Elastin in the Pathogenesis of Abdominal Aortic Aneurysm
Abstract
1. Clinical Background, Epidemiology, and Current Management of AAA
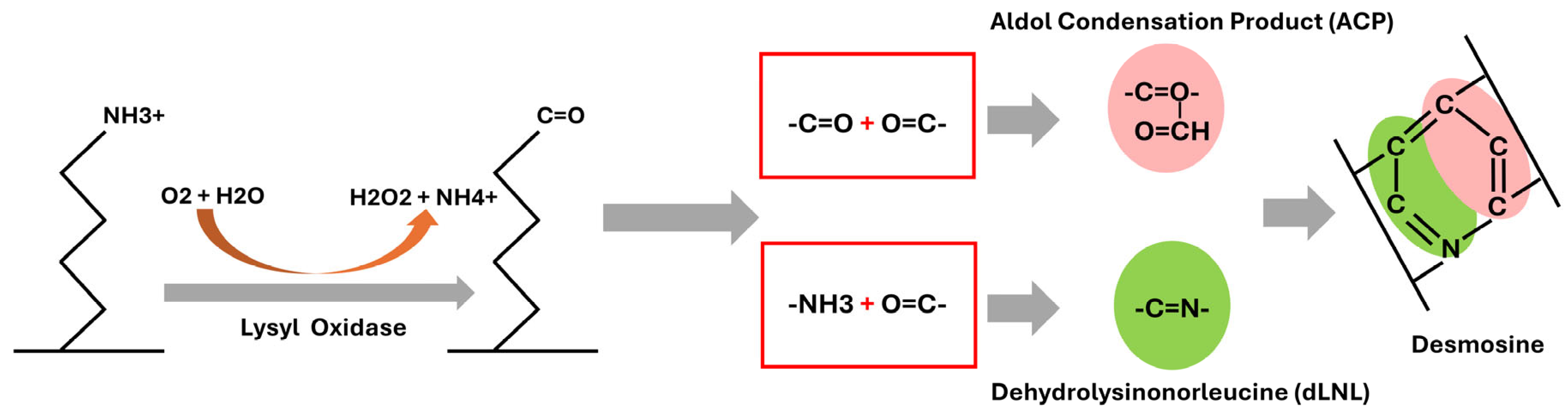
2. Elastin Structure and Cross-Linking in the Aortic Wall
3. Molecular Mechanism Involved in Elastin Degradation in AAA
3.1. Protease-Driven Elastinolysis in AAA
3.2. VSMC Loss–Driven Elastin Depletion
3.3. Inflammatory Orchestration of Elastinolysis in AAA
3.4. Links Between AAA Risk Factors and Elastin Degradation
4. Current and Potential Novel AAA Therapy Targeting Elastin
4.1. Elastin Stabilization (“Tanning” the Elastic Lamellae)
4.2. Elastin-Targeted Drug Delivery (Active Targeting to Sites of Damage)
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acosta, S.; Ogren, M.; Bengtsson, H.; Bergqvist, D.; Lindblad, B.; Zdanowski, Z. Increasing incidence of ruptured abdominal aortic aneurysm: A population-based study. J. Vasc. Surg. 2006, 44, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, H.; Bergqvist, D. Ruptured abdominal aortic aneurysm: A population-based study. J. Vasc. Surg. 1993, 18, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.T.; Laukontaus, S.J.; Kantonen, I.; Venermo, M. Population-based study of ruptured abdominal aortic aneurysm. Br. J. Surg. 2016, 103, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Scaife, M.; Giannakopoulos, T.; Al-Khoury, G.E.; Chaer, R.A.; Avgerinos, E.D. Contemporary Applications of Ultrasound in Abdominal Aortic Aneurysm Management. Front. Surg. 2016, 3, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.W.; Rutherford, R.B.; Tilson, M.D.; Shah, D.M.; Hollier, L.; Stanley, J.C. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J. Vasc. Surg. 1991, 13, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Abdominal Aortic Aneurysm: Diagnosis and Management; Clinical Guidelines; National Institute for Health and Care Excellence: London, UK, 2020.
