Journal Description
Cells
Cells
is an international, peer-reviewed, open access journal on cell biology, molecular biology, and biophysics, published semimonthly online by MDPI. The Nordic Autophagy Society (NAS) and the Spanish Society of Hematology and Hemotherapy (SEHH) are affiliated with Cells and their members receive discounts on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, MEDLINE, PMC, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q2 (Cell Biology) / CiteScore - Q1 (General Biochemistry, Genetics and Molecular Biology)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 16 days after submission; acceptance to publication is undertaken in 2.7 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
- Sections: published in 21 topical sections.
- Companion journal: Organoids.
Impact Factor:
5.2 (2024);
5-Year Impact Factor:
6.1 (2024)
Latest Articles
Muscle-Bone Crosstalk and Metabolic Dysregulation in Children and Young People Affected with Type 1 Diabetes: Mechanisms and Clinical Implications
Cells 2025, 14(20), 1611; https://doi.org/10.3390/cells14201611 - 16 Oct 2025
Abstract
Pediatric type 1 diabetes (T1D) disrupts musculoskeletal development during critical windows of growth, puberty, and peak bone mass accrual. Beyond classic micro- and macrovascular complications, accumulating evidence shows a dual burden of diabetic bone disease—reduced bone mineral density, microarchitectural deterioration, and higher fracture
[...] Read more.
Pediatric type 1 diabetes (T1D) disrupts musculoskeletal development during critical windows of growth, puberty, and peak bone mass accrual. Beyond classic micro- and macrovascular complications, accumulating evidence shows a dual burden of diabetic bone disease—reduced bone mineral density, microarchitectural deterioration, and higher fracture risk—and diabetic myopathy, characterized by loss of muscle mass, diminished strength, and metabolic dysfunction. Mechanistically, chronic hyperglycemia, absolute or functional insulin deficiency, and glycemic variability converge to suppress PI3K–AKT–mTOR signaling, activate FoxO-driven atrogenes (atrogin-1, MuRF1), and impair satellite-cell biology; advanced glycation end-products (AGEs) and RAGE signaling stiffen extracellular matrix and promote low-grade inflammation (IL-6, TNF-α/IKK/NF-κB), while oxidative stress and mitochondrial dysfunction further compromise the bone–muscle unit. In vitro, ex vivo, and human studies consistently link these pathways to lower BMD and trabecular/cortical quality, reduced muscle performance, and increased fractures—associations magnified by poor metabolic control and longer disease duration. Prevention prioritizes tight, stable glycemia, daily physical activity with weight-bearing and progressive resistance training, and optimized nutrition (adequate protein, calcium, vitamin D). Treatment is individualized: supervised exercise-based rehabilitation (including neuromuscular and flexibility training) is the cornerstone of skeletal muscle health. This review provides a comprehensive analysis of the mechanisms underlying the impact of type 1 diabetes on musculoskeletal system. It critically appraises evidence from in vitro studies, animal models, and clinical research in children, it also explores the effects of prevention and treatment.
Full article
(This article belongs to the Special Issue Diabetes-Induced Organ Damage: Cellular Mechanisms and Therapeutic Opportunities)
Open AccessReview
The Emerging Role of Peroxisome Proliferator-Activated Receptors in Cancer Stemness
by
Beatriz Parejo-Alonso, Marta Mascaraque, Alba Royo-García and Patricia Sancho
Cells 2025, 14(20), 1610; https://doi.org/10.3390/cells14201610 - 16 Oct 2025
Abstract
The peroxisome proliferator-activated receptors (PPAR-α, PPAR-δ, and PPAR-γ) are transcription factors that belong to the nuclear hormone receptor superfamily. Upon activation by specific lipids, they regulate gene expression by directly binding to PPAR response elements (PPREs) in the DNA. Although the functions of
[...] Read more.
The peroxisome proliferator-activated receptors (PPAR-α, PPAR-δ, and PPAR-γ) are transcription factors that belong to the nuclear hormone receptor superfamily. Upon activation by specific lipids, they regulate gene expression by directly binding to PPAR response elements (PPREs) in the DNA. Although the functions of the different PPARs are specific to the isoform, tissue, and context, all three PPARs are generally involved in energy homeostasis through lipid sensing in physiological conditions. Importantly, there is increasing evidence linking PPARs with malignant behavior in cancer, regulating features frequently attributed to the aggressive subpopulation of cancer stem cells (CSCs): self-renewal, tumor initiation, chemoresistance, metastasis, and immune evasion. However, contradictory effects have been described for each isoform in various cancer types, and their implication in these malignant features may not consistently follow a pro- or anti-tumoral pattern. In this review, we revise the current knowledge on the role of the PPAR family members in cancer, with a special focus on cancer stemness, and discuss the potential for PPARs as therapeutic targets in CSC-driven relapse and resistance.
Full article
Open AccessReview
NSUN-Mediated m5C RNA Modification in Stem Cell Regulation
by
Jiin Moon, Hyohi Lee, Yeonju Jang and Seung-Kyoon Kim
Cells 2025, 14(20), 1609; https://doi.org/10.3390/cells14201609 - 16 Oct 2025
Abstract
RNA modifications comprise a core epigenetic dimension of gene regulation; among these, N6-methyladenosine (m6A) and 5-methylcytosine (m5C) have been most intensively investigated. While the functions of m6A in stem cell biology have been well characterized, the contributions of m5C remain comparatively less well
[...] Read more.
RNA modifications comprise a core epigenetic dimension of gene regulation; among these, N6-methyladenosine (m6A) and 5-methylcytosine (m5C) have been most intensively investigated. While the functions of m6A in stem cell biology have been well characterized, the contributions of m5C remain comparatively less well defined. This review focuses on m5C modifications catalyzed by the NSUN family of RNA methyltransferases and their roles in regulating stem cell identity, pluripotency, and differentiation. Evidence from embryonic and mesenchymal stem cells, as well as animal models, demonstrates that NSUN-mediated m5C is deposited on diverse RNA substrates, including rRNA, tRNA, mRNA, mitochondrial RNA, and enhancer RNAs, thereby influencing processes such as self-renewal, cell cycle progression, RNA stability, metabolic activation, and lineage specification. Disruption of m5C regulation often leads to developmental defects, underscoring its essential role during embryogenesis. Collectively, these findings establish m5C as a versatile and dynamic regulator in stem cell biology and underscore the need for future studies to delineate the roles of the NSUN family in stem cells and define the RNA targets of m5C. In addition, its broader implications for development, regenerative medicine, and disease, including cancer, as well as its potential interplay with other RNA modifications such as m6A and pseudouridine, remain important areas for further investigation.
Full article
(This article belongs to the Special Issue Advances and Breakthroughs in Stem Cell Research)
►▼
Show Figures
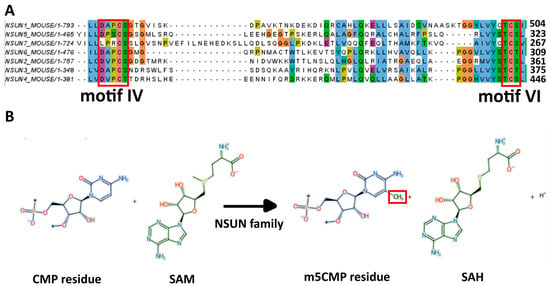
Figure 1
Open AccessArticle
Description of T-Cell and Monocyte Populations in the Circulation of People with HIV Prior to AIDS-NHL Diagnosis
by
Laura E. Martínez, Begoña Comin-Anduix, Miriam Güemes-Aragon, Javier Ibarrondo, Roger Detels, Matthew J. Mimiaga and Marta Epeldegui
Cells 2025, 14(20), 1608; https://doi.org/10.3390/cells14201608 - 16 Oct 2025
Abstract
People with HIV (PWH) are at an increased risk for AIDS-associated non-Hodgkin lymphoma (AIDS-NHL); however, the immune signatures underlying this risk are not well understood. In this study, we utilized mass cytometry by time-of-flight (CyTOF) to analyze T-cells and monocytes in the PBMCs
[...] Read more.
