Drosophila Models Reveal NAT Complex Roles in Heart Development and Enable Functional Validation of Congenital Heart Disease Variants
Abstract
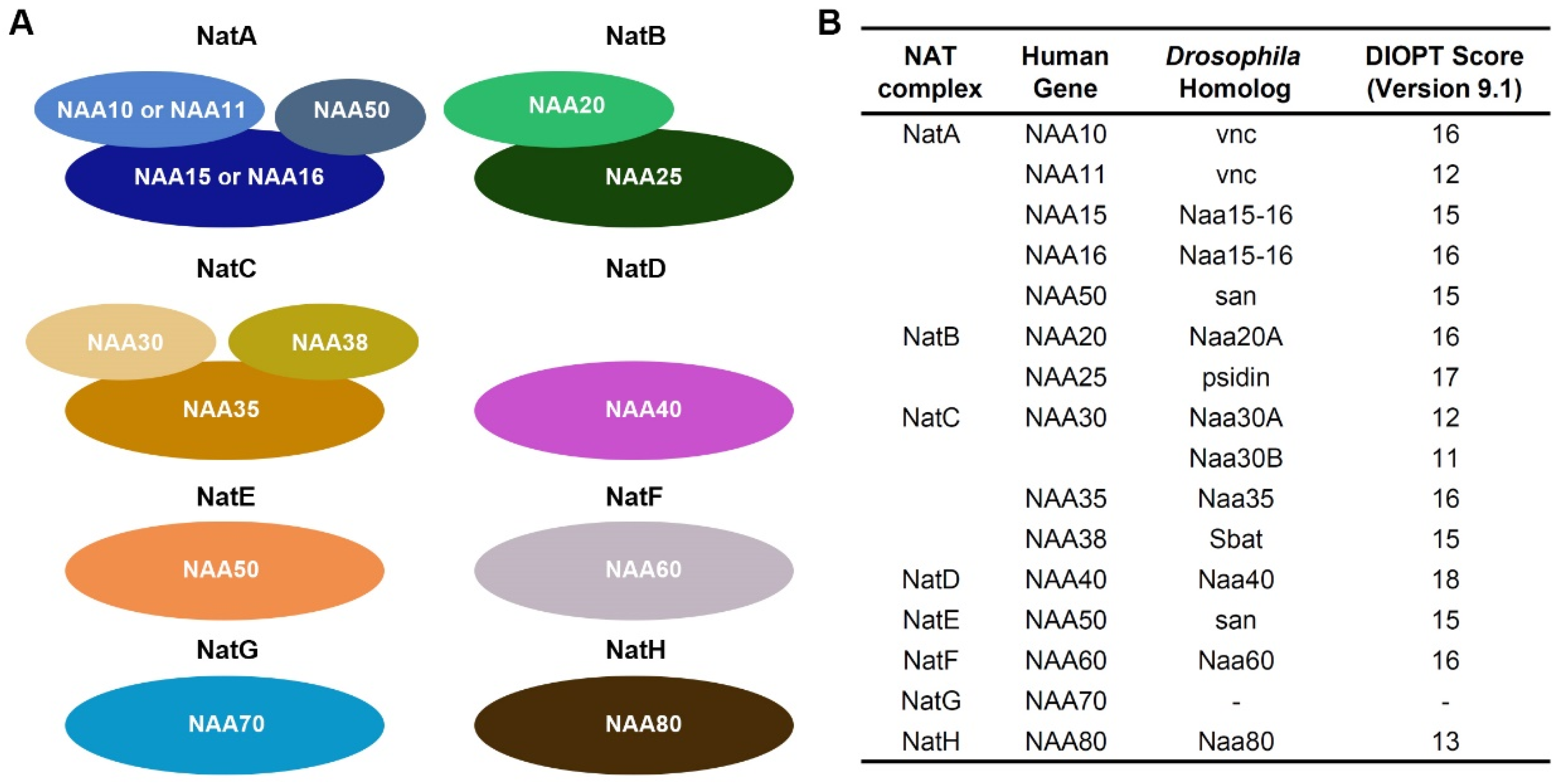
1. Introduction
2. Results
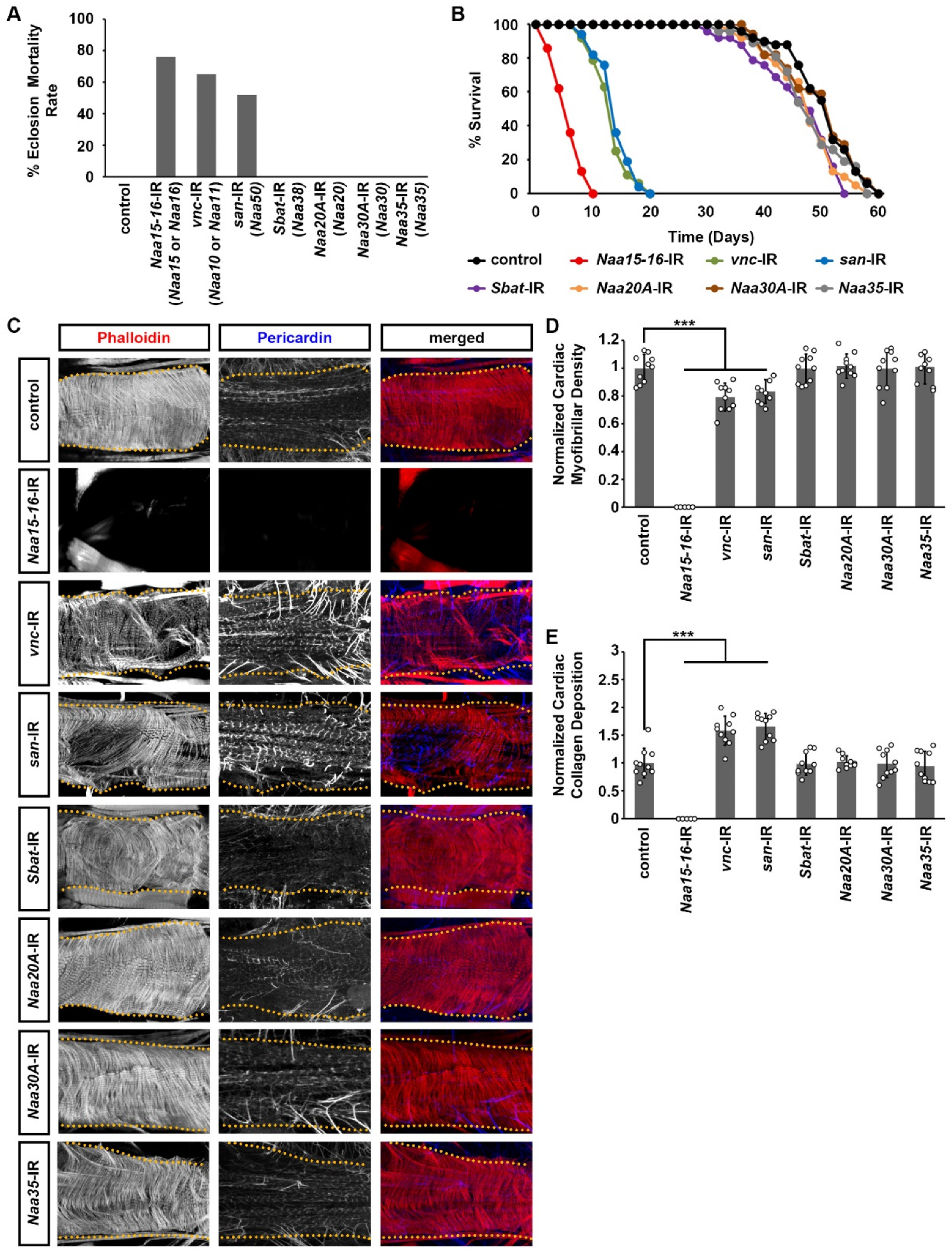
2.1. Silencing NatA Complex Components Naa15-16, Vnc, or San Impaired Drosophila Survival
2.2. Silencing NatA Complex Components Naa15-16, Vnc, or San Induced Cardiac Structural and Functional Defects in Adult Drosophila
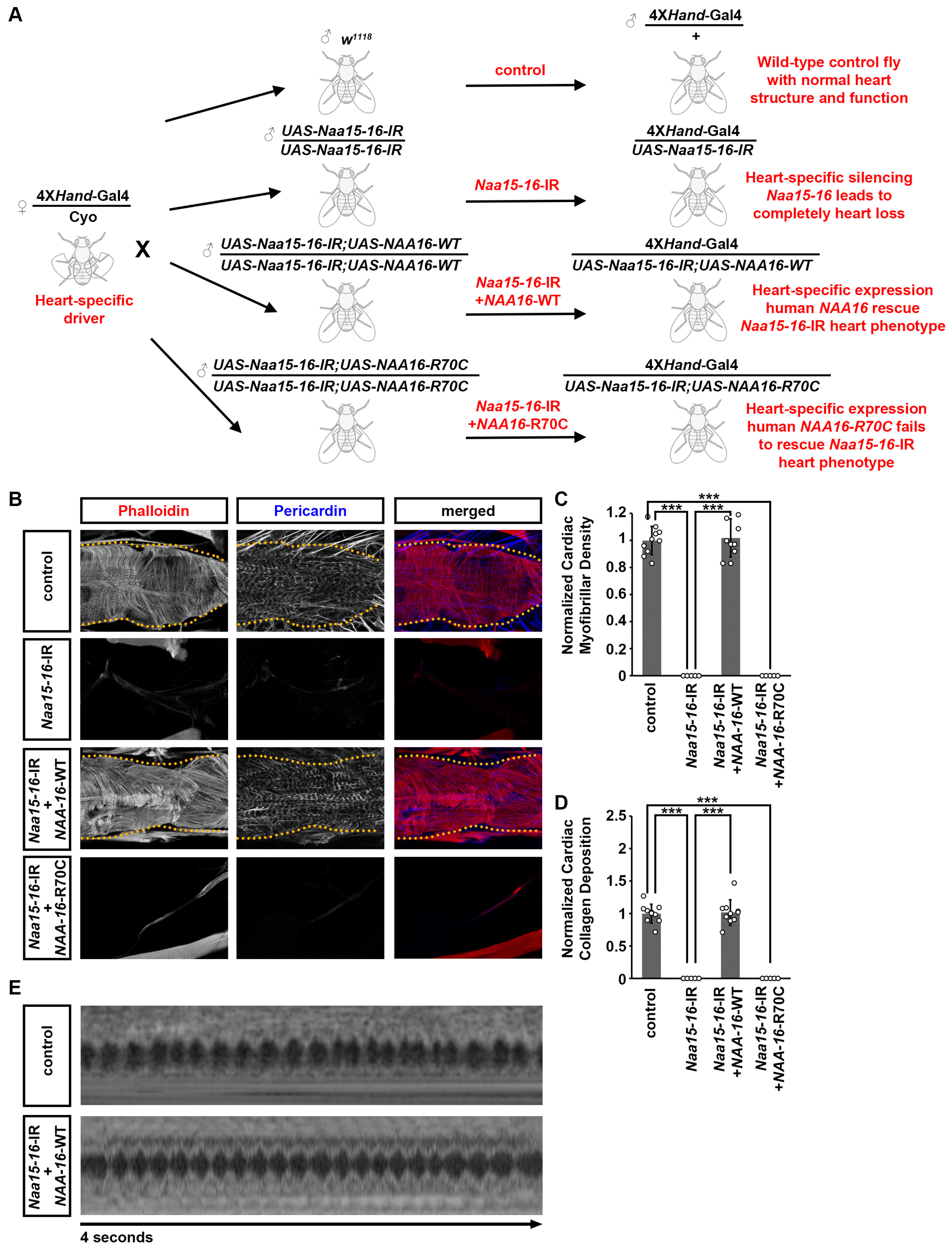
2.3. Expression of Wild-Type Human NAA16 and Its CHD-Associated Variant in the Drosophila Heart Did Not Cause Any Detectable Structural and Functional Cardiac Defects
2.4. Cardiac Structural and Functional Defects Caused by Naa15-16 Silencing in Drosophila Can Be Rescued by Expression of Wild-Type but Not Mutant Human NAA16
3. Discussion
3.1. Drosophila as a High-Throughput Platform for Identifying Genes Required for Heart Development
3.2. The NatA Complex Is Required for Heart Development and Function
3.3. Drosophila Enables Variant-Level Validation
3.4. Potentials of Drosophila as a Preclinical Model for CHD-Linked Ogden Syndrome and Therapeutic Testing
4. Materials and Methods
4.1. Drosophila Lines
4.2. Fly Quantitative RT-PCR Analysis
4.3. Lethality at Eclosion
4.4. Adult Drosophila Survival Assay
4.5. Immunochemistry
4.6. Heart Structural Analysis and Quantitation
4.7. Optical Coherence Tomography (OCT)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varland, S.; Osberg, C.; Arnesen, T. N-Terminal Modifications of Cellular Proteins: The Enzymes Involved, Their Substrate Specificities and Biological Effects. Proteomics 2015, 15, 2385–2401. [Google Scholar] [CrossRef]
- Varland, S.; Silva, R.D.; Kjosås, I.; Faustino, A.; Bogaert, A.; Billmann, M.; Boukhatmi, H.; Kellen, B.; Costanzo, M.; Drazic, A.; et al. N-Terminal Acetylation Shields Proteins from Degradation and Promotes Age-Dependent Motility and Longevity. Nat. Commun. 2023, 14, 6774. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Marmorstein, R. Protein N-Terminal Acetylation: Structural Basis, Mechanism, Versatility, and Regulation. Trends Biochem. Sci. 2021, 46, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Øye, H.; Lundekvam, M.; Caiella, A.; Hellesvik, M.; Arnesen, T. Protein N-Terminal Modifications: Molecular Machineries and Biological Implications. Trends Biochem. Sci. 2025, 50, 290–310. