Epidemiological Survey of Porcine Circovirus Type 2 (PCV2) in Large-Scale Pig Farms in Hubei Province and Comprehensive Evaluation of Commercial Vaccine Efficacy
Abstract
1. Introduction
2. Materials and Methods
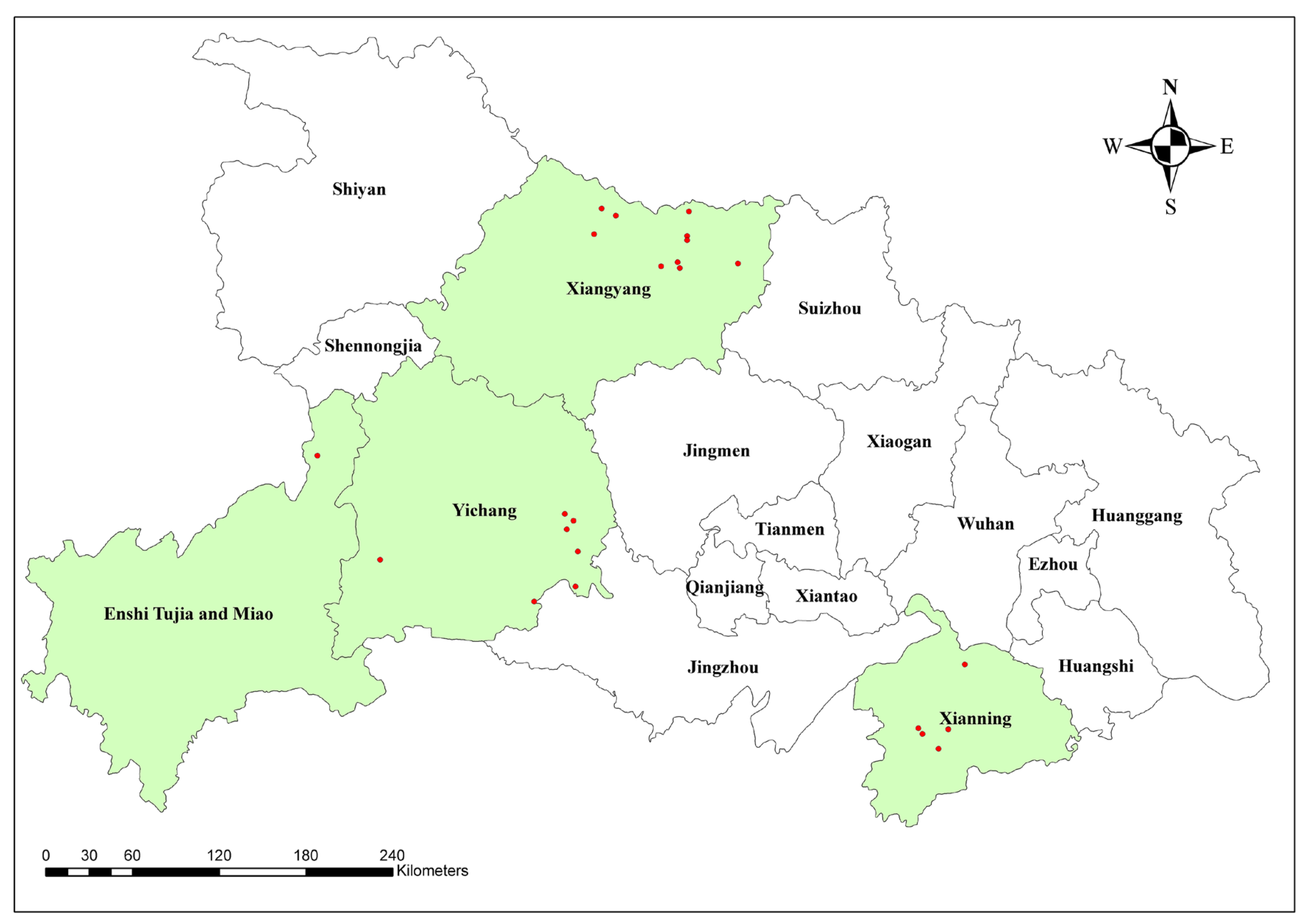
2.1. Sampling Strategy for Epidemiology Survey
2.2. PCV2 Quantitative Real-Time PCR Antigen Detection
2.3. PCV2 ELISA Antibody Detection
2.4. Evaluation of Commercial PCV2 Vaccine Efficacy in a Fattening Farm
2.5. Statistical Analysis
3. Results
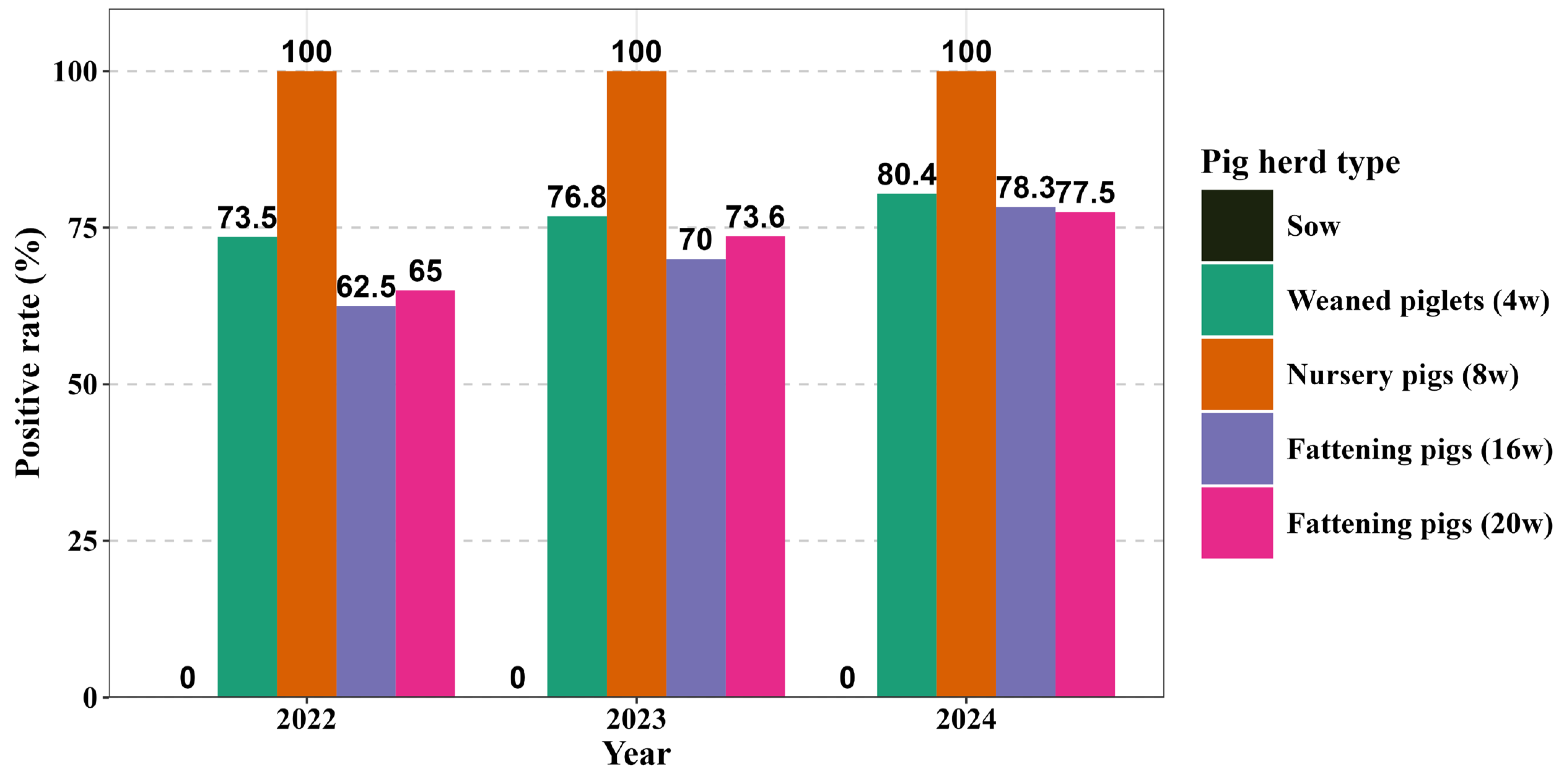
3.1. Epidemiological Survey of PCV2 Antigen
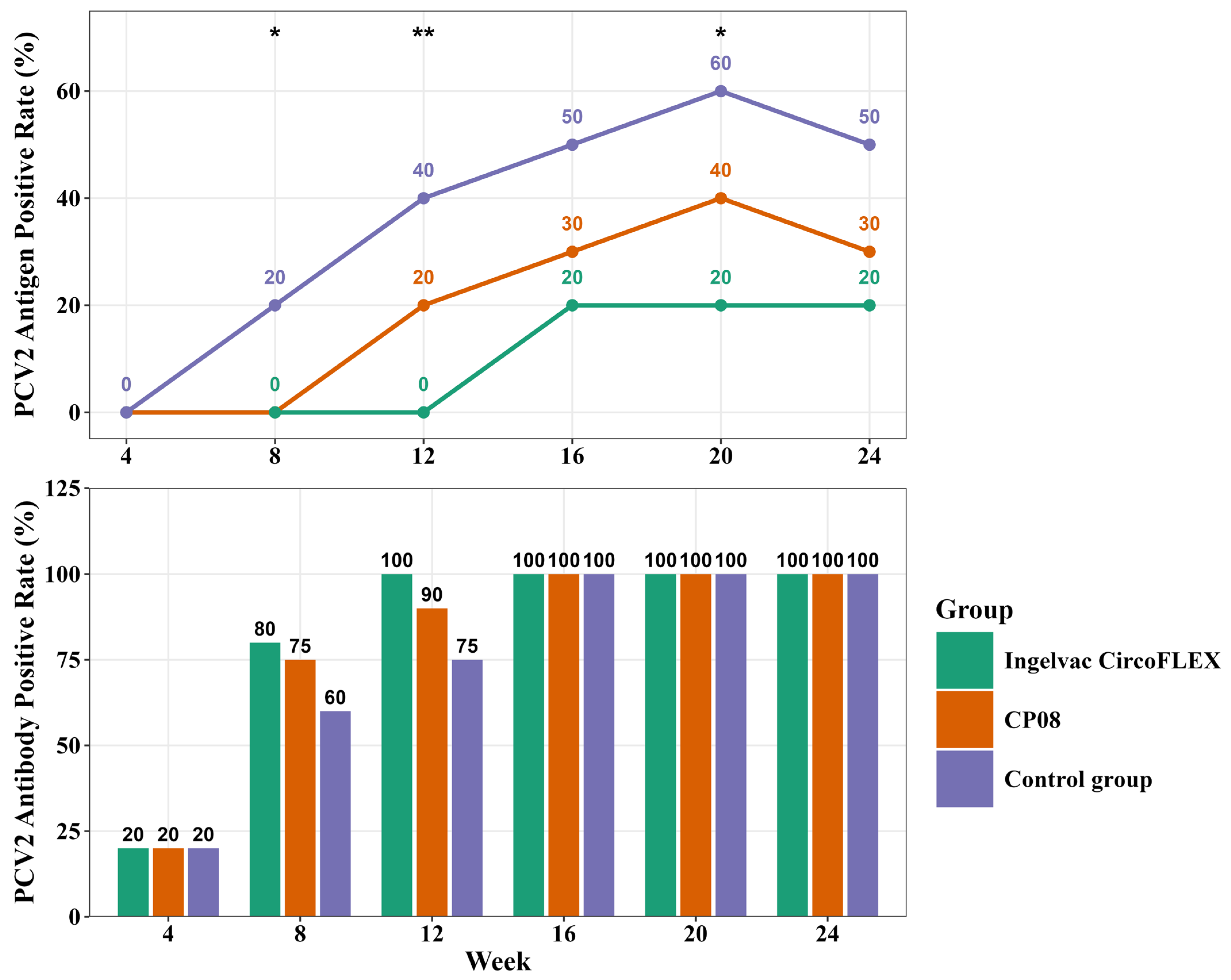
3.2. Efficacy of Commercial Vaccines: Antigen and Antibody Dynamics
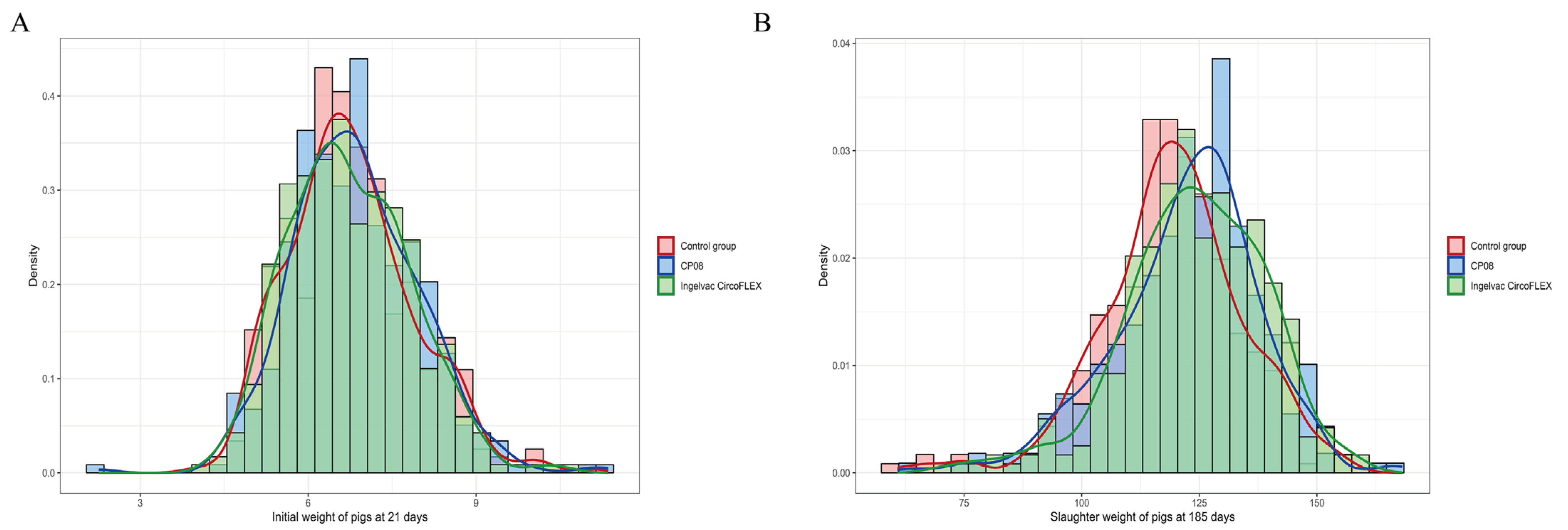
3.3. Growth Performance and Mortality Analysis
3.4. Economic Benefits of Commercial PCV2 Vaccines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCV2 | Porcine circovirus type 2 |
| Ct | Cycle threshold |
| OD | Optical density |
| PCV-AD | Porcine circovirus-associated disease |
| PCV2-SD | Porcine circovirus type 2 systemic disease |
| ORF | Open reading frames |
| ADG | Average daily weight gain |
| ELISA | Enzyme-linked immunosorbent assay |
| PMWS | Postweaning multisystemic wasting syndrome |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
References
- Segalés, J.; Kekarainen, T.; Cortey, M. The natural history of porcine circovirus type 2: From an inoffensive virus to a devastating swine disease? Vet. Microbiol. 2013, 165, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.C.S. The clinical expression and emergence of porcine circovirus 2. Vet. Microbiol. 