People with HIV (PWH) are at an increased risk for AIDS-associated non-Hodgkin lymphoma (AIDS-NHL); however, the immune signatures underlying this risk are not well understood. In this study, we utilized mass cytometry by time-of-flight (CyTOF) to analyze T-cells and monocytes in the PBMCs of treatment-naïve PWH, including those 3 to 36 months before an AIDS-NHL diagnosis (HIV-positive pre-NHL), as well as people without HIV (PWoH). Mass cytometry is an advanced single-cell analysis platform that combines flow cytometry principles with mass spectrometry. Unlike conventional flow cytometry, this technology employs antibodies conjugated to unique metal isotopes instead of fluorescent markers, enabling simultaneous measurement of over 40 distinct cellular markers per individual cell without spectral overlap limitations. Participants were enrolled at the Los Angeles site of the MACS/WIHS Combined Cohort Study (MWCCS). Unsupervised clustering and Uniform Manifold Approximation and Projection (UMAP) analysis identified CD3+ T-cell and CD14+ monocyte metaclusters, and Spearman’s rank correlation assessed their relationships with B-cell subsets exhibiting aberrant phenotypes. We observed elevated levels of CD8+CD20+ T-cells, CD8+CD14+ T-cells, and M2-like CD14+CD163+ monocytes in HIV-positive pre-NHL individuals compared to HIV-negative controls. Positive correlations were found between CD19+ AICDA+ cMYC+ B-cells and M1-like CD14+cMYC+ monocytes (metacluster, MC02), and between metaclusters of CD8+PD-1+CD27+CXCR4− T-cells (MC05) and CD4+FoxP3+PD-1+CD27+CD28+CXCR4− ICOS+ T-cells (MC08). In addition, a different CD19+ B-cell metacluster (FoxP3+AICDA+cMYC+) was positively associated with a metacluster of CD8+PD-1+CD27+CD28+CXCR4+ T-cells (MC03). Moreover, the metacluster of CD8+PD-1+CD27+CXCR4− T-cells (MC05) negatively correlated with M2-like CD14+CD163+ monocytes (MC06), while CD8+CD14+ T-cells positively correlated with AICDA+ Bregs and IL-10+ B-regs in HIV-positive pre-NHL individuals. Unsupervised analysis revealed increased frequencies of CD8+CD20+ T-cells in HIV-positive individuals compared to HIV-negative controls. These immune alterations provide valuable insights into potential biomarkers for early detection, monitoring, and therapeutic strategies for AIDS-NHL.
Full article
(This article belongs to the Special Issue Immune Response in HIV Infection, Pathogenesis and Persistence)
►▼
Show Figures
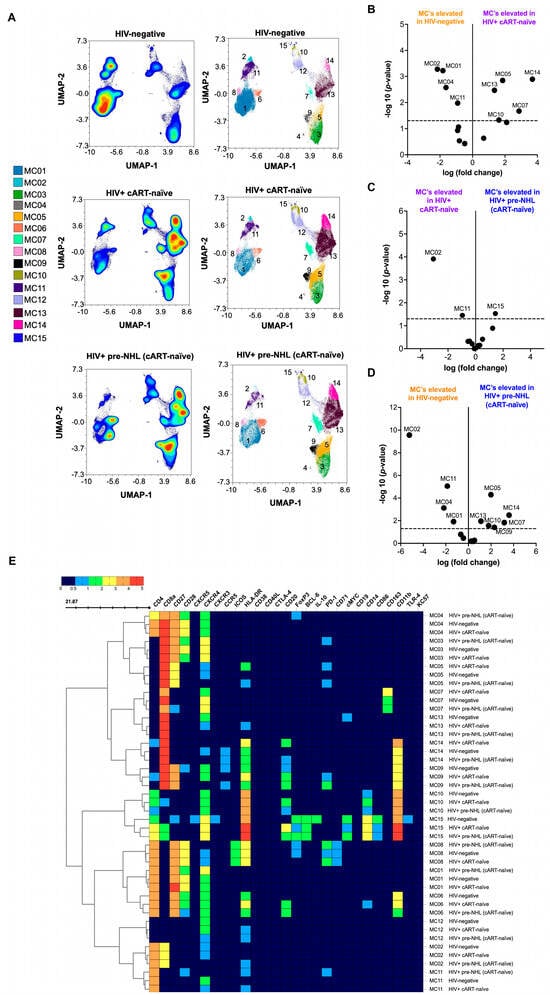
Figure 1
Open AccessArticle
Neuronal Primary Cilia Mediate Noggin Release to Enable Extracellular Signaling
by
Sara R. Dunlop, Justin A. Geier, Chian-Yu Peng and John A. Kessler
Cells 2025, 14(20), 1607; https://doi.org/10.3390/cells14201607 - 16 Oct 2025
Abstract
The primary cilium is generally viewed as a sensory organelle that transduces chemical and mechanical stimuli from the environment. In the adult hippocampus, primary cilia mediate the effects of sonic hedgehog (Shh) and other signals on neurogenesis and hippocampal function, and loss of
[...] Read more.
The primary cilium is generally viewed as a sensory organelle that transduces chemical and mechanical stimuli from the environment. In the adult hippocampus, primary cilia mediate the effects of sonic hedgehog (Shh) and other signals on neurogenesis and hippocampal function, and loss of cilia leads to cognitive and behavioral deficits. The secreted peptide noggin is a bone morphogenetic protein (BMP) antagonist and plays a critical role in regulating adult hippocampal neurogenesis (AHN) and hippocampus-dependent behavior. Here, we show that noggin is expressed by mature granule cell neurons, that it is apically targeted and localized intracellularly near the pocket region of primary cilia, and that cilia regulate noggin release through Shh and somatostatin (SST) pathways. Further, granule cell activation modulates noggin dynamics both in vitro and in vivo. Together, these findings demonstrate synergy between Shh and noggin and the positive regulatory action of neuronal activity on regulating BMP antagonism within the neurogenic niche. Thus, the primary cilium is not only an organelle that transduces signals to neurons but also one that mediates extracellular signaling. Significance statement: Primary cilia are organelles that protrude from the surface of most vertebrate cell types. Defects in primary ciliary structure and function are associated with human disease. Primary cilia are generally viewed as exclusively sensory organelles that respond to environmental signals to regulate both cell development and adult cell function. This study demonstrates that the primary cilia in hippocampal granule cell neurons mediate the release of the BMP antagonist, noggin. These observations expand the current understanding of ciliary signaling and may inform future studies exploring the connection between hippocampal activity and cognition in ciliopathies.