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Mun, S.-H.; Lee, C.-S.; Hwang, C.-S. Control of Protein Degradation by N-Terminal Acetylation and the N-End Rule Pathway. Exp. Mol. Med. 2018, 50, 1–8. [Google Scholar] [CrossRef]
- McTiernan, N.; Kjosås, I.; Arnesen, T. Illuminating the Impact of N-Terminal Acetylation: From Protein to Physiology. Nat. Commun. 2025, 16, 703. [Google Scholar] [CrossRef] [PubMed]
- Ree, R.; Varland, S.; Arnesen, T. Spotlight on Protein N-Terminal Acetylation. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef]
- Koufaris, C.; Demetriadou, C.; Nicolaidou, V.; Kirmizis, A. Bioinformatic Analysis Reveals the Association of Human N-Terminal Acetyltransferase Complexes with Distinct Transcriptional and Post-Transcriptional Processes. Biochem. Genet. 2024, 63, 2987–3008. [Google Scholar] [CrossRef]
- Deng, S.; Magin, R.S.; Wei, X.; Pan, B.; Petersson, E.J.; Marmorstein, R. Structure and Mechanism of Acetylation by the N-Terminal Dual Enzyme NatA/Naa50 Complex. Structure 2019, 27, 1057–1070.e4. [Google Scholar] [CrossRef]
- Eiyama, A.; Okamoto, K. Protein N-Terminal Acetylation by the NatA Complex Is Critical for Selective Mitochondrial Degradation. J. Biol. Chem. 2015, 290, 25034–25044. [Google Scholar] [CrossRef]
- Jin, S.C.; Homsy, J.; Zaidi, S.; Lu, Q.; Morton, S.; DePalma, S.R.; Zeng, X.; Qi, H.; Chang, W.; Sierant, M.C.; et al. Contribution of Rare Inherited and de Novo Variants in 2,871 Congenital Heart Disease Probands. Nat. Genet. 2017, 49, 1593–1601. [Google Scholar] [CrossRef]
- Homsy, J.; Zaidi, S.; Shen, Y.; Ware, J.S.; Samocha, K.E.; Karczewski, K.J.; DePalma, S.R.; McKean, D.; Wakimoto, H.; Gorham, J.; et al. De Novo Mutations in Congenital Heart Disease with Neurodevelopmental and Other Congenital Anomalies. Science 2015, 350, 1262–1266. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; Choi, M.; Wakimoto, H.; Ma, L.; Jiang, J.; Overton, J.D.; Romano-Adesman, A.; Bjornson, R.D.; Breitbart, R.E.; Brown, K.K.; et al. De Novo Mutations in Histone-Modifying Genes in Congenital Heart Disease. Nature 2013, 498, 220–223. [Google Scholar] [CrossRef]
