA-Based Respiratory Syncytial Virus Vaccine, mRNA-1345, Under Extended Vaccination Campaigns Among Older Adults in the United Kingdom: A Modelling Study
by
Mariia Dronova, Anna Tytuła, Zuzanna Janusz, Parinaz Ghaswalla, Stuart Carroll, Orsolya Balogh and Keya Joshi
Vaccines 2025, 13(10), 1065; https://doi.org/10.3390/vaccines13101065 - 18 Oct 2025
Abstract
Background/Objectives: Respiratory syncytial virus (RSV) is a leading cause of severe respiratory disease in older adults. Despite growing recognition of RSV as a public health concern, vaccination options remain limited. This study assessed the potential long-term public health impact of extended mRNA-1345
[...] Read more.
Background/Objectives: Respiratory syncytial virus (RSV) is a leading cause of severe respiratory disease in older adults. Despite growing recognition of RSV as a public health concern, vaccination options remain limited. This study assessed the potential long-term public health impact of extended mRNA-1345 RSV vaccination campaigns. Methods: A dynamic transmission model, stratified by age, was developed to evaluate the epidemiological and clinical impact of RSV vaccination in the UK over a 20-year time horizon. Eight vaccination strategies were assessed: two reflecting the JCVI recommendation for the 2024–2025 season and its recent extension, and six extended strategies considering broader eligible age groups, higher coverage, and/or revaccination every 2 or 3 years. Two exploratory analyses and extensive model validation versus reported data were also conducted. Results: Strategies combining broader age eligibility (≥60 years), higher coverage (80%), and 2-year revaccination achieved the greatest impact, preventing 310,000 hospitalisations over 20 years in the total UK population. Exploratory analyses showed that the expected public health impact might exceed the estimates presented in this analysis, if an alternative vaccine efficacy profile or the projected demographic shift would be confirmed. Conclusions: Extended RSV vaccination strategies including broader age eligibility and routine revaccination could offer substantial public health benefits in the UK. Targeting adults aged ≥60 years is expected to be particularly efficient in achieving a sustainable reduction in RSV burden. These findings could provide valuable support for national policy discussions on optimising RSV vaccination strategies in older adults, particularly regarding target age groups, revaccination schedules, and long-term programme planning.
Full article
(This article belongs to the Special Issue Respiratory Syncytial Virus (RSV) Vaccine)
►▼
Show Figures

Figure 1
Open AccessSystematic Review
Health System Determinants of Delivery and Uptake of HPV Vaccination Services Among Involuntary Migrant Populations: A Qualitative Systematic Review
by
Jennifer Nyawira Githaiga, Jill Olivier, Susanne Noll and Edina Amponsah-Dacosta
Vaccines 2025, 13(10), 1064; https://doi.org/10.3390/vaccines13101064 - 18 Oct 2025
Abstract
Background: Migrant populations are commonly under-immunised relative to general populations in host countries. The evidence base on routine vaccination among migrant children suggests that higher priority is given to infants and younger children compared to adolescents. Though migrants are often classified as a
[...] Read more.
Background: Migrant populations are commonly under-immunised relative to general populations in host countries. The evidence base on routine vaccination among migrant children suggests that higher priority is given to infants and younger children compared to adolescents. Though migrants are often classified as a homogenous group, different sub-populations of migrants exist, including voluntary migrants who choose to move and involuntary migrants forcibly displaced by humanitarian crises. The human papillomavirus (HPV) vaccine, a relatively recent addition to global routine immunisation schedules for adolescents, is a useful proxy for understanding vaccine equity for this under-prioritised group. This qualitative systematic review explores health system determinants of delivery and uptake of HPV vaccination services among involuntary migrants. Methods: A literature search was conducted across ten electronic databases. An analytical framework tailored to the migrant context aided in capturing the complexity and magnitude of systemic factors that determine vaccine delivery and uptake among involuntary migrants. Of the 676 records retrieved, 27 studies were included in this review. Results: Key determinants of vaccine delivery include adaptation of immunisation policies for migrant inclusiveness, implementation of migrant-targeted interventions, health provider recommendations, electronic health records, and free vaccines. Uptake determinants include access dependent on legal status, awareness-related determinants akin to culturally appropriate health messaging, and acceptance-related determinants associated with sociocultural beliefs, misinformation, and distrust. Conclusions: Prioritising vaccination programmes linked with non-outbreak-related diseases is challenging in the disruptive context of humanitarian crises given fragile health systems, limited resources, loss of health infrastructure and deployment of health personnel to emergency care. We strongly advocate for global actors at all health systems levels to actively reform national HPV vaccination programmes to enhance inclusivity of adolescent girls in crises settings or resettled in host countries.
Full article
(This article belongs to the Special Issue Inequality in Immunization 2025)
►▼
Show Figures

