Antimicrobial Use in Pig Farms in the Midwestern Region of Minas Gerais, Brazil
Abstract
1. Introduction
2. Results
2.1. Size and Productivity of Swine Farms Evaluated
2.2. Use of Antimicrobials