- Yoshinaga, D.; Craven, I.; Feng, R.; Prondzynski, M.; Shani, K.; Tharani, Y.; Mayourian, J.; Joseph, M.; Walker, D.; Bortolin, R.H.; et al. Dysregulation of N-Terminal Acetylation Causes Cardiac Arrhythmia and Cardiomyopathy. Nat. Commun. 2025, 16, 3604. [Google Scholar] [CrossRef]
- Ward, T.; Tai, W.; Morton, S.; Impens, F.; Van Damme, P.; Van Haver, D.; Timmerman, E.; Venturini, G.; Zhang, K.; Jang, M.Y.; et al. Mechanisms of Congenital Heart Disease Caused by NAA15 Haploinsufficiency. Circ. Res. 2021, 128, 1156–1169. [Google Scholar] [CrossRef]
- Li, F.; Wang, W.; Li, Y.; Liu, X.; Zhu, Z.; Tang, J.; Hu, Y. NAA10 Gene Related Ogden Syndrome with Obstructive Hypertrophic Cardiomyopathy: A Rare Case Report. Medicine 2024, 103, e36034. [Google Scholar] [CrossRef]
- Wu, Y.; Lyon, G.J. NAA10-Related Syndrome. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Makwana, R.; Patel, R.; O’Neill, R.; Marchi, E.; Lyon, G.J. The Cardiovascular Manifestations and Management Recommendations for Ogden Syndrome. Pediatr. Cardiol. 2025. [Google Scholar] [CrossRef]
- Varland, S.; Brønstad, K.M.; Skinner, S.J.; Arnesen, T. A Nonsense Variant in the N-Terminal Acetyltransferase NAA30 May Be Associated with Global Developmental Delay and Tracheal Cleft. Am. J. Med. Genet. A 2023, 191, 2402–2410. [Google Scholar] [CrossRef]
- Forrest, I.S.; Chaudhary, K.; Vy, H.M.T.; Petrazzini, B.O.; Bafna, S.; Jordan, D.M.; Rocheleau, G.; Loos, R.J.F.; Nadkarni, G.N.; Cho, J.H.; et al. Population-Based Penetrance of Deleterious Clinical Variants. JAMA 2022, 327, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.B.; Kurki, M.I.; Foley, C.N.; Mechakra, A.; Chen, C.-Y.; Marshall, E.; Wilk, J.B.; Biogen Biobank Team; Chahine, M.; Chevalier, P.; et al. Genetic Associations of Protein-Coding Variants in Human Disease. Nature 2022, 603, 95–102. [Google Scholar] [CrossRef]
- Souidi, A.; Jagla, K. Drosophila Heart as a Model for Cardiac Development and Diseases. Cells 2021, 10, 3078. [Google Scholar] [CrossRef] [PubMed]