- Crane, C. Arteriosclerotic aneurysm of the abdominal aorta; some pathological and clinical correlations. N. Engl. J. Med. 1955, 253, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Du, X.; Chen, Y.Q.; Tan, Y.S.; Liu, L. Potential Medication Treatment According to Pathological Mechanisms in Abdominal Aortic Aneurysm. J. Cardiovasc. Pharmacol. 2018, 71, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Moxon, J.V.; Parr, A.; Emeto, T.I.; Walker, P.; Norman, P.E.; Golledge, J. Diagnosis and monitoring of abdominal aortic aneurysm: Current status and future prospects. Curr. Probl. Cardiol. 2010, 35, 512–548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brady, A.R.; Thompson, S.G.; Fowkes, F.G.; Greenhalgh, R.M.; Powell, J.T.; on behalf of the UK Small Aneurysm Trial Participants. Abdominal aortic aneurysm expansion: Risk factors and time intervals for surveillance. Circulation 2004, 110, 16–21. [Google Scholar] [CrossRef] [PubMed]
- De Haro, J.; Acin, F.; Bleda, S.; Varela, C.; Medina, F.J.; Esparza, L. Prediction of asymptomatic abdominal aortic aneurysm expansion by means of rate of variation of C-reactive protein plasma levels. J. Vasc. Surg. 2012, 56, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Jormsjo-Pettersson, S.; Brady, A.R.; Deguchi, H.; Hamsten, A.; Powell, J.T. Genotype-phenotype relationships in an investigation of the role of proteases in abdominal aortic aneurysm expansion. Br. J. Surg. 2005, 92, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hussien, H.; Hanemaaijer, R.; Verheijen, J.H.; van Bockel, J.H.; Geelkerken, R.H.; Lindeman, J.H. Doxycycline therapy for abdominal aneurysm: Improved proteolytic balance through reduced neutrophil content. J. Vasc. Surg. 2009, 49, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.T.; Matsumura, J.; Curci, J.; McBride, R.; Blackwelder, W.C.; Liu, X.; Larson, L.; Terrin, M.L.; N-TA3CT Investigators. Non-invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (N-TA(3)CT): Design of a Phase IIb, placebo-controlled, double-blind, randomized clinical trial of doxycycline for the reduction of growth of small abdominal aortic aneurysm. Contemp. Clin. Trials 2016, 48, 91–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hackmann, A.E.; Rubin, B.G.; Sanchez, L.A.; Geraghty, P.A.; Thompson, R.W.; Curci, J.A. A randomized, placebo-controlled trial of doxycycline after endoluminal aneurysm repair. J. Vasc. Surg. 2008, 48, 519–526; discussion 526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, M.; Chen, C.; Cao, Y.; Qi, R. Inhibitory effects of doxycycline on the onset and progression of abdominal aortic aneurysm and its related mechanisms. Eur. J. Pharmacol. 2017, 811, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, H.N.; Canner, J.K.; Malas, M. Effect of Beta Blockers on Mortality After Open Repair of Abdominal Aortic Aneurysm. Ann. Surg. 2018, 267, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Brooke, B.S.; Dominici, F.; Makary, M.A.; Pronovost, P.J. Use of beta-blockers during aortic aneurysm repair: Bridging the gap between evidence and effective practice. Health Aff. 2009, 28, 1199–1209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hofmann Bowman, M.A.; Eagle, K.A.; Milewicz, D.M. Update on Clinical Trials of Losartan with and Without beta-Blockers to Block Aneurysm Growth in Patients with Marfan Syndrome: A Review. JAMA Cardiol. 2019, 4, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Kertai, M.D.; Boersma, E.; Westerhout, C.M.; Klein, J.; Van Urk, H.; Bax, J.J.; Roelandt, J.R.; Poldermans, D. A combination of statins and beta-blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, K.E.; Meijer, C.A.; Hinnen, J.W.; Dalman, R.L.; Xu, B.; Hamming, J.F.; Lindeman, J.H. ACE inhibitors potently reduce vascular inflammation, results of an open proof-of-concept study in the abdominal aortic aneurysm. PLoS ONE 2014, 9, e111952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.D.; Liu, Z.J.; Ren, J.; Xiang, M.X. Pharmacological Therapy of Abdominal Aortic Aneurysm: An Update. Curr. Vasc. Pharmacol. 2018, 16, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Wemmelund, H.; Hogh, A.; Hundborg, H.H.; Johnsen, S.P.; Lindholt, J.S. Preadmission use of renin-angiotensin blockers and rupture of abdominal aortic aneurysm: A nationwide, population-based study. Pharmacoepidemiol. Drug Saf. 2016, 25, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Periard, D.; Guessous, I.; Mazzolai, L.; Haesler, E.; Monney, P.; Hayoz, D. Reduction of small infrarenal abdominal aortic aneurysm expansion rate by statins. Vasa 2012, 41, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Schouten, O.; van Laanen, J.H.; Boersma, E.; Vidakovic, R.; Feringa, H.H.; Dunkelgrun, M.; Bax, J.J.; Koning, J.; van Urk, H.; Poldermans, D. Statins are associated with a reduced infrarenal abdominal aortic aneurysm growth. Eur. J. Vasc. Endovasc. Surg. 2006, 32, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Sukhija, R.; Aronow, W.S.; Sandhu, R.; Kakar, P.; Babu, S. Mortality and size of abdominal aortic aneurysm at long-term follow-up of patients not treated surgically and treated with and without statins. Am. J. Cardiol. 