Full article
(This article belongs to the Special Issue Advanced Research in Neurogenesis and Neuroinflammation)
►▼
Show Figures
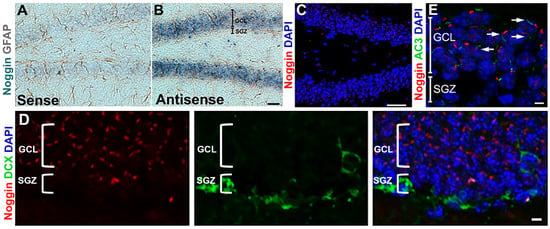
Figure 1
Open AccessArticle
Estrogen Receptor Regulates Male Satellite Cells in a Female, but Not Male, Environment
by
Ahmed S. Shams, Brian P. Sullivan, Erik A. Toso, Dawn A. Lowe and Michael Kyba
Cells 2025, 14(20), 1606; https://doi.org/10.3390/cells14201606 - 16 Oct 2025
Abstract
►▼
Show Figures
Skeletal muscle homeostasis is dependent on the satellite cell pool, which is regulated by numerous signaling pathways. Estradiol (E2) function via estrogen receptor alpha (ERα, Esr1) plays an important role in satellite cell regulation in females, being necessary for satellite cell maintenance,
[...] Read more.
Skeletal muscle homeostasis is dependent on the satellite cell pool, which is regulated by numerous signaling pathways. Estradiol (E2) function via estrogen receptor alpha (ERα, Esr1) plays an important role in satellite cell regulation in females, being necessary for satellite cell maintenance, proliferation and differentiation. Here we investigate this signaling axis in male satellite cells. Male satellite cells express Esr1 mRNA at similar levels to female satellite cells, and E2 enhances the proliferation of male satellite cell-derived myoblasts in vitro. Deletion of Esr1 specifically in male satellite cells has no effect on satellite cell number, nor on their ability to self-renew after injury, during regeneration, or when transplanted into male hosts. However, Esr1 deletion severely reduces self-renewal of male satellite cells when transplanted into female hosts. These data suggest that male satellite cells are competent for E2-ERα signaling, but that this signaling is not efficacious in the male environment, though E2-ERα signaling does become necessary when the male cells are transplanted into a female environment.
Full article
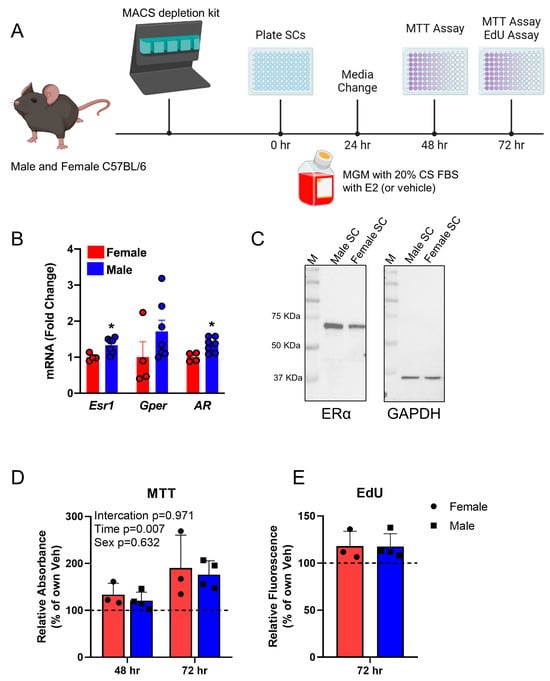
Figure 1
Open AccessReview
Joint Acidosis and Acid-Sensing Receptors and Ion Channels in Osteoarthritis Pathobiology and Therapy
by
William N. Martin, Colette Hyde, Adam Yung, Ryan Taffe, Bhakti Patel, Ajay Premkumar, Pallavi Bhattaram, Hicham Drissi and Nazir M. Khan
Cells 2025, 14(20), 1605; https://doi.org/10.3390/cells14201605 - 16 Oct 2025
Abstract
Osteoarthritis (OA) lacks disease-modifying therapies, in part because key features of the joint microenvironment remain underappreciated. One such feature is localized acidosis, characterized by sustained reductions in extracellular pH within the cartilage, meniscus, and the osteochondral interface despite near-neutral bulk synovial fluid. We
[...] Read more.
Osteoarthritis (OA) lacks disease-modifying therapies, in part because key features of the joint microenvironment remain underappreciated. One such feature is localized acidosis, characterized by sustained reductions in extracellular pH within the cartilage, meniscus, and the osteochondral interface despite near-neutral bulk synovial fluid. We synthesize current evidence on the origins, sensing, and consequences of joint acidosis in OA. Metabolic drivers include hypoxia-biased glycolysis in avascular cartilage, cytokine-driven reprogramming in the synovium, and limits in proton/lactate extrusion (e.g., monocarboxylate transporters (MCTs)), with additional contributions from fixed-charge matrix chemistry and osteoclast-mediated acidification at the osteochondral junction. Acidic niches shift proteolysis toward cathepsins, suppress anabolic control, and trigger chondrocyte stress responses (calcium overload, autophagy, senescence, apoptosis). In the nociceptive axis, protons engage ASIC3 and sensitize TRPV1, linking acidity to pain. Joint cells detect pH through two complementary sensor classes: proton-sensing GPCRs (GPR4, GPR65/TDAG8, GPR68/OGR1, GPR132/G2A), which couple to Gs, Gq/11, and G12/13 pathways converging on MAPK, NF-κB, CREB, and RhoA/ROCK; and proton-gated ion channels (ASIC1a/3, TRPV1), which convert acidity into electrical and Ca2+ signals. Therapeutic implications include inhibition of acid-enabled proteases (e.g., cathepsin K), pharmacologic modulation of pH-sensing receptors (with emerging interest in GPR68 and GPR4), ASIC/TRPV1-targeted analgesia, metabolic control of lactate generation, and pH-responsive intra-articular delivery systems. We outline research priorities for pH-aware clinical phenotyping and imaging, cell-type-resolved signaling maps, and targeted interventions in ‘acidotic OA’ endotypes. Framing acidosis as an actionable component of OA pathogenesis provides a coherent basis for mechanism-anchored, locality-specific disease modification.
Full article
(This article belongs to the Special Issue Molecular Mechanisms Underlying Inflammatory Pain)
►▼
Show Figures
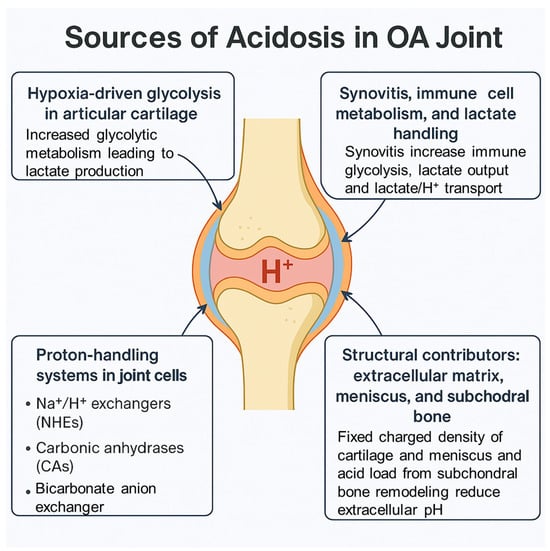
Figure 1
Open AccessArticle
The Immunomodulatory Role of Gemcitabine in Triple Negative Breast Cancer
by
Cory Fines, Syed Umbreen, Elaine Gilmore, Helen McCarthy and Niamh Buckley
Cells 2025, 14(20), 1604; https://doi.org/10.3390/cells14201604 - 16 Oct 2025
Abstract
Triple negative breast cancer (TNBC), defined for its lack of expression/amplification of three major receptors, makes up ~15% of all BC cases but a majority of all BC deaths. TNBC has been found to be the most immune-rich among BC subtypes, and progress
[...] Read more.