- Taghli-Lamallem, O.; Plantié, E.; Jagla, K. Drosophila in the Heart of Understanding Cardiac Diseases: Modeling Channelopathies and Cardiomyopathies in the Fruitfly. J. Cardiovasc. Dev. Dis. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.M. Conserved Signaling Mechanisms in Drosophila Heart Development. Dev. Dyn. 2017, 246, 641–656. [Google Scholar] [CrossRef]
- Curtis, N.J.; Ringo, J.M.; Dowse, H.B. Morphology of the Pupal Heart, Adult Heart, and Associated Tissues in the Fruit Fly, Drosophila Melanogaster. J. Morphol. 1999, 240, 225–235. [Google Scholar] [CrossRef]
- Rotstein, B.; Paululat, A. On the Morphology of the Drosophila Heart. J. Cardiovasc. Dev. Dis. 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-Y.; Fu, Y.; Nettleton, M.; Richman, A.; Han, Z. High Throughput in Vivo Functional Validation of Candidate Congenital Heart Disease Genes in Drosophila. eLife 2017, 6, e22617. [Google Scholar] [CrossRef]
- Alabdi, L.; Altuwaijri, N.; Zhu, J.-Y.; Efthymiou, S.; Lee, H.; Duan, J.; Salem, I.; Yu, P.; Abdullah, N.L.; Alzahrani, F.; et al. SLK Is Mutated in Individuals with a Neurodevelopmental Disorder. eBioMedicine 2025, 116, 105725. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Hannan, S.B.; Dräger, N.M.; Vereshchagina, N.; Krahl, A.-C.; Fu, Y.; Elliott, C.J.H.; Han, Z.; Jahn, T.R.; Rasse, T.M. Autophagy Inhibition Rescues Structural and Functional Defects Caused by the Loss of Mitochondrial Chaperone Hsc70-5 in Drosophila. Autophagy 2021, 17, 3160–3174. [Google Scholar] [CrossRef]
- Huang, X.; Fu, Y.; Lee, H.; Zhao, Y.; Yang, W.; van de Leemput, J.; Han, Z. Single-Cell Profiling of the Developing Embryonic Heart in Drosophila. Development 2023, 150, dev201936. [Google Scholar] [CrossRef]
- Duffy, J.B. GAL4 System in Drosophila: A Fly Geneticist’s Swiss Army Knife. Genesis 2002, 34, 1–15. [Google Scholar] [CrossRef]
- Vaughan, L.; Marley, R.; Miellet, S.; Hartley, P.S. The Impact of SPARC on Age-Related Cardiac Dysfunction and Fibrosis in Drosophila. Exp. Gerontol. 2018, 109, 59–66. [Google Scholar] [CrossRef]
- Zheng, W.; Ocorr, K.; Tatar, M. Extra-Cellular Matrix Induced by Steroids and Aging through a G-Protein Coupled Receptor in a Drosophila Model of Renal Fibrosis. Dis. Model. Mech. 2020, 13, dmm041301. [Google Scholar] [CrossRef]