2006, 97, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Nagasawa, A.; Kudo, J.; Onoda, M.; Morikage, N.; Furutani, A.; Aoki, H.; Hamano, K. Inhibitory effect of statins on inflammation-related pathways in human abdominal aortic aneurysm tissue. Int. J. Mol. Sci. 2015, 16, 11213–11228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorice, G.P.; Folli, F. A combination of PPAR-gamma agonists and HMG CoA reductase inhibitors (statins) as a new therapy for the conservative treatment of AAS (aortic aneurysm syndromes). Med. Hypotheses 2009, 73, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Abdulrasak, M.; Sonesson, B.; Singh, B.; Resch, T.; Dias, N.V. Long-term outcomes of infrarenal endovascular aneurysm repair with a commercially available stent graft. J. Vasc. Surg. 2020, 72, 520–530.e521. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.; Szeberin, Z.; Benevento, D.; Abdallah, F.; Palasciano, G.; Lescan, M.; Uberoi, R.; Setacci, C. A comparison of clinical outcomes of abdominal aortic aneurysm patients with favorable and hostile neck angulation treated by endovascular repair with the Treovance stent graft. J. Vasc. Surg. 2020, 71, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Vaaramaki, S.; Salenius, J.; Pimenoff, G.; Uurto, I.; Suominen, V. Overall outcome after endovascular aneurysm repair with a first-generation stent graft (Vanguard): A 20-year single-center experience. J. Vasc. Surg. 2020, 72, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Fang, K.; Wang, T.; Li, Q.; Li, M.; Li, X. Operation experience of atypical ruptured abdominal aortic aneurysm. Zhonghua Wai Ke Za Zhi 2015, 53, 831–835. [Google Scholar] [PubMed]
- Zhang, H.; Zhao, Y.; Naha, G.; Hou, C.; Wang, Z.; Yang, X. Successful Management of Extracranial Vertebral Artery Aneurysm by Artificial Vessel Reconstruction. World Neurosurg. 2018, 116, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Bonardelli, S.; Parrinello, G.; De Lucia, M.; Nodari, F.; Maffeis, R.; Cervi, E.; Viotti, F.; Piardi, T.; Portolani, N.; Giulini, S.M. Risk factors for immediate results and long-term survival following elective open surgery for AAA. Statistical analysis of 1111 consecutively-treated patients. Ann. Ital. Chir. 2007, 78, 265–276. [Google Scholar] [PubMed]
- Lemaire, A.; Cook, C.; Tackett, S.; Mendes, D.M.; Shortell, C.K. The impact of race and insurance type on the outcome of endovascular abdominal aortic aneurysm (AAA) repair. J. Vasc. Surg. 2008, 47, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Song, Y.; Mai, W.; Hu, Y.; Cai, X.; Wu, Y.; Qiu, R.; Kuang, J. Association of N-terminal pro brain natriuretic peptide and impaired aortic elastic property in hypertensive patients. Clin. Chim. Acta 2011, 412, 2272–2276. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Hasegawa, G.; Yabe, Y.; Arai, C.; Kashikura, Y. Pulse wave and arterial elastic property--the principle and the essence of pulse wave velocity method (author’s transl). Kokyu Junkan 1976, 24, 376–387. [Google Scholar] [PubMed]
- Yanagisawa, H.; Davis, E.C. Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. Int. J. Biochem. Cell Biol. 2010, 42, 1084–1093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mecham, R.P. Overview of extracellular matrix. Curr. Protoc. Cell Biol. 2012, 57, 10.1.1–10.1.16. [Google Scholar] [CrossRef] [PubMed]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cruise, A.J. Anisotropic structures in human elastin. Nature 1957, 179, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H. Three dimensional structures of pulmonary elastin; airway vs vascular elastin. Yonsei Med. J. 1994, 35, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D.; Endicott, S.K.; Province, M.A.; Pierce, J.A.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chow, M.J.; Mondonedo, J.R.; Johnson, V.M.; Zhang, Y. Progressive structural and biomechanical changes in elastin degraded aorta. Biomech. Model. Mechanobiol. 2013, 12, 361–372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kristensen, J.H.; Larsen, L.; Dasgupta, B.; Brodmerkel, C.; Curran, M.; Karsdal, M.A.; Sand, J.M.; Willumsen, N.; Knox, A.J.; Bolton, C.E.; et al. Levels of circulating MMP-7 degraded elastin are elevated in pulmonary disorders. Clin. Biochem. 2015, 48, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Skjot-Arkil, H.; Clausen, R.E.; Nguyen, Q.H.; Wang, Y.; Zheng, Q.; Martinez, F.J.; Hogaboam, C.M.; Han, M.; Klickstein, L.B.; Larsen, M.R.; et al. Measurement of MMP-9 and -12 degraded elastin (ELM) provides unique information on lung tissue degradation. BMC Pulm. Med. 2012, 12, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hansen, N.U.; Karsdal, M.A.; Brockbank, S.; Cruwys, S.; Ronnow, S.; Leeming, D.J. Tissue turnover of collagen type I, III and elastin is elevated in the PCLS model of IPF and can be restored back to vehicle levels using a phosphodiesterase inhibitor. Respir. Res. 2016, 17, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuhn, C., 3rd; Engleman, W.; Chraplyvy, M.; Starcher, B.C. Degradation of elastin in experimental elastase-induced emphysema measured by a radioimmunoassay for desmosine. Exp. Lung Res. 1983, 5, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Rucker, R.B. Aorta elastin turnover in normal and hypercholesterolemic Japanese quail. Biochim. Biophys. Acta 1980, 630, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.; Leaf, E.M.; El-Abbadi, M.; Giachelli, C.M. Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am. J. Pathol. 2011, 178, 764–773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sivan, S.S.; Van El, B.; Merkher, Y.; Schmelzer, C.E.; Zuurmond, A.M.; Heinz, A.; Wachtel, E.; Varga, P.P.; Lazary, A.; Brayda-Bruno, M.; et al. Longevity of elastin in human intervertebral disc as probed by the racemization of aspartic acid. Biochim. Biophys. Acta 2012, 1820, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Stolz, D.; Leeming, D.J.; Kristensen, J.H.E.; Karsdal, M.A.; Boersma, W.; Louis, R.; Milenkovic, B.; Kostikas, K.; Blasi, F.; Aerts, J.; et al. Systemic Biomarkers of Collagen and Elastin Turnover Are Associated with Clinically Relevant Outcomes in COPD. Chest 2017, 151, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Daamen, W.F.; van Moerkerk, H.T.; Hafmans, T.; Buttafoco, L.; Poot, A.A.; Veerkamp, J.H.; van Kuppevelt, T.H. Preparation and evaluation of molecularly-defined collagen-elastin-glycosaminoglycan scaffolds for tissue engineering. Biomaterials 2003, 24, 4001–4009. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Turino, G.M.; Lin, Y.Y. Characterization of peptide fragments from lung elastin degradation in chronic obstructive pulmonary disease. Exp. Lung Res. 2010, 36, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Mecham, R.P. Elastin in lung development and disease pathogenesis. Matrix Biol. 2018, 73, 6–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wagner, W.; Bennett, R.D.; Ackermann, M.; Ysasi, A.B.; Belle, J.; Valenzuela, C.D.; Pabst, A.; Tsuda, A.; Konerding, M.A.; Mentzer, S.J. Elastin Cables Define the Axial Connective Tissue System in the Murine Lung. Anat. Rec. 2015, 298, 1960–1968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fazio, M.J.; Mattei, M.G.; Passage, E.; Chu, M.L.; Black, D.; Solomon, E.; Davidson, J.M.; Uitto, J. Human elastin gene: New evidence for localization to the long arm of chromosome 7. Am. J. Hum. Genet. 1991, 48, 696–703. [Google Scholar] [PubMed] [PubMed Central]
- Mariani, T.J.; Reed, J.J.; Shapiro, S.D. Expression profiling of the developing mouse lung: Insights into the establishment of the extracellular matrix. Am. J. Respir. Cell Mol. Biol. 2002, 26, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.L.; Looker, T.; Germain, J. The growth and development of the rat aorta. I. Morphological aspects. J. Anat. 1972, 113, 1–16. [Google Scholar] [PubMed] [PubMed Central]
- Haviarova, Z.; Weismann, P.; Stvrtinova, V.; Benuska, J. The determination of the collagen and elastin amount in the human varicose vein by the computer morphometric method. Gen. Physiol. Biophys. 1999, 18 (Suppl. S1), 30–33. [Google Scholar] [PubMed]
- Jackson, D.S.; Cleary, E.G. The determination of collagen and elastin. Methods Biochem. Anal. 1967, 15, 25–76. [Google Scholar] [CrossRef] [PubMed]
- Neuman, R.E.; Logan, M.A. The determination of collagen and elastin in tissues. J. Biol. Chem. 1950, 186, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, K. Lysyl oxidases: A novel multifunctional amine oxidase family. Prog. Nucleic Acid. Res. Mol. Biol. 2001, 70, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Mäki, J.M.; Räsänen, J.; Tikkanen, H.; Sormunen, R.; Mäkikallio, K.; Kivirikko, K.I.; Soininen, R. Inactivation of the Lysyl Oxidase Gene Lox Leads to Aortic Aneurysms, Cardiovascular Dysfunction, and Perinatal Death in Mice. Circulation 2002, 106, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Coutard, M. Experimental cerebral aneurysms in the female heterozygous Blotchy mouse. Int. J. Exp. Pathol. 1999, 80, 357–367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCallum, H.M. Experimental Lathyrism in Mice. J. Pathol. Bacteriol. 1965, 89, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, Y.; Kanematsu, M.; Kurihara, C.; Tsou, T.L.; Nuki, Y.; Liang, E.I.; Makino, H.; Hashimoto, T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension 2010, 55, 1267–1274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bjorck, M.; Wanhainen, A. Pathophysiology of AAA: Heredity vs environment. Prog. Cardiovasc. Dis. 2013, 56, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, P.; La Mattina, V.; Bernacchia, A.; Magnoni, R.; Cerri, F.; Cox, G.; Quattrini, A.; Casari, G.; Rugarli, E.I. Genetic interaction between the m-AAA protease isoenzymes reveals novel roles in cerebellar degeneration. Hum. Mol. Genet. 2009, 18, 2001–2013. [Google Scholar] [CrossRef] [PubMed]