Triple negative breast cancer (TNBC), defined for its lack of expression/amplification of three major receptors, makes up ~15% of all BC cases but a majority of all BC deaths. TNBC has been found to be the most immune-rich among BC subtypes, and progress has been made in the development of immunotherapies; however, not all patients are eligible, and response can be limited. Therefore, there is a significant clinical need to enhance the response to these treatments. Given chemotherapy is the core component of TNBC treatment, and is given in combination with immunotherapy, its potential immunomodulatory impact warrants exploration. Gemcitabine, currently used for the treatment of metastatic TNBC, has been reported to have potential immunomodulatory properties that create a more immune-favourable TME for combination with immunotherapies and/or improved outcome. We therefore investigated the use of gemcitabine as an immunomodulator in a primary 4T1 TNBC mouse model. Gemcitabine was able to reduce pro-tumour immune cells including macrophages and MDSCs while increasing T-cell abundance, therefore resulting in a less immunosuppressive TME. We demonstrated that this immune response was both temporal and dose-dependent, which has impact for planning and scheduling combination treatments. In conclusion, we have demonstrated that gemcitabine modulates the TME in ways that could not only enhance the direct anti-tumour effects of gemcitabine itself but also potentially enhance responsiveness to immunotherapy. This work has laid the foundation for further studies investigating combination therapy for the treatment of TNBC.
Full article
(This article belongs to the Special Issue How Can We Optimise Cancer Therapy? Tumour Microenvironment and Immune Evasion Strategies)
►▼
Show Figures
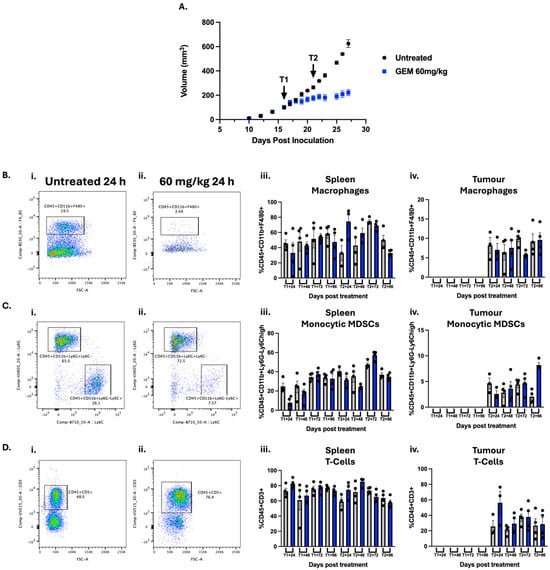
Figure 1
Open AccessArticle
Dental Pulp Stem Cell-Derived Organoids: Advancing the Development of 3D Structures
by
Loreto Lancia, Fanny Pulcini, Emanuela Mari, Luca Piccoli, Leda Assunta Biordi, Luciano Mutti, Claudio Festuccia, Giovanni Luca Gravina, Vincenzo Mattei, Annunziata Mauro, Valentina Notarstefano and Simona Delle Monache
Cells 2025, 14(20), 1603; https://doi.org/10.3390/cells14201603 - 15 Oct 2025
Abstract
Two-dimensional cell cultures are crucial research tools, and they have been widely used, although they are not completely representative of biological processes in vivo due to the lack of tissue architecture and complexity. Recent advances in organoid technology have addressed these limitations and
[...] Read more.
Two-dimensional cell cultures are crucial research tools, and they have been widely used, although they are not completely representative of biological processes in vivo due to the lack of tissue architecture and complexity. Recent advances in organoid technology have addressed these limitations and are revolutionizing the tools available for in vitro culture. Although there are no unified protocols for generating organoids, they can be obtained with various techniques, leading to cell aggregation by promoting cell adhesion. This work aims to generate and characterise organoid models of dental pulp from dental pulp stem cells (DPSCs), a type of mesenchymal stem/stromal cells known for their high regenerative potential and ease of accessibility, to establish a model for translational studies. The organoids were subjected to osteogenic differentiation conditions. Cell viability was evaluated using a CCK-8 assay, while osteogenic morphology and mineralization were confirmed by Alizarin red analysis, Raman microspectroscopy, and by immunofluorescence for the lineage markers expression. The Alizarin red analysis indicated a higher presence of calcium phosphate deposits in the differentiated organoids than in the control group (CTR). These results were confirmed by spectral profiles obtained using Raman microspectroscopy, which were attributable to a hydroxyapatite-based biomaterial. Immunofluorescence analysis also revealed increased expression of odonto/osteogenic markers (RUNX and OSX), alongside reduced expression of stemness markers. In conclusion, the organoids appeared to have successfully differentiated into an osteogenic lineage, forming a mineralized matrix containing hydroxyapatite and showing increased expression of relevant lineage markers.
Full article
(This article belongs to the Special Issue 3D Cultures and Organ-on-a-Chip in Cell and Tissue Cultures)
►▼
Show Figures
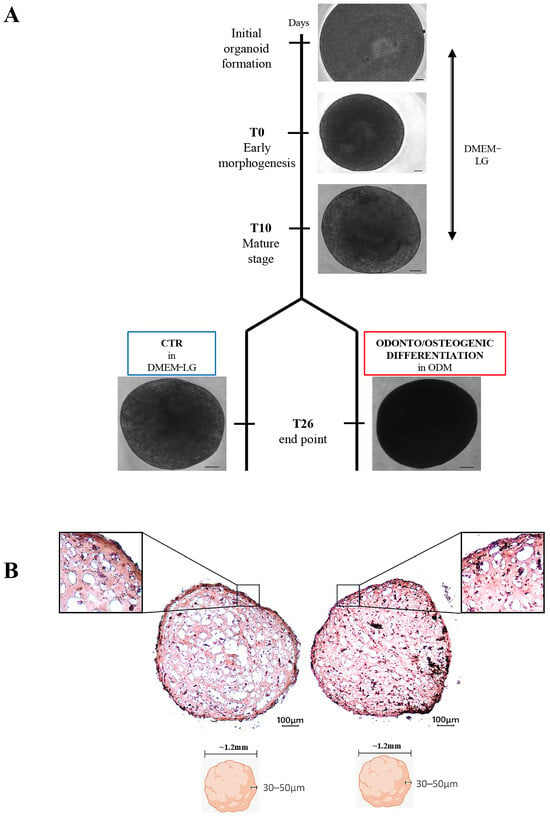
Figure 1
Open AccessArticle
IL-6 Blockade Enhances the Efficacy of CDK4/6 Inhibitor in BRCA1-Mutant Triple-Negative Breast Cancer Cells
by
Li Pan, Changyou Shi, Joungil Choi and Jiayuh Lin
Cells 2025, 14(20), 1602; https://doi.org/10.3390/cells14201602 - 15 Oct 2025
Abstract
►▼
Show Figures
Breast cancer gene 1 (BRCA1) is a tumor suppressor gene essential for DNA repair, and its mutations are linked to aggressive breast cancers with poor prognosis. While poly (ADP-ribose) polymerase (PARP) inhibitors benefit some patients with BRCA1-mutant, human epidermal growth
[...] Read more.