- Choma, M.A.; Izatt, S.D.; Wessells, R.J.; Bodmer, R.; Izatt, J.A. Images in Cardiovascular Medicine: In Vivo Imaging of the Adult Drosophila Melanogaster Heart with Real-Time Optical Coherence Tomography. Circulation 2006, 114, e35–e36. [Google Scholar] [CrossRef] [PubMed]
- Yelbuz, T.M.; Choma, M.A.; Thrane, L.; Kirby, M.L.; Izatt, J.A. Optical Coherence Tomography: A New High-Resolution Imaging Technology to Study Cardiac Development in Chick Embryos. Circulation 2002, 106, 2771–2774. [Google Scholar] [CrossRef] [PubMed]
- Zeytuni, N.; Zarivach, R. Structural and Functional Discussion of the Tetra-Trico-Peptide Repeat, a Protein Interaction Module. Structure 2012, 20, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.P.; Franklin, R.C.G.; Béland, M.J.; Spicer, D.E.; Colan, S.D.; Walters, H.L., 3rd; Bailliard, F.; Houyel, L.; St Louis, J.D.; Lopez, L.; et al. Nomenclature for Pediatric and Congenital Cardiac Care: Unification of Clinical and Administrative Nomenclature-the 2021 International Paediatric and Congenital Cardiac Code (IPCCC) and the Eleventh Revision of the International Classification of Diseases (ICD-11). World J. Pediatr. Congenit. Heart Surg. 2021, 12, E1–E18. [Google Scholar]
- Zhu, J.-Y.; Fu, Y.; Richman, A.; Zhao, Z.; Ray, P.E.; Han, Z. A Personalized Model of COQ2 Nephropathy Rescued by the Wild-Type COQ2 Allele or Dietary Coenzyme Q10 Supplementation. J. Am. Soc. Nephrol. 2017, 28, 2607–2617. [Google Scholar] [CrossRef]
- Zhao, F.; Zhu, J.-Y.; Richman, A.; Fu, Y.; Huang, W.; Chen, N.; Pan, X.; Yi, C.; Ding, X.; Wang, S.; et al. Mutations in NUP160 Are Implicated in Steroid-Resistant Nephrotic Syndrome. J. Am. Soc. Nephrol. 2019, 30, 840–853. [Google Scholar] [CrossRef]
- Olson, E.N. Gene Regulatory Networks in the Evolution and Development of the Heart. Science 2006, 313, 1922–1927. [Google Scholar] [CrossRef]
- Cui, M.; Wang, Z.; Bassel-Duby, R.; Olson, E.N. Genetic and Epigenetic Regulation of Cardiomyocytes in Development, Regeneration and Disease. Development 2018, 145, dev171983. [Google Scholar] [CrossRef]