- Ohama, T.; Osawa, S.; Watanabe, K.; Jukes, T.H. Evolution of the mitochondrial genetic code. IV. AAA as an asparagine codon in some animal mitochondria. J. Mol. Evol. 1990, 30, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Kobel, F.; Dorazilova, V.; Kummel, L. Human heart aneurysm: Biochemical and morphological characteristics. Recent. Adv. Stud. Cardiac Struct. Metab. 1975, 6, 431–436. [Google Scholar] [PubMed]
- Ramsbottom, D.; Fitzgerald, P.; Grace, P.A.; McAnena, O.; Burke, P.; Collins, P.; Johnson, A.; Croke, D.T.; Bouchier-Hayes, D. Biochemical and molecular genetic studies of abdominal aortic aneurysm in an Irish population. Eur. J. Vasc. Surg. 1994, 8, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Fragai, M.; Luchinat, C.; Melikian, M.; Venturi, C. Characterisation of the MMP-12-elastin adduct. Chemistry 2009, 15, 7842–7845. [Google Scholar] [CrossRef] [PubMed]
- Brassart, B.; Randoux, A.; Hornebeck, W.; Emonard, H. Regulation of matrix metalloproteinase-2 (gelatinase A, MMP-2), membrane-type matrix metalloproteinase-1 (MT1-MMP) and tissue inhibitor of metalloproteinases-2 (TIMP-2) expression by elastin-derived peptides in human HT-1080 fibrosarcoma cell line. Clin. Exp. Metastasis 1998, 16, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Miekus, N.; Luise, C.; Sippl, W.; Baczek, T.; Schmelzer, C.E.H.; Heinz, A. MMP-14 degrades tropoelastin and elastin. Biochimie 2019, 165, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Pellicoro, A.; Aucott, R.L.; Ramachandran, P.; Robson, A.J.; Fallowfield, J.A.; Snowdon, V.K.; Hartland, S.N.; Vernon, M.; Duffield, J.S.; Benyon, R.C.; et al. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology 2012, 55, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Bank, A.J.; Wang, H.; Holte, J.E.; Mullen, K.; Shammas, R.; Kubo, S.H. Contribution of collagen, elastin, and smooth muscle to in vivo human brachial artery wall stress and elastic modulus. Circulation 1996, 94, 3263–3270. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.A.; Starcher, B.C. Urinary excretion of elastin peptides containing desmosin after intratracheal injection of elastase in hamsters. J. Clin. Investig. 1978, 61, 1286–1290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anidjar, S.; Salzmann, J.L.; Gentric, D.; Lagneau, P.; Camilleri, J.P.; Michel, J.B. Elastase-induced experimental aneurysms in rats. Circulation 1990, 82, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Lysgaard Poulsen, J.; Stubbe, J.; Lindholt, J.S. Animal Models Used to Explore Abdominal Aortic Aneurysms: A Systematic Review. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 487–499. [Google Scholar] [CrossRef]
- Berman, A.G.; Romary, D.J.; Kerr, K.E.; Gorazd, N.E.; Wigand, M.M.; Patnaik, S.S.; Finol, E.A.; Cox, A.D.; Goergen, C.J. Experimental aortic aneurysm severity and growth depend on topical elastase concentration and lysyl oxidase inhibition. Sci. Rep. 2022, 12, 99. [Google Scholar] [CrossRef]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Qu, C.; Zhang, Y.; Fang, J.; Teng, L.; Shen, C. Perfusion pressure of elastase impacts the formation ratio and diameters of abdominal aortic aneurysms in rats. Exp. Ther. Med. 2023, 25, 190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bontekoe, J.; Upchurch, G.; Morgan, C.; Liu, B. Advanced Abdominal Aortic Aneurysm Modeling in Mice by Combination of Topical Elastase and Oral ß-aminopropionitrile. J. Vis. Exp. 2024, 209, e66812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mordi, I.R.; Forsythe, R.O.; Gellatly, C.; Iskandar, Z.; McBride, O.M.; Saratzis, A.; Chalmers, R.; Chin, C.; Bown, M.J.; Newby, D.E.; et al. Plasma Desmosine and Abdominal Aortic Aneurysm Disease. J. Am. Heart Assoc. 2019, 8, e013743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Longo, G.M.; Xiong, W.; Greiner, T.C.; Zhao, Y.; Fiotti, N.; Baxter, B.T. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Investig. 2002, 110, 625–632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, Y.; Cao, X.; Guo, J.; Zhang, Y.; Pan, L.; Zhang, H.; Li, H.; Tang, C.; Du, J.; Shi, G.P. Deficiency of cathepsin S attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Cardiovasc. Res. 2012, 96, 401–410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, J.; Sukhova, G.K.; Zhang, J.; Chen, H.; Sjöberg, S.; Libby, P.; Xia, M.; Xiong, N.; Gelb, B.D.; Shi, G.P. Cathepsin K deficiency reduces elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 15–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eliason, J.L.; Hannawa, K.K.; Ailawadi, G.; Sinha, I.; Ford, J.W.; Deogracias, M.P.; Roelofs, K.J.; Woodrum, D.T.; Ennis, T.L.; Henke, P.K.; et al. Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation 2005, 112, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Hance, K.A.; Tataria, M.; Ziporin, S.J.; Lee, J.K.; Thompson, R.W. Monocyte chemotactic activity in human abdominal aortic aneurysms: Role of elastin degradation peptides and the 67-kD cell surface elastin receptor. J. Vasc. Surg. 2002, 35, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Dale, M.A.; Xiong, W.; Carson, J.S.; Suh, M.K.; Karpisek, A.D.; Meisinger, T.M.; Casale, G.P.; Baxter, B.T. Elastin-Derived Peptides Promote Abdominal Aortic Aneurysm Formation by Modulating M1/M2 Macrophage Polarization. J. Immunol. 2016, 196, 4536–4543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vorp, D.A. Biomechanics of abdominal aortic aneurysm. J. Biomech. 2007, 40, 1887–1902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Isenburg, J.C.; Simionescu, D.T.; Starcher, B.C.; Vyavahare, N.R. Elastin Stabilization for Treatment of Abdominal Aortic Aneurysms. Circulation 2007, 115, 1729–1737. [Google Scholar] [CrossRef]