2004, 98, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hou, L.; Zhou, J.; Wang, D.; Cui, Y.; Feng, X.; Liu, J. Porcine circovirus type 2 vaccines: Commercial application and research advances. Viruses 2022, 14, 2005. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Cao, M.; Wei, Y.; Liu, J.; Zhang, H.; Liu, C.; Feng, L.; Huang, L. Evaluation of cross-immunity among major porcine circovirus type 2 genotypes by infection with PCV2b and PCV2d circulating strains. Vet. Microbiol. 2023, 283, 109796. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Domingo, M. Postweaning multisystemic wasting syndrome (PMWS) in pigs. A review. Vet. Quart. 2002, 24, 109–124. [Google Scholar] [CrossRef]
- Opriessnig, T.; Meng, X.J.; Halbur, P.G. Porcine circovirus type 2 associated disease: Update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet. Diagn. Invest. 2007, 19, 591–615. [Google Scholar] [CrossRef]
- Finsterbusch, T.; Mankertz, A. Porcine circoviruses—Small but powerful. Virus Res. 2009, 143, 177–183. [Google Scholar] [CrossRef]
- Tischer, I.; Gelderblom, H.; Vettermann, W.; Koch, M.A. A very small porcine virus with circular single-stranded DNA. Nature 1982, 295, 64–66. [Google Scholar] [CrossRef]
- Nayar, G.P.; Hamel, A.; Lin, L. Detection and characterization of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. Can. Vet. J. 1997, 38, 385–386. [Google Scholar]
- Hamel, A.L.; Lin, L.L.; Nayar, G.P. Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J. Virol. 1998, 72, 5262–5267. [Google Scholar] [CrossRef]
- Karuppannan, A.K.; Opriessnig, T. Porcine Circovirus Type 2 (PCV2) Vaccines in the Context of Current Molecular Epidemiology. Viruses 2017, 9, 99. [Google Scholar] [CrossRef]