Breast cancer gene 1 (BRCA1) is a tumor suppressor gene essential for DNA repair, and its mutations are linked to aggressive breast cancers with poor prognosis. While poly (ADP-ribose) polymerase (PARP) inhibitors benefit some patients with BRCA1-mutant, human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer, issues such as limited efficacy and drug resistance persist. This is especially critical for triple-negative breast cancer (TNBC), which lacks targeted therapies. Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors such as abemaciclib—FDA-approved for estrogen receptor (ER)-positive/HER2-negative breast cancer—are emerging as potential treatments for TNBC. We evaluated abemaciclib in BRCA1-mutant TNBC cell lines (SUM149, HCC1937, and MDA-MB-436) and found them to be sensitive to the drug. However, treatment induced cellular senescence and Interleukin-6 (IL-6) secretion, which may promote drug resistance. To address this, we inhibited IL-6 signaling using bazedoxifene or glycoprotein 130 (GP130) siRNA, and both of which enhanced abemaciclib sensitivity. Combination treatment with bazedoxifene and abemaciclib synergistically inhibited cell migration and invasion, and induced apoptosis. In a mammary fat pad TNBC tumor model, the combination treatment significantly suppressed SUM149 tumor growth more than either agent alone. These findings support co-targeting IL-6 and CDK4/6 as a novel therapeutic strategy for BRCA1-mutant TNBC.
Full article
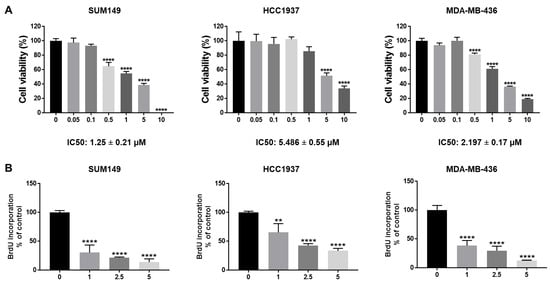
Figure 1
Open AccessArticle
Mitochondrial Fragmentation Induced by the CFTR Modulators Lumacaftor and Ivacaftor in Immortalized Cystic Fibrosis Cell Lines
by
Camila Dib, Pablo A. Iglesias González, María de los Ángeles Aguilar, Guillermo L. Taminelli, Tatiana Limpias del Valle, Nadia E. Nuñez, Analía G. Karadayian, Tomás A. Santa-Coloma and Ángel G. Valdivieso
Cells 2025, 14(20), 1601; https://doi.org/10.3390/cells14201601 - 15 Oct 2025
Abstract
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the CFTR gene, which encodes a cAMP-activated chloride channel essential for epithelial function. Beyond its canonical role, evidence suggests CFTR also influences mitochondrial function. Previous studies have identified CFTR- and Cl-dependent
[...] Read more.
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the CFTR gene, which encodes a cAMP-activated chloride channel essential for epithelial function. Beyond its canonical role, evidence suggests CFTR also influences mitochondrial function. Previous studies have identified CFTR- and Cl-dependent genes, including MTND4 and CISD1, which are downregulated in CF cells and play a critical role in mitochondrial function. CF cells exhibit altered mitochondrial complex I (mCx-I) activity and impaired electron transport chain function, although the underlying mechanisms remain unclear. In this study, the impact of the CFTR modulators lumacaftor (VX-809) and ivacaftor (VX-770) on mitochondrial morphology and function was investigated in heterozygous ΔF508/W1282X CF IB3-1 cells. Combined treatment with VX-809 (10 μM, CFTR corrector) and VX-770 (0.1 μM, CFTR potentiator) induced a fragmented mitochondrial morphology in both CF and CF expressing wt-CFTR cells, without affecting cell viability or mitochondrial membrane potential (ΔΨm). While individual treatments differentially modulated ROS production and ΔΨm, these effects were not statistically significant under combined treatment. These results highlight a previously unrecognized role for CFTR modulators in shaping mitochondrial morphology. A better understanding of these effects may reveal novel mechanisms underlying the regulation of mitochondrial structure and function.
Full article
(This article belongs to the Special Issue Mechanisms of Respiratory Diseases)
►▼
Show Figures

Figure 1
Open AccessReview
The Articular Chromatin Landscape in Osteoarthritis
by
George D. Kalliolias, Efthimia K. Basdra and Athanasios G. Papavassiliou
Cells 2025, 14(20), 1600; https://doi.org/10.3390/cells14201600 - 15 Oct 2025
Abstract
►▼
Show Figures
Recent technological breakthroughs have enabled multidimensional phenotyping, with unprecedented single-cell resolution and genome-wide coverage, across multiple osteoarthritis (OA)-relevant tissues, such as articular cartilage, synovium, infrapatellar fat pad, and subchondral bone. The majority of the single nucleotide variations (SNVs) that have been associated with
[...] Read more.
Recent technological breakthroughs have enabled multidimensional phenotyping, with unprecedented single-cell resolution and genome-wide coverage, across multiple osteoarthritis (OA)-relevant tissues, such as articular cartilage, synovium, infrapatellar fat pad, and subchondral bone. The majority of the single nucleotide variations (SNVs) that have been associated with OA are located in non-protein coding regions and confer risk for disease by altering the expression level, instead of the amino acid sequence of the gene product. These data have shaped the concept of OA as a polygenic disease, where genetic factors disrupt the chromatin landscape in disease-relevant cells, leading to aberrant expression of effector genes. Pharmacologic manipulation of the OA-driving epigenetic landscape has recently emerged as an attractive path for the development of disease-modifying drugs. Novel clustered regulatory interspaced short palindromic repeats (CRISPR)-based technologies provide opportunities for precise epigenetic editing at the desired genomic regions and may allow a targeted transcriptional regulation of disease-relevant genes in disease-relevant cells. The aim of the present narrative review is to summarize the emerging data on the role of epigenetic factors and chromatin structure as calibrators of the risk for developing OA and to discuss the opportunities and challenges arising from the use of chromatin landscape to guide drug discovery.
Full article
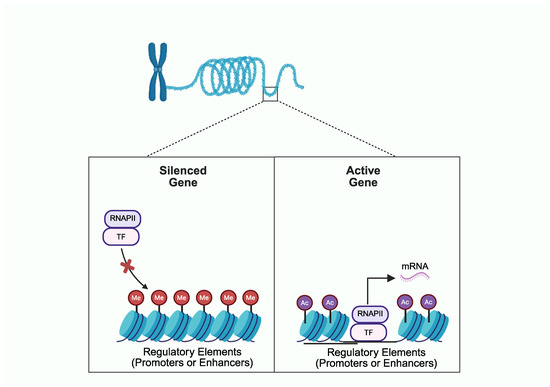
Figure 1
Open AccessArticle
Dual HDAC and PI3K Inhibitor CUDC-907 Inhibits Growth of Pleural Mesothelioma: The Impact of Cisplatin Sensitivity and Myc Expression
by
Luca Hegedüs, Silvia Qaisieh, Christian Stülpnagel, Yavar Ganjeh Khor Dezfouli, Winny Tambo, Fabian Doerr, Natalie Baldes, Dirk Theegarten, Martin Schuler, Servet Bölükbas and Balazs Hegedüs
Cells 2025, 14(20), 1599; https://doi.org/10.3390/cells14201599 - 15 Oct 2025
Abstract
Objectives: Pleural mesothelioma (PM) is a rare cancer that often develops after a decades-long latency period and confers a grim prognosis. Novel, biomarker-based therapeutic modalities are expected to improve the outcome of patients with advanced PM. CUDC-907 (fimepinostat) is a dual inhibitor that
[...] Read more.