- Nosoudi, N.; Chowdhury, A.; Siclari, S.; Parasaram, V.; Karamched, S.; Vyavahare, N. Systemic Delivery of Nanoparticles Loaded with Pentagalloyl Glucose Protects Elastic Lamina and Prevents Abdominal Aortic Aneurysm in Rats. J. Cardiovasc. Transl. Res. 2016, 9, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Thanigaimani, S.; Phie, J. A Systematic Review and Meta-Analysis of the Effect of Pentagalloyl Glucose Administration on Aortic Expansion in Animal Models. Biomedicines 2021, 9, 1442. [Google Scholar] [CrossRef] [PubMed]
- Kabay, L. Clinical aspects and pathology of so-called spontaneous abdominal hemorrhages; spontaneous rupture of splenic artery aneurysm. Orv. Hetil. 1951, 92, 1462–1465. [Google Scholar] [PubMed]
- Schoenberg, S.O.; Wunsch, C.; Knopp, M.V.; Essig, M.; Hawighorst, H.; Laub, G.; Prince, M.R.; Allenberg, J.R.; Van Kaick, G. Abdominal aortic aneurysm. Detection of multilevel vascular pathology by time-resolved multiphase 3D gadolinium MR angiography: Initial report. Investig. Radiol. 1999, 34, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Davis, V.; Persidskaia, R.; Baca-Regen, L.; Itoh, Y.; Nagase, H.; Persidsky, Y.; Ghorpade, A.; Baxter, B.T. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.W.; Holmes, D.R.; Mertens, R.A.; Liao, S.; Botney, M.D.; Mecham, R.P.; Welgus, H.G.; Parks, W.C. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J. Clin. Investig. 1995, 96, 318–326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Longo, G.M.; Buda, S.J.; Fiotta, N.; Xiong, W.; Griener, T.; Shapiro, S.; Baxter, B.T. MMP-12 has a role in abdominal aortic aneurysms in mice. Surgery 2005, 137, 457–462. [Google Scholar] [CrossRef]
- Rizas, K.D.; Ippagunta, N.; Tilson, M.D., 3rd. Immune cells and molecular mediators in the pathogenesis of the abdominal aortic aneurysm. Cardiol. Rev. 2009, 17, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Takino, T.; Miyamori, H.; Watanabe, Y.; Yoshioka, K.; Seiki, M.; Sato, H. Membrane type 1 matrix metalloproteinase regulates collagen-dependent mitogen-activated protein/extracellular signal-related kinase activation and cell migration. Cancer Res. 2004, 64, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Knispel, R.; MacTaggart, J.; Greiner, T.C.; Weiss, S.J.; Baxter, B.T. Membrane-type 1 matrix metalloproteinase regulates macrophage-dependent elastolytic activity and aneurysm formation in vivo. J. Biol. Chem. 2009, 284, 1765–1771. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, C.; Jiang, B.; Shi, X.; Fan, C. Hes3 Enhances the Malignant Phenotype of Lung Cancer through Upregulating Cyclin D1, Cyclin D3 and MMP7 Expression. Int. J. Med. Sci. 2019, 16, 470–476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kastelijn, E.A.; van Moorsel, C.H.; Ruven, H.J.; Karthaus, V.; Kwakkel-van Erp, J.M.; van de Graaf, E.A.; Zanen, P.; van Kessel, D.A.; Grutters, J.C.; van den Bosch, J.M. Genetic polymorphisms in MMP7 and reduced serum levels associate with the development of bronchiolitis obliterans syndrome after lung transplantation. J. Heart Lung Transplant. 2010, 29, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Qiao, C.H.; Zhang, X.; Luo, H.; Sun, X.K. The expression of MMP-7 in serum and aneurysm tissues of patients with abdominal aortic aneurysm associated with hypertension and the clinical efficacy of endovascular exclusion. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4623–4631. [Google Scholar] [PubMed]
- Johnson, J.L.; George, S.J.; Newby, A.C.; Jackson, C.L. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc. Natl. Acad. Sci. USA 2005, 102, 15575–15580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, H.; Johnson, J.L.; Jackson, C.L.; White, S.J.; George, S.J. MMP-7 mediates cleavage of N-cadherin and promotes smooth muscle cell apoptosis. Cardiovasc. Res. 2010, 87, 137–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batra, J.; Robinson, J.; Soares, A.S.; Fields, A.P.; Radisky, D.C.; Radisky, E.S. Matrix metalloproteinase-10 (MMP-10) interaction with tissue inhibitors of metalloproteinases TIMP-1 and TIMP-2: Binding studies and crystal structure. J. Biol. Chem. 2012, 287, 15935–15946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamarina, N.A.; McMillan, W.D.; Shively, V.P.; Pearce, W.H. Expression of matrix metalloproteinases and their inhibitors in aneurysms and normal aorta. Surgery 1997, 122, 264–271; discussion 262–271. [Google Scholar] [CrossRef] [PubMed]