- Cannon, L.; Zambon, A.C.; Cammarato, A.; Zhang, Z.; Vogler, G.; Munoz, M.; Taylor, E.; Cartry, J.; Bernstein, S.I.; Melov, S.; et al. Expression Patterns of Cardiac Aging in Drosophila. Aging Cell 2017, 16, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, R. Drosophila as a Model for Congenital Heart Disease and Arrhythmia. J. Card. Fail. 2006, 12, S153. [Google Scholar] [CrossRef]
- Dorn, G.W., 2nd; Clark, C.F.; Eschenbacher, W.H.; Kang, M.-Y.; Engelhard, J.T.; Warner, S.J.; Matkovich, S.J.; Jowdy, C.C. MARF and Opa1 Control Mitochondrial and Cardiac Function in Drosophila. Circ. Res. 2011, 108, 12–17. [Google Scholar] [CrossRef]
- Smeir, E.; Leberer, S.; Blumrich, A.; Vogler, G.; Vasiliades, A.; Dresen, S.; Jaeger, C.; Gloaguen, Y.; Klose, C.; Beule, D.; et al. Depletion of Cardiac Cardiolipin Synthase Alters Systolic and Diastolic Function. iScience 2021, 24, 103314. [Google Scholar] [CrossRef]
- Birker, K.; Ge, S.; Kirkland, N.J.; Theis, J.L.; Marchant, J.; Fogarty, Z.C.; Missinato, M.A.; Kalvakuri, S.; Grossfeld, P.; Engler, A.J.; et al. Mitochondrial MICOS Complex Genes, Implicated in Hypoplastic Left Heart Syndrome, Maintain Cardiac Contractility and Actomyosin Integrity. eLife 2023, 12, e83385. [Google Scholar] [CrossRef]
- Wessells, R.J.; Bodmer, R. Screening Assays for Heart Function Mutants in Drosophila. Biotechniques 2004, 37, 58–60, 62, 64 passim. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.E.; Tripoli, B.A.; Schoborg, T.A.; Smyth, J.T. Analysis of Drosophila Cardiac Hypertrophy by Microcomputerized Tomography for Genetic Dissection of Heart Growth Mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H296–H309. [Google Scholar] [CrossRef]
- Gomez, A.; Gonzalez, S.; Oke, A.; Luo, J.; Duong, J.B.; Esquerra, R.M.; Zimmerman, T.; Capponi, S.; Fung, J.C.; Nystul, T.G. A High-Throughput Method for Quantifying Drosophila Fecundity. Toxics 2024, 12, 658. [Google Scholar] [CrossRef]
- van de Kooij, B.; de Vries, E.; Rooswinkel, R.W.; Janssen, G.M.C.; Kok, F.K.; van Veelen, P.A.; Borst, J. N-Terminal Acetylation Can Stabilize Proteins Independent of Their Ubiquitination. Sci. Rep. 2023, 13, 5333. [Google Scholar] [CrossRef]