- Franzo, G.; Cortey, M.; Segales, J.; Hughes, J.; Drigo, M. Phylodynamic analysis of porcine circovirus type 2 reveals global waves of emerging genotypes and the circulation of recombinant forms. Mol. Phylogenet. Evol. 2016, 100, 269–280. [Google Scholar] [CrossRef]
- Firth, C.; Charleston, M.A.; Duffy, S.; Shapiro, B.; Holmes, E.C. Insights into the evolutionary history of an emerging livestock pathogen: Porcine circovirus 2. J. Virol. 2009, 83, 12813–12821. [Google Scholar] [CrossRef]
- Xiao, C.T.; Halbur, P.G.; Opriessnig, T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 2015, 96, 1830–1841. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, T.; Wen, J.; Yang, L.; Lai, S.; Sun, X.; Xu, Z.; Zhu, L. Prevalence and phylogenetic analysis of porcine circovirus type 2 (PCV2) and type 3 (PCV3) in the Southwest of China during 2020–2022. Front. Vet. Sci. 2022, 9, 1042792. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhu, Q.; Xu, T.; Chen, X.; Li, H.; Ma, M.; Zhang, Y.; He, Z.; Chen, H. Detection and genetic characteristics of porcine circovirus type 2 and 3 in Henan province of China. Mol. Cell. Probes 2022, 61, 101790. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Huang, L.; Xie, Y.; Zhang, X.; Wei, Y.; Liu, D.; Zhu, H.; Bian, H.; Feng, L.; Liu, C. The prevalence and genetic diversity of porcine circovirus types 2 and 3 in Northeast China from 2015 to 2018. Arch. Virol. 2019, 164, 2435–2449. [Google Scholar] [CrossRef]
- Yang, K.; Wang, Z.; Wang, X.; Bi, M.; Hu, S.; Li, K.; Pan, X.; Wang, Y.; Ma, D.; Mo, X.J.V.J. Epidemiological investigation and analysis of the infection of porcine circovirus in Xinjiang. Virol. J. 2024, 21, 230. [Google Scholar] [CrossRef]