- Acilan, C.; Serhatli, M.; Kacar, O.; Adiguzel, Z.; Tuncer, A.; Hayran, M.; Baysal, K. Smooth muscle cells isolated from thoracic aortic aneurysms exhibit increased genomic damage, but similar tendency for apoptosis. DNA Cell Biol. 2012, 31, 1523–1534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bokeriia, L.A.; Arakelian, V.S.; Ivanova, S.M.; Serov, R.A.; Sukhareva, T.V.; Shubaeva, N.O. Smooth muscle cell apoptosis in aneurysmal aortic lesion in the light of surgical treatment results. Vestn. Ross. Akad. Med. Nauk. 2005, 4, 75–81. [Google Scholar] [PubMed]
- Charbonneau, C.; Liberelle, B.; Hebert, M.J.; De Crescenzo, G.; Lerouge, S. Stimulation of cell growth and resistance to apoptosis in vascular smooth muscle cells on a chondroitin sulfate/epidermal growth factor coating. Biomaterials 2011, 32, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.C.; Littlewood, T.D.; Figg, N.; Maguire, J.J.; Davenport, A.P.; Goddard, M.; Bennett, M.R. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ. Res. 2008, 102, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Lopez-Candales, A.; Liao, S.; Thompson, R.W. Smooth muscle cell apoptosis and p53 expression in human abdominal aortic aneurysms. Ann. N. Y. Acad. Sci. 1996, 800, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Misfeld, M.; Hanke, T.; Charitos, E.I.; Bullerdiek, J.; Belge, G.; Kuehnel, W.; Sievers, H.H. Inhibition of caspase-3 differentially affects vascular smooth muscle cell apoptosis in the concave versus convex aortic sites in ascending aneurysms with a bicuspid aortic valve. Ann. Anat. 2010, 192, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Nataatmadja, M.; West, M.; West, J.; Summers, K.; Walker, P.; Nagata, M.; Watanabe, T. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation 2003, 108 (Suppl. S1), II329–II334. [Google Scholar] [CrossRef] [PubMed]
- Sakaki, T.; Kohmura, E.; Kishiguchi, T.; Yuguchi, T.; Yamashita, T.; Hayakawa, T. Loss and apoptosis of smooth muscle cells in intracranial aneurysms. Studies with in situ DNA end labeling and antibody against single-stranded DNA. Acta Neurochir 1997, 139, 469–474; discussion 465–474. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.W.; Liao, S.; Curci, J.A. Vascular smooth muscle cell apoptosis in abdominal aortic aneurysms. Coron. Artery Dis. 1997, 8, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, L.; Yan, H.; Yu, L.; Shan, W.; Jiang, H. LUCAT1 contributes to MYRF-dependent smooth muscle cell apoptosis and may facilitate aneurysm formation via the sequestration of miR-199a-5p. Cell Biol. Int. 2020, 44, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Schmidt, J.; Ryschich, E.; Schumacher, H.; Allenberg, J.R. Increased apoptosis and decreased density of medial smooth muscle cells in human abdominal aortic aneurysms. Chin. Med. J. 2003, 116, 1549–1552. [Google Scholar] [PubMed]
- Haimovici, H.; Maier, N. Fate of aortic homografts in canine atherosclerosis. 3. study of fresh abdominal and thoracic aortic implants into thoracic aorta: Role of tissue susceptibility in atherogenesis. Arch. Surg. 1964, 89, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Colbert, M.C.; Robbins, J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ. Res. 2006, 98, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, W.M.; Zhang, L.; Schuiki, I.; Curi, R.; Volchuk, A.; Alba-Loureiro, T.C. NADPH oxidase-dependent production of reactive oxygen species induces endoplasmatic reticulum stress in neutrophil-like HL60 cells. PLoS ONE 2015, 10, e0116410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rohm, M.; Grimm, M.J.; D’Auria, A.C.; Almyroudis, N.G.; Segal, B.H.; Urban, C.F. NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infect. Immun. 2014, 82, 1766–1777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCormick, M.L.; Gavrila, D.; Weintraub, N.L. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, S.; Koga, J.I.; Matoba, T.; Umezu, R.; Nakashiro, S.; Nakano, K.; Tsutsui, H.; Egashira, K. Nanoparticle-Mediated Delivery of Pitavastatin to Monocytes/Macrophages Inhibits Angiotensin II-Induced Abdominal Aortic Aneurysm Formation in Apoe(/) Mice. J. Atheroscler. Thromb. 2021, 29, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Potteaux, S.; Tedgui, A. Monocytes, Macrophages and Other Inflammatory Mediators of Abdominal Aortic Aneurysm. Curr. Pharm. Des. 2015, 21, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Raffort, J.; Lareyre, F.; Clement, M.; Hassen-Khodja, R.; Chinetti, G.; Mallat, Z. Monocytes and macrophages in abdominal aortic aneurysm. Nat. Rev. Cardiol. 2017, 14, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Komutrattananont, P.; Mahakkanukrauh, P.; Das, S. Morphology of the human aorta and age-related changes: Anatomical facts. Anat. Cell Biol. 2019, 52, 109–114. [Google Scholar] [CrossRef]
- Greenwald, S.E. Ageing of the conduit arteries. J. Pathol. 2007, 211, 157–172. [Google Scholar] [CrossRef]