- François, C.M.; Pihl, T.; Dunoyer de Segonzac, M.; Hérault, C.; Hudry, B. Metabolic Regulation of Proteome Stability via N-Terminal Acetylation Controls Male Germline Stem Cell Differentiation and Reproduction. Nat. Commun. 2023, 14, 6737. [Google Scholar] [CrossRef]
- Shishido, A.; Morisada, N.; Tominaga, K.; Uemura, H.; Haruna, A.; Hanafusa, H.; Nozu, K.; Iijima, K. A Japanese Boy with NAA10-Related Syndrome and Hypertrophic Cardiomyopathy. Hum. Genome Var. 2020, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, M.; Brady, L.; Tarnopolsky, M.; Ronen, G.M. Clinical Manifestations Associated with the N-Terminal-Acetyltransferase NAA10 Gene Mutation in a Girl: Ogden Syndrome. Pediatr. Neurol. 2017, 76, 82–85. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; van de Leemput, J.; Han, Z. Promoting Mitochondrial Dynamics by Inhibiting the PINK1/PRKN Pathway to Relieve Diabetic Nephropathy. Dis. Model. Mech. 2024, 17, dmm050471. [Google Scholar] [CrossRef] [PubMed]
- Kaun, K.R.; Devineni, A.V.; Heberlein, U. Drosophila Melanogaster as a Model to Study Drug Addiction. Hum. Genet. 2012, 131, 959–975. [Google Scholar] [CrossRef]
- Kruger, L.; Denton, T.T. A Standardized Method for Incorporation of Drugs into Food for Use with Drosophila Melanogaster. Anal. Biochem. 2020, 599, 113740. [Google Scholar] [CrossRef]
- DeLoriea, J.; Millet-Boureima, C.; Gamberi, C. Protocol to Build a Drug-Testing Pipeline Using Large Populations of Drosophila Melanogaster. STAR Protoc. 2023, 4, 102747. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; van de Leemput, J.; Han, Z. Distinct Roles of COMPASS Subunits to Drosophila Heart Development. Biol. Open 2024, 13, bio061736. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Lee, H.; Huang, X.; van de Leemput, J.; Han, Z. Distinct Roles for COMPASS Core Subunits Set1, Trx, and Trr in the Epigenetic Regulation of Drosophila Heart Development. Int. J. Mol. Sci. 2023, 24, 17314. [Google Scholar] [CrossRef]
- Spletter, M.L.; Barz, C.; Yeroslaviz, A.; Zhang, X.; Lemke, S.B.; Bonnard, A.; Brunner, E.; Cardone, G.; Basler, K.; Habermann, B.H.; et al. A Transcriptomics Resource Reveals a Transcriptional Transition during Ordered Sarcomere Morphogenesis in Flight Muscle. eLife 2018, 7, e34058. [Google Scholar] [CrossRef] [PubMed]