- Chae, C. Commercial porcine circovirus type 2 vaccines: Efficacy and clinical application. Vet. J. 2012, 194, 151–157. [Google Scholar] [CrossRef]
- Segalés, J. Best practice and future challenges for vaccination against porcine circovirus type 2. Expert Rev. Vaccines 2015, 14, 473–487. [Google Scholar] [CrossRef]
- Jeong, J.; Park, C.; Choi, K.; Chae, C. Comparison of three commercial one-dose porcine circovirus type 2 (PCV2) vaccines in a herd with concurrent circulation of PCV2b and mutant PCV2b. Vet. Microbiol. 2015, 177, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J. Porcine circovirus: A historical perspective. Vet. Pathol. 2014, 51, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Ferrando, S.; Segalés, J.; López-Soria, S.; Callén, A.; Merdy, O.; Joisel, F.; Sibila, M. Evaluation of natural porcine circovirus type 2 (PCV2) subclinical infection and seroconversion dynamics in piglets vaccinated at different ages. Vet. Res. 2016, 47, 121. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Xiao, C.T.; Halbur, P.G.; Gerber, P.F.; Matzinger, S.R.; Meng, X.J. A commercial porcine circovirus (PCV) type 2a-based vaccine reduces PCV2d viremia and shedding and prevents PCV2d transmission to naïve pigs under experimental conditions. Vaccine 2017, 35, 248–254. [Google Scholar] [CrossRef]
- Bandrick, M.; Balasch, M.; Heinz, A.; Taylor, L.; King, V.; Toepfer, J.; Foss, D. A bivalent porcine circovirus type 2 (PCV2), PCV2a-PCV2b, vaccine offers biologically superior protection compared to monovalent PCV2 vaccines. Vet. Res. 2022, 53, 12. [Google Scholar] [CrossRef]