Objectives: Pleural mesothelioma (PM) is a rare cancer that often develops after a decades-long latency period and confers a grim prognosis. Novel, biomarker-based therapeutic modalities are expected to improve the outcome of patients with advanced PM. CUDC-907 (fimepinostat) is a dual inhibitor that affects both histone deacetylases and PI3K enzymes. Its antitumor activity was described in several cancer types, but it has not yet been explored in PM. Materials and Methods: The sensitivity of 22 PM cell lines—including 18 models established in our laboratory—to cisplatin and CUDC-907 was determined using a cell viability assay. BAP1, PTEN, and c-Myc expression, as well as MYC copy number variation, were measured. The effect of combination treatment with cisplatin was assessed with cell viability, cell cycle, and 3D spheroid formation assays. Results: Most PM cell lines were sensitive to CUDC-907 treatment, and the CUDC-907 response was significantly higher in cell lines with higher c-Myc expression due to MYC copy number gain or amplification. Importantly, all cisplatin-insensitive cell lines were sensitive to CUDC-907. Combination treatment with cisplatin synergistically decreased cell viability and induced G2/M arrest or cell death. We tested cisplatin-sensitive P31WT and cisplatin resistant P31cis isogeneic pair and found that in both 2D and 3D assays the cisplatin-resistant cells showed a higher sensitivity to CUDC-907 single treatment. Combining CUDC-907 with cisplatin further decreased cell growth even in cisplatin-resistant cells. Conclusions: The majority of PM cell models are sensitive to CUDC-907, which may be a potent therapeutic agent in PM.
Full article
(This article belongs to the Special Issue Emerging Targets and Therapeutic Potential Drugs in Tumor Progression and Therapeutic Resistance)
►▼
Show Figures
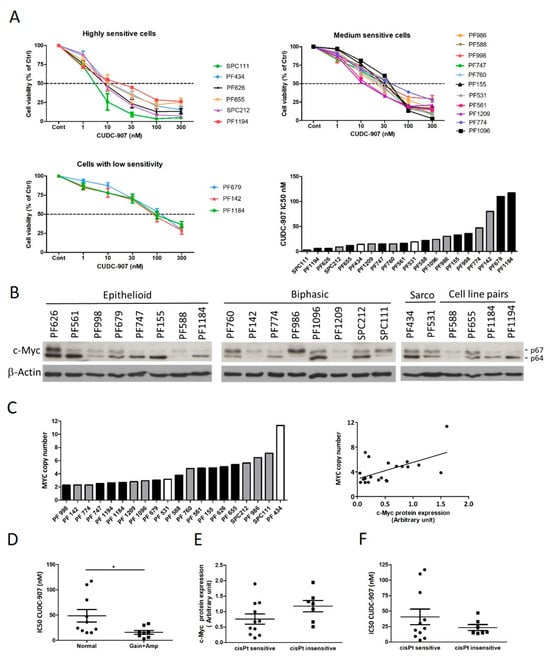
Figure 1
Open AccessArticle
House Dust Mite Nebulization Drives Alarmin and Complement Activation in a Murine Tracheal Air–Liquid Interface Culture System
by
Janti Haj Ahmad, Philip Einwohlt, Mareike Ohms, Doris Wilflingseder and Jörg Köhl
Cells 2025, 14(20), 1598; https://doi.org/10.3390/cells14201598 - 14 Oct 2025
Abstract
Air–liquid interface (ALI) cultures offer a physiologically relevant in vitro model of the airway epithelium (AE), capable of recapitulating key structural and functional features observed in vivo. In this study, we established and validated a murine ALI culture system comprising pseudostratified epithelia with
[...] Read more.
Air–liquid interface (ALI) cultures offer a physiologically relevant in vitro model of the airway epithelium (AE), capable of recapitulating key structural and functional features observed in vivo. In this study, we established and validated a murine ALI culture system comprising pseudostratified epithelia with functional tight junctions, ciliated cells and goblet cells. To assess their innate immune functions, we designed and 3D-printed an autoclavable aerosol deposition chamber, which allowed us to expose differentiated AE cultures to house dust mite (HDM) allergen. Upon HDM exposure, AE cells mounted a time-dependent innate immune response characterized by the secretion of complement component C3, the generation of its active cleavage products C3a and increased expression of C3aR and C5aR1. This was associated with increased intracellular TSLP and IL-25 production and TSLP release in AE cells. Progressive loss of tight junction integrity and reduced transepithelial electrical resistance (TEER) demonstrated epithelial susceptibility to allergen protease-induced cell damage. Together, we established a murine ALI system preserving airway epithelial architecture and a nebulization system to study innate immune activation of AE cells in response to HDM mimicking the initial phase of allergen sensitization. More generally, we described a powerful and accessible platform for studying epithelial-driven mechanisms in murine airway immune responses.
Full article
(This article belongs to the Special Issue Novel Insights into Molecular Mechanisms and Therapy of Asthma)
►▼
Show Figures
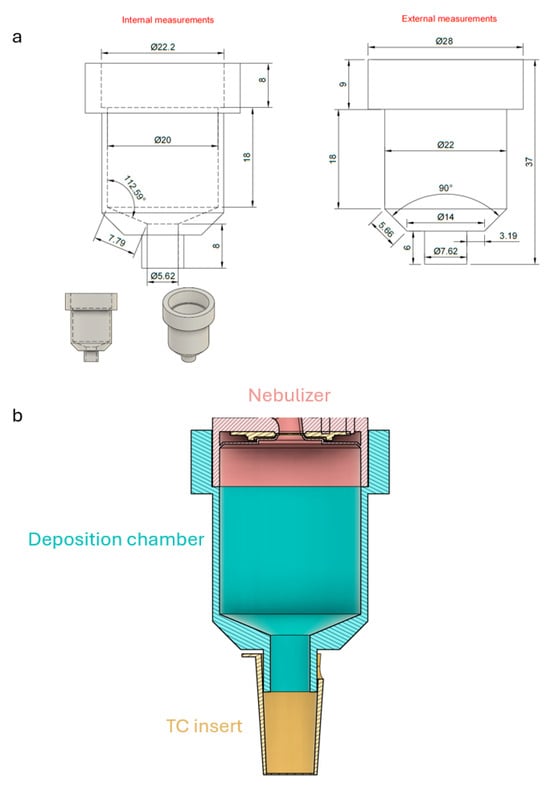
Figure 1
Open AccessReview
Elastin in the Pathogenesis of Abdominal Aortic Aneurysm
by
Dunpeng Cai and Shi-You Chen
Cells 2025, 14(20), 1597; https://doi.org/10.3390/cells14201597 - 14 Oct 2025
Abstract
Abdominal aortic aneurysms (AAAs) are progressive, life-threatening vascular disorders characterized by focal dilation of the abdominal aorta due to chronic weakening of the arterial wall. The condition often remains asymptomatic until rupture, which carries mortality rates exceeding 70–85%. Among the various etiological theories
[...] Read more.
