- Article
Integrating Syndromic Molecular Assays into Routine Diagnostic Microbiology: Benefits and Challenges
- Sara Comini,
- Anna Maria Priori and
- Francesca Brecciaroli
- + 5 authors
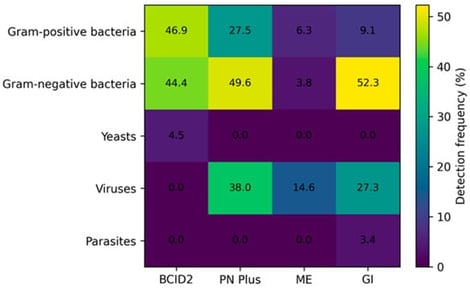
Background/Objectives: Rapid pathogen identification is essential to optimize antimicrobial therapy and improve patient outcomes, particularly in severe infections. Syndromic molecular diagnostics have been introduced to overcome the limitations of conventional culture-based methods. This study evaluated the diagnostic performance and real-life implementation of BioFire® FilmArray® syndromic panels compared with routine microbiological diagnostics. Methods: A total of 955 clinical specimens collected between 2022 and June 2025 were retrospectively analyzed, including positive blood cultures (n = 400), lower respiratory tract samples (n = 309), cerebrospinal fluid (n = 158) and stool specimens (n = 88). FilmArray® BCID2, Pneumonia Plus, Meningitis/Encephalitis and Gastrointestinal panels were performed on the Biofire Fimarray® instrument according to clinical indication and compared with conventional culture-based identification and phenotypic antimicrobial susceptibility testing. Results: Overall diagnostic concordance between BioFire® FilmArray® syndromic panels and conventional methods was high across all specimen types, with the highest positive percent agreement (PPA) observed for bloodstream infections (97.7%) and gastrointestinal pathogens (100%). In respiratory samples, the Pneumonia Plus panel detected a considerable number of microorganisms that could not be identified by culture, including viral pathogens and fastidious bacteria. Molecular detection of antimicrobial resistance markers showed excellent concordance with phenotypic profiles, with 100% agreement for CTX-M, carbapenemases (KPC, NDM, OXA-48-like, IMP), and vanA/B, while lower concordance was observed for mecA/C in staphylococci. In parallel, semi-quantitative bacterial loads provided by the Pneumonia Plus panel showed a strong essential agreement with culture-based quantification (97.4%, ±1 log10). Across all panels, syndromic testing significantly reduced diagnostic turnaround time. Conclusions: Syndromic molecular panels provide rapid and reliable simultaneous detection of pathogens, as well as early resistance marker detection, thereby supporting timely antimicrobial optimization and stewardship when integrated with conventional microbiological diagnostics.
7 February 2026