- Schlepers, M.; Gelauf, J.; Wertenbroek, N.; Nielen, M. Improvement of technical results following use of Ingelvac CircoFLEX in a Dutch organic breeding and fattening farm: A case report. Tijdschr. Voor Diergeneeskd. 2013, 138, 26–33. [Google Scholar]
- Thrusfield, M. Veterinary Epidemiology; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Fleiss, J.L.; Levin, B.; Paik, M.C. Statistical Methods for Rates and Proportions, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Haukanes, B.-I.; Kvam, C. Application of magnetic beads in bioassays. Biotechnology 1993, 11, 60–63. [Google Scholar] [CrossRef]
- Festing, M.F.; Altman, D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002, 43, 244–258. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- McKight, P.E.; Najab, J. Kruskal-Wallis Test; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 2. [Google Scholar] [CrossRef]
- Dunn, O.J.; Clark, V.A. Basic Statistics: A Primer for the Biomedical Sciences, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Pearson, K.X. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Lond. Edinb. Dubl. Phil. Mag. 1900, 50, 157–175. [Google Scholar] [CrossRef]
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant Graphics for Data Analysis. Meas. Interdiscip. Res. Perspect. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Fan, M.; Bian, L.; Tian, X.; Hu, Z.; Wu, W.; Sun, L.; Yuan, G.; Li, S.; Yue, L.; Wang, Y. Infection characteristics of porcine circovirus type 2 in different herds from intensive farms in China, 2022. Front. Vet. Sci. 2023, 10, 1187753. [Google Scholar] [CrossRef]
- Ham, S.; Suh, J.; Kim, C.; Seo, B.J.; Park, G.S.; Chae, C.J.V.M. A field evaluation of a new porcine circovirus type 2d and Mycoplasma hyopneumoniae bivalent vaccine in herds suffering from subclinical PCV2d infection and enzootic pneumonia. Vet. Med. Sci. 2024, 10, e70001. [Google Scholar] [CrossRef]