Abdominal aortic aneurysms (AAAs) are progressive, life-threatening vascular disorders characterized by focal dilation of the abdominal aorta due to chronic weakening of the arterial wall. The condition often remains asymptomatic until rupture, which carries mortality rates exceeding 70–85%. Among the various etiological theories of AAA development, degradation of the extracellular matrix (ECM) has emerged as the most widely accepted paradigm, with the breakdown of elastin representing a central and irreversible hallmark event. Elastin, a highly cross-linked and durable structural protein, provides elasticity and recoil to the aortic wall. In human AAA specimens, reduced elastin content, impaired cross-linking, and extensive fiber fragmentation are consistently observed, while experimental studies across multiple animal models confirm that elastin degradation directly correlates with aneurysm initiation, expansion, and rupture risk. Elastin loss is driven by a complex interplay of proteolytic enzymes coupled with inflammatory cell infiltration and oxidative stress. Furthermore, elastin-derived peptides perpetuate immune cell recruitment and matrix degradation, creating a vicious cycle of wall injury. Genetic and epigenetic factors, including variants in ECM regulators and dysregulation of non-coding RNAs, further modulate elastin homeostasis in AAA pathobiology. Clinically, biomarkers of elastin turnover and elastin-targeted molecular imaging techniques are emerging as tools for risk stratification. Therapeutically, novel strategies aimed at stabilizing elastin fibers, enhancing cross-linking, or delivering drugs directly to sites of elastin damage have shown promise in preclinical models and early translational studies. In parallel, regenerative approaches employing stem cells, exosomes, and bioengineered elastin scaffolds are under development to restore structural integrity. Collectively, these advances underscore the pivotal roles of elastin not only as a structural determinant of aneurysm development but also as a diagnostic and therapeutic target. This review summarizes and integrates recent discoveries on elastin biology in AAA, with a particular emphasis on molecular mechanisms of elastin degradation and the translational potential of elastin-centered interventions for the prevention and treatment of AAA.
Full article
(This article belongs to the Special Issue Molecular Pathogenesis of Cardiovascular Diseases)
►▼
Show Figures

Figure 1
Open AccessArticle
Drosophila Models Reveal NAT Complex Roles in Heart Development and Enable Functional Validation of Congenital Heart Disease Variants
by
Jun-Yi Zhu, Hannah Seah, Hangnoh Lee, Hanhan Liu and Zhe Han
Cells 2025, 14(20), 1596; https://doi.org/10.3390/cells14201596 - 14 Oct 2025
Abstract
N-terminal acetylation, catalyzed by N-terminal acetyltransferase (NAT) complexes, is one of the most prevalent protein modifications in eukaryotic cells, yet its role in heart development remains poorly understood. Here, we use Drosophila as an in vivo platform to investigate the functions of NAT
[...] Read more.
N-terminal acetylation, catalyzed by N-terminal acetyltransferase (NAT) complexes, is one of the most prevalent protein modifications in eukaryotic cells, yet its role in heart development remains poorly understood. Here, we use Drosophila as an in vivo platform to investigate the functions of NAT complex components in cardiac development and congenital heart disease (CHD). Focusing on the NatA complex, we showed that cardiac-specific knockdown of each of its three subunits (Naa15-16, vnc, and san) led to developmental lethality, structural disorganization, fibrosis, and impaired cardiac function in Drosophila. Remarkably, human NAA16 completely rescued the cardiac defects in Naa15-16 silenced Drosophila, whereas a CHD-associated variant (NAA16-R70C) failed to do so, providing direct functional evidence of its pathogenicity. Together, these findings suggest the NatA complex as a critical regulator of heart development and provide functional validation linking variants in NatA complex genes to CHD. Further studies in mammalian models will be required to provide additional supporting evidence.
Full article
(This article belongs to the Special Issue Drosophila as a Model for Understanding Human Disease)
►▼
Show Figures

Figure 1
Open AccessReview
Advances in β-Thalassemia Gene Therapy: CRISPR/Cas Systems and Delivery Innovations
by
Hongmei Liu and Peng Zhang
Cells 2025, 14(20), 1595; https://doi.org/10.3390/cells14201595 - 14 Oct 2025
Abstract
β-thalassemia is an inherited blood disorder caused by mutations in the β-globin (HBB) gene, leading to reduced or absent β-globin production, resulting in chronic anemia. While current therapies, including blood transfusions and hematopoietic stem cell transplantation, offer symptomatic relief, they are limited by
[...] Read more.
β-thalassemia is an inherited blood disorder caused by mutations in the β-globin (HBB) gene, leading to reduced or absent β-globin production, resulting in chronic anemia. While current therapies, including blood transfusions and hematopoietic stem cell transplantation, offer symptomatic relief, they are limited by complications and their limited accessibility. CRISPR-based gene editing technologies provide new therapeutic avenues by enabling the precise correction of HBB mutations or the reactivation of fetal hemoglobin (HbF) through the targeting of regulatory elements such as BCL11A. These approaches have shown promising preclinical and clinical outcomes. However, efficient and safe delivery remains a major challenge. Viral vectors offer high efficiency but raise concerns about immunogenicity and insertional mutagenesis, whereas non-viral systems such as lipid nanoparticles and engineered exosomes offer lower toxicity and modularity but face targeting limitations. This review highlights recent progress in CRISPR-based therapies for β-thalassemia and emerging delivery strategies to enhance clinical translation.
Full article
(This article belongs to the Special Issue CRISPR-Based Genome Editing in Translational Research—Third Edition)
►▼
Show Figures
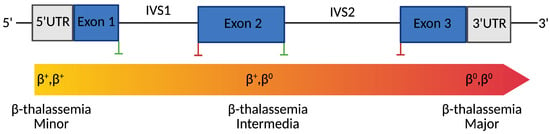
Figure 1
Open AccessReview
Crosstalk Between Inflammasome Signalling and Epithelial-Mesenchymal Transition in Cancer and Benign Disease: Mechanistic Insights, Context-Dependence, and Therapeutic Opportunities
by
Abdul L. Shakerdi, Emma Finnegan, Yin-Yin Sheng, Karlo Vidovic, Jessica M. Logan, Mark P. Ward, Sharon A. O’Toole, Cara Martin, Stavros Selemidis, Doug Brooks, John J. O’Leary and Prerna Tewari
Cells 2025, 14(20), 1594; https://doi.org/10.3390/cells14201594 - 14 Oct 2025
Abstract
Epithelial-mesenchymal transition (EMT) and inflammasome signalling are intercon-nected processes which underpin tumour progression, metastasis, and therapeutic re-sistance. Inflammasomes such as NLRP3 encourage pro-inflammatory states (IL-1β, IL-18, NF-κB) and the activation of signalling pathways like TGF-β that promote mes-enchymal traits crucial for EMT. EMT
[...] Read more.
Epithelial-mesenchymal transition (EMT) and inflammasome signalling are intercon-nected processes which underpin tumour progression, metastasis, and therapeutic re-sistance. Inflammasomes such as NLRP3 encourage pro-inflammatory states (IL-1β, IL-18, NF-κB) and the activation of signalling pathways like TGF-β that promote mes-enchymal traits crucial for EMT. EMT transcriptional programmes can then in turn modulate the inflammasome via NF-κB/TGF-β signalling, creating self-perpetuating mechanisms of cellular plasticity and dysregulated therapeutic response. We have re-viewed the mechanistic evidence for EMT–inflammasome crosstalk in cancer and discussed the potential therapeutic implications. The function of the EMT-inflammasome axis is clearly context-dependent, with the cancer type, stage, and the complexity of the tumour microenvironment heavily contributing. The crosstalk between EMT and the inflammasome is an overlooked mechanism of tumour evolution, and targeting inflammasomes like NLRP3, or their downstream signalling pathways, offers a promising therapeutic avenue, with the objective of inhibiting metastasis and overcoming drug resistance.