| Patient ID | Cardiac Category | Cardiac Diagnoses | Human Gene | AA Changes | Effect |
|---|---|---|---|---|---|
| 1-01119 | CTD | Atrial septal defect, secundum; Transposition D-loop with ventricular septal defect; Usual coronary arteries in D-loop TGA | NAA16 | R70C | Missense |
| 1-01943 | TOF | Tetralogy of Fallot | NAA16 | L765fs | Frameshift |
| 1-06626 | TOF | LSVC to coronary sinus; Tetralogy of Fallot with pulmonary atresia | NAA16 | E630fs | Frameshift |
| 1-00455 | HTX | Aortic valve position relative to the pulmonary valve, anterior; Atrial inversion; Congenital tricuspid valve abnormality; D-looped ventricles; Dextrocardia; DORV, ventricular defect uncommitted; Heterotaxy; Hypoplastic right ventricle; Hypoplastic tricuspid valve; IDD; Left superior vena cava to right atrium; Pulmonary stenosis, bilateral branch pulmonary artery; Pulmonary stenosis, valvar; Right aortic arch; Right superior vena cava absent; Totally anomalous pulmonary venous return, mixed | NAA15 | K336fs | Frameshift |
| 1-00141 | TOF | Pulmonary stenosis, valvar; Single left coronary; Tetralogy of Fallot | NAA15 | S761X | Nonsense |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.-Y.; Seah, H.; Lee, H.; Liu, H.; Han, Z. Drosophila Models Reveal NAT Complex Roles in Heart Development and Enable Functional Validation of Congenital Heart Disease Variants. Cells 2025, 14, 1596. https://doi.org/10.3390/cells14201596
Zhu J-Y, Seah H, Lee H, Liu H, Han Z. Drosophila Models Reveal NAT Complex Roles in Heart Development and Enable Functional Validation of Congenital Heart Disease Variants. Cells. 2025; 14(20):1596. https://doi.org/10.3390/cells14201596
Chicago/Turabian StyleZhu, Jun-Yi, Hannah Seah, Hangnoh Lee, Hanhan Liu, and Zhe Han. 2025. "Drosophila Models Reveal NAT Complex Roles in Heart Development and Enable Functional Validation of Congenital Heart Disease Variants" Cells 14, no. 20: 1596. https://doi.org/10.3390/cells14201596
APA StyleZhu, J.-Y., Seah, H., Lee, H., Liu, H., & Han, Z. (2025). Drosophila Models Reveal NAT Complex Roles in Heart Development and Enable Functional Validation of Congenital Heart Disease Variants. Cells, 14(20), 1596. https://doi.org/10.3390/cells14201596