- Sagrera, M.; Garza-Moreno, L.; Sibila, M.; Oliver-Ferrando, S.; Cárceles, S.; Casanovas, C.; Prieto, P.; García-Flores, A.; Espigares, D.; Segalés, J.J.P.h.m. Frequency of PCV-2 viremia in nursery piglets from a Spanish swine integration system in 2020 and 2022 considering PRRSV infection status. Porc. Health Manag. 2024, 10, 4. [Google Scholar] [CrossRef]
| Year | Number of Pig Farms | Pig Herd Type | Total | ||||
|---|---|---|---|---|---|---|---|
| Sow | Weaned Piglets (4 Weeks) | Nursery Pigs (8 Weeks) | Fattening Pigs (16 Weeks) | Fattening Pigs (20 Weeks) | |||
| 2022 | 20 | 1200 | 200 | 200 | 200 | 200 | 2000 |
| 2023 | 22 | 1320 | 220 | 220 | 220 | 220 | 2200 |
| 2024 | 24 | 1440 | 240 | 240 | 240 | 240 | 2400 |
| Total | 66 | 3960 | 660 | 660 | 660 | 660 | 6600 |
| Group | Weight (kg) | Total (Head) | Average Weight (kg) | |||||
|---|---|---|---|---|---|---|---|---|
| 4.32–5.32 | 5.32–6.32 | 6.32–7.32 | 7.32–8.32 | 8.32–9.32 | 9.32–10.32 | |||
| Ingelvac CircoFLEX® | 26 | 107 | 122 | 80 | 29 | 1 | 365 | 6.76 |
| CP08 | 24 | 99 | 138 | 81 | 23 | 0 | 365 | 6.78 |
| Control group | 31 | 99 | 137 | 62 | 34 | 2 | 365 | 6.77 |
| Total (head) | 81 | 305 | 397 | 223 | 86 | 3 | 1095 | 6.77 |
| Group | Number of Pig Missing Ear Tags | Number of Pig Deaths | Mortality Rate (%) | χ2 Values | p Value a | Weighing Head Number | Average Weight Gain per Head (kg) (Mean ± Sd) b |
|---|---|---|---|---|---|---|---|
| Ingelvac CircoFLEX® | 23 | 21 | 6.14 | 5.991 | 0.732 | 321 | 117.29 ± 14.70 a |
| CP08 | 45 | 26 | 8.12 | 294 | 115.59 ± 15.02 a | ||
| Control group | 28 | 25 | 7.42 | 312 | 112.65 ± 14.75 b |
| Group | Vaccine (¥/Head) | Price (¥/Head) a | Average Revenue (¥/Head) b | Difference Among Groups (¥/Head) | ||
|---|---|---|---|---|---|---|
| Ingelvac CircoFLEX® vs. COP08 | Ingelvac CircoFLEX® vs. Control Group | CP08 vs. Control Group | ||||
| Ingelvac CircoFLEX® | 19.6 | 23 | 2678.07 | 29.5 | 87.12 | 57.62 |
| CP08 | 10 | 2648.57 | ||||
| Control group | 0 | 2590.95 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, W.; Xi, Z.; Fang, R.; Shen, B.; Zhao, J. Epidemiological Survey of Porcine Circovirus Type 2 (PCV2) in Large-Scale Pig Farms in Hubei Province and Comprehensive Evaluation of Commercial Vaccine Efficacy. Vaccines 2025, 13, 1066. https://doi.org/10.3390/vaccines13101066
Liao W, Xi Z, Fang R, Shen B, Zhao J. Epidemiological Survey of Porcine Circovirus Type 2 (PCV2) in Large-Scale Pig Farms in Hubei Province and Comprehensive Evaluation of Commercial Vaccine Efficacy. Vaccines. 2025; 13(10):1066. https://doi.org/10.3390/vaccines13101066
Chicago/Turabian StyleLiao, Wenjun, Zhaofang Xi, Rui Fang, Bang Shen, and Junlong Zhao. 2025. "Epidemiological Survey of Porcine Circovirus Type 2 (PCV2) in Large-Scale Pig Farms in Hubei Province and Comprehensive Evaluation of Commercial Vaccine Efficacy" Vaccines 13, no. 10: 1066. https://doi.org/10.3390/vaccines13101066
APA StyleLiao, W., Xi, Z., Fang, R., Shen, B., & Zhao, J. (2025). Epidemiological Survey of Porcine Circovirus Type 2 (PCV2) in Large-Scale Pig Farms in Hubei Province and Comprehensive Evaluation of Commercial Vaccine Efficacy. Vaccines, 13(10), 1066. https://doi.org/10.3390/vaccines13101066