Full article
(This article belongs to the Special Issue Cell Migration and Invasion)
►▼
Show Figures
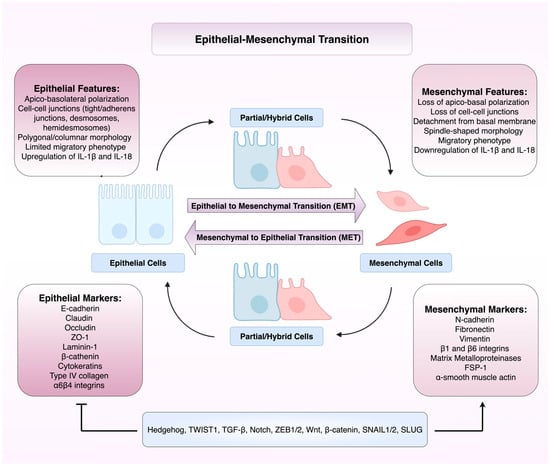
Figure 1
Open AccessArticle
Oligosaccharyltransferase Is Involved in Targeting to ER-Associated Degradation
by
Marina Shenkman, Navit Ogen-Shtern, Chaitanya Patel, Haddas Saad, Bella Groisman, Metsada Pasmanik-Chor, Sonya M. Schermann, Roman Körner and Gerardo Z. Lederkremer
Cells 2025, 14(20), 1593; https://doi.org/10.3390/cells14201593 - 14 Oct 2025
Abstract
Most membrane and secretory proteins undergo N-glycosylation, catalyzed by oligosaccharyltransferase (OST), a membrane-bound complex in the endoplasmic reticulum (ER). Proteins failing quality control are degraded via ER-associated degradation (ERAD), involving retrotranslocation to cytosolic proteasomes, or relegated to ER subdomains and eliminated via ER-phagy.
[...] Read more.
Most membrane and secretory proteins undergo N-glycosylation, catalyzed by oligosaccharyltransferase (OST), a membrane-bound complex in the endoplasmic reticulum (ER). Proteins failing quality control are degraded via ER-associated degradation (ERAD), involving retrotranslocation to cytosolic proteasomes, or relegated to ER subdomains and eliminated via ER-phagy. Using stable isotope labeling by amino acids in cell culture (SILAC) proteomics, we identified OST subunits as differential key interactors with a misfolded ER protein bait upon proteasomal inhibition, suggesting unexpected involvement in ERAD. Previous reports implied additional roles for OST subunits beyond N-glycosylation, such as quality control by ribophorin I. We tested OST engagement in glycoprotein and non-glycosylated protein ERAD; overexpression or partial knockdown of OST subunits interfered with ERAD in conditions that did not affect glycosylation. We studied the effects on model misfolded type I and II membrane-bound proteins, BACE476 and asialoglycoprotein receptor H2a, respectively, and on a soluble luminal misfolded glycoprotein, α1-antitrypsin NHK variant. OST subunits appear to participate in late ERAD stages, interacting with the E3 ligase HRD1 and facilitating retrotranslocation. Molecular dynamics simulations suggest membrane thinning by OST transmembrane domains, possibly assisting retrotranslocation via membrane distortion.
Full article
(This article belongs to the Section Intracellular and Plasma Membranes)
►▼
Show Figures

Figure 1
Open AccessReview
Evolutionary Perspective of Nonclassical MHC Class I and Innate-like T Cells Relevance in Immune Surveillance
by
Jacques Robert and Elnaz Najafi-Majd
Cells 2025, 14(20), 1592; https://doi.org/10.3390/cells14201592 - 14 Oct 2025
Abstract
Unlike conventional T cells, which express a highly diverse repertoire of dimeric αβ T-cell receptors (TCRs) restricted by classical, polymorphic MHC class I molecules (MHC-Ia), a distinct group of T cells—collectively termed “innate-like T (iT) cells”—exhibits limited TCR diversity and depends instead on
[...] Read more.
Unlike conventional T cells, which express a highly diverse repertoire of dimeric αβ T-cell receptors (TCRs) restricted by classical, polymorphic MHC class I molecules (MHC-Ia), a distinct group of T cells—collectively termed “innate-like T (iT) cells”—exhibits limited TCR diversity and depends instead on nonclassical, nonpolymorphic MHC class I molecules (MHC-Ib) for their development and function. While mounting evidence supports the role of iT cells as pivotal regulators and effectors in both innate and adaptive immune responses, many aspects of their biology remain incompletely understood. In humans, iT cells represent a significant fraction of the total T cell population, and evolutionarily conserved subsets have also been identified in other mammals and amphibians. Moreover, the expanding catalog of nonpolymorphic MHC-Ib genes and lineages—distinct from polymorphic MHC-Ia genes—across jawed vertebrate genomes suggests a broader and potentially more integral role for MHC-Ib molecules in T cell function and immune surveillance. In this review, we explore the immunological significance of MHC-Ib molecules and iT cells through an evolutionary lens, highlighting recent advances that shed light on their contributions to immune homeostasis and defense.
Full article
(This article belongs to the Special Issue T Cells in Inflammation and Cancer)
►▼
Show Figures

Figure 1

Journal Menu
► ▼ Journal Menu-
- Cells Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Analytica, Metabolites, Toxins, Molecules, Cells, Chemosensors
Application of Analytical Technology in Metabolomics
Topic Editors: Preeti Chandra, Raúl G. EnríquezDeadline: 31 October 2025
Topic in
Cells, Chemistry, IJMS, Molecules, Metabolites
Bioactive Compounds and Therapeutics: Molecular Aspects, Metabolic Profiles, and Omics Studies 2nd Edition
Topic Editors: Michele Costanzo, Giovanni N. Roviello, Armando CeveniniDeadline: 20 November 2025
Topic in
Animals, Cells, Life, Veterinary Sciences
Application of Animal Models: From Physiology to Pathology
Topic Editors: Juan Carlos Illera del Portal, Sara Cáceres Ramos, Felisbina Luisa QueirogaDeadline: 20 December 2025
Topic in
Biology, Biomolecules, Cancers, Cells, IJMS
RNAs and Phase Separation Phenomena
Topic Editors: Ana Lúcia Leitão, Afshin Beheshti, Francisco J. EnguitaDeadline: 31 December 2025

Conferences
Special Issues
Special Issue in
Cells
Sperm Biology and Reproductive Health—Second Edition
Guest Editors: Yi-Xun Liu, Shou-Long DengDeadline: 20 October 2025
Special Issue in
Cells
Therapeutic Targets in Glioblastoma
Guest Editors: Candice Mazewski, Frank EckerdtDeadline: 20 October 2025
Special Issue in
Cells
Immune Response in HIV Infection, Pathogenesis and Persistence
Guest Editor: Suvadip MallickDeadline: 20 October 2025
Special Issue in
Cells
Molecular Mechanisms of Autism Spectrum Disorder
Guest Editors: Tanya Gandhi, Charles LeeDeadline: 20 October 2025
Topical Collections
Topical Collection in
Cells
Insulin-Like Growth Factors in Development, Cancers and Aging
Collection Editor: Haim Werner
Topical Collection in
Cells
Advances in Epithelial-Mesenchymal Transition (EMT)
Collection Editors: Oriana Trubiani, Francesca Diomede, Jacopo Pizzicannella, Guya Diletta Marconi
Topical Collection in
Cells
How Perinatal Stress Affects Brain Plasticity in Ontogenesis
Collection Editors: Alexander V. Arutjunyan, Natalia V. Gulyaeva