Figure 1
Open AccessArticle
A Novel Flow Cytometry Array for High Throughput Detection of SARS-CoV-2 Antibodies
by
Benyue Zhang, Zhuo Zhang, Yichao Zhao, Jingqiao Lu, Jianmin Fang, Brianne Petritis, Kelly Whittaker, Rani Huang and Ruo-Pan Huang
Vaccines 2025, 13(10), 1063; https://doi.org/10.3390/vaccines13101063 - 17 Oct 2025
Abstract
Background/Objectives: Although the U.S. Food and Drug Administration (FDA) has approved one antiviral treatment and authorized others for emergency use, there is no fully effective antiviral therapy for coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2
[...] Read more.
Background/Objectives: Although the U.S. Food and Drug Administration (FDA) has approved one antiviral treatment and authorized others for emergency use, there is no fully effective antiviral therapy for coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Assays detecting virus-specific immunoglobulins (Ig) or nucleic acids in large-scale epidemiological, vaccine, and drug development studies remain limited due to high costs, reagent accessibility, and cumbersome protocols. Methods: A multiplex bead-based assay was developed to simultaneously detect human IgM, IgG, and IgA antibodies against the SARS-CoV-2 spike receptor binding domain (RBD) in serum using flow cytometry. Assay performance was evaluated for sensitivity, specificity, reproducibility, and cross-reactivity and compared to another immunoassay platform. Results: The assay enabled simultaneous measurement of three antibody isotypes across 624 samples within 2 h. Intra-plate coefficients of variation (CVs) ranged from 3.16 to 6.71%, and inter-plate CVs ranged from 3.33 to 5.49%, demonstrating high reproducibility. The platform also quantified background noise from nonspecific binding, facilitating straightforward data interpretation. Conclusions: This novel, flexible multiplex bead-based assay utilizing a well-established platform provides a rapid and reproducible approach for detecting SARS-CoV-2-specific antibodies. Its high throughput capacity and low variability make it well suited for large-scale epidemiological, vaccine, and therapeutic studies. The platform’s adaptability further supports application to other infectious diseases, offering an ideal tool for broad immunological surveillance.
Full article
(This article belongs to the Special Issue COVID Vaccines: Design, Development, and Immune Response Studies: 2nd Edition)
►▼
Show Figures

Figure 1

Journal Menu
► ▼ Journal Menu-
- Vaccines Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Animals, Arthropoda, Insects, Vaccines, Veterinary Sciences, Pathogens
Ticks and Tick-Borne Pathogens: 2nd Edition
Topic Editors: Alina Rodriguez-Mallon, Alejandro Cabezas-CruzDeadline: 31 March 2026

Conferences
Special Issues
Special Issue in
Vaccines
Viral Vector-Based Vaccines and Therapeutics
Guest Editor: Sujit PujhariDeadline: 31 October 2025
Special Issue in
Vaccines
Immunization Strategies for Animal Health
Guest Editors: Eva Calvo-Pinilla, Alejandro Marin-LopezDeadline: 31 October 2025
Special Issue in
Vaccines
Research in Vaccine Adjuvants: Innovations and Challenges
Guest Editors: Haibo Li, Hongwu SunDeadline: 31 October 2025
Special Issue in
Vaccines
Pandemic Influenza Vaccination
Guest Editors: Francisco Nogareda, Shoshanna GoldinDeadline: 31 October 2025
Topical Collections
Topical Collection in
Vaccines
Research on Monoclonal Antibodies and Antibody Engineering
Collection Editor: Tatsuya Yamazaki
Topical Collection in
Vaccines
COVID-19 Vaccine Development and Vaccination
Collection Editors: Ralph Tripp, Scott Anthony
Topical Collection in
Vaccines
Topic Advisory Panel Members’ Collection Series: Immunization and Vaccines for Infectious Diseases
Collection Editors: Shumaila Hanif, Ravinder Kumar