- Aune, D.; Schlesinger, S.; Norat, T.; Riboli, E. Tobacco smoking and the risk of abdominal aortic aneurysm: A systematic review and meta-analysis of prospective studies. Sci. Rep. 2018, 8, 14786. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.E.; Curci, J.A. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1473–1477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wagenhäuser, M.U.; Schellinger, I.N.; Yoshino, T.; Toyama, K.; Kayama, Y.; Deng, A.; Guenther, S.P.; Petzold, A.; Mulorz, J.; Mulorz, P.; et al. Chronic Nicotine Exposure Induces Murine Aortic Remodeling and Stiffness Segmentation—Implications for Abdominal Aortic Aneurysm Susceptibility. Front. Physiol. 2018, 9, 1459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makrygiannis, G.; Courtois, A.; Drion, P.; Defraigne, J.-O.; Kuivaniemi, H.; Sakalihasan, N. Sex Differences in Abdominal Aortic Aneurysm: The Role of Sex Hormones. Ann. Vasc. Surg. 2014, 28, 1946–1958. [Google Scholar] [CrossRef]
- Laser, A.; Ghosh, A.; Roelofs, K.; Sadiq, O.; McEvoy, B.; DiMusto, P.; Eliason, J.; Upchurch, G.R., Jr. Increased estrogen receptor alpha in experimental aortic aneurysms in females compared with males. J. Surg. Res. 2014, 186, 467–474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charolidi, N.; Pirianov, G.; Torsney, E.; Pearce, S.; Laing, K.; Nohturfft, A.; Cockerill, G.W. Pioglitazone Identifies a New Target for Aneurysm Treatment: Role of Egr1 in an Experimental Murine Model of Aortic Aneurysm. J. Vasc. Res. 2015, 52, 81–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koole, D.; van Herwaarden, J.A.; Schalkwijk, C.G.; Lafeber, F.P.J.G.; Vink, A.; Smeets, M.B.; Pasterkamp, G.; Moll, F.L. A potential role for glycated cross-links in abdominal aortic aneurysm disease. J. Vasc. Surg. 2017, 65, 1493–1503.e3. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Guo, J.; Samura, M.; Ge, Y.; Zhao, S.; Li, G.; Chen, X.; Shoji, T.; Ikezoe, T.; et al. Treatment with Small Molecule Inhibitors of Advanced Glycation End-Products Formation and Advanced Glycation End-Products-Mediated Collagen Cross-Linking Promotes Experimental Aortic Aneurysm Progression in Diabetic Mice. J. Am. Heart Assoc. 2023, 12, e028081. [Google Scholar] [CrossRef]
- Lu, H.Y.; Huang, C.Y.; Shih, C.M.; Chang, W.H.; Tsai, C.S.; Lin, F.Y.; Shih, C.C. Dipeptidyl peptidase-4 inhibitor decreases abdominal aortic aneurysm formation through GLP-1-dependent monocytic activity in mice. PLoS ONE 2015, 10, e0121077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nordness, M.J.; Baxter, B.T.; Matsumura, J.; Terrin, M.; Zhang, K.; Ye, F.; Webb, N.R.; Dalman, R.L.; Curci, J.A. The effect of diabetes on abdominal aortic aneurysm growth over 2 years. J. Vasc. Surg. 2022, 75, 1211–1222.e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thanigaimani, S.; Singh, T.P.; Unosson, J.; Phie, J.; Moxon, J.; Wanhainen, A.; Golledge, J. Editor’s Choice—Association Between Metformin Prescription and Abdominal Aortic Aneurysm Growth and Clinical Events: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiong, J.; Wu, Z.; Chen, C.; Wei, Y.; Guo, W. Association between diabetes and prevalence and growth rate of abdominal aortic aneurysms: A meta-analysis. Int. J. Cardiol. 2016, 221, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Isenburg, J.C.; Karamchandani, N.V.; Simionescu, D.T.; Vyavahare, N.R. Structural requirements for stabilization of vascular elastin by polyphenolic tannins. Biomaterials 2006, 27, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Kloster, B.O.; Lund, L.; Lindholt, J.S. Inhibition of early AAA formation by aortic intraluminal pentagalloyl glucose (PGG) infusion in a novel porcine AAA model. Ann. Med. Surg. 2016, 7, 65–70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dhital, S.; Vyavahare, N.R. Nanoparticle-based targeted delivery of pentagalloyl glucose reverses elastase-induced abdominal aortic aneurysm and restores aorta to the healthy state in mice. PLoS ONE 2020, 15, e0227165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nosoudi, N.; Nahar-Gohad, P.; Sinha, A.; Chowdhury, A.; Gerard, P.; Carsten, C.G.; Gray, B.H.; Vyavahare, N.R. Prevention of abdominal aortic aneurysm progression by targeted inhibition of matrix metalloproteinase activity with batimastat-loaded nanoparticles. Circ. Res. 2015, 117, e80–e89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, D.; Chen, S.-Y. Elastin in the Pathogenesis of Abdominal Aortic Aneurysm. Cells 2025, 14, 1597. https://doi.org/10.3390/cells14201597
Cai D, Chen S-Y. Elastin in the Pathogenesis of Abdominal Aortic Aneurysm. Cells. 2025; 14(20):1597. https://doi.org/10.3390/cells14201597
Chicago/Turabian StyleCai, Dunpeng, and Shi-You Chen. 2025. "Elastin in the Pathogenesis of Abdominal Aortic Aneurysm" Cells 14, no. 20: 1597. https://doi.org/10.3390/cells14201597
APA StyleCai, D., & Chen, S.-Y. (2025). Elastin in the Pathogenesis of Abdominal Aortic Aneurysm. Cells, 14(20), 1597. https://doi.org/10.3390/cells14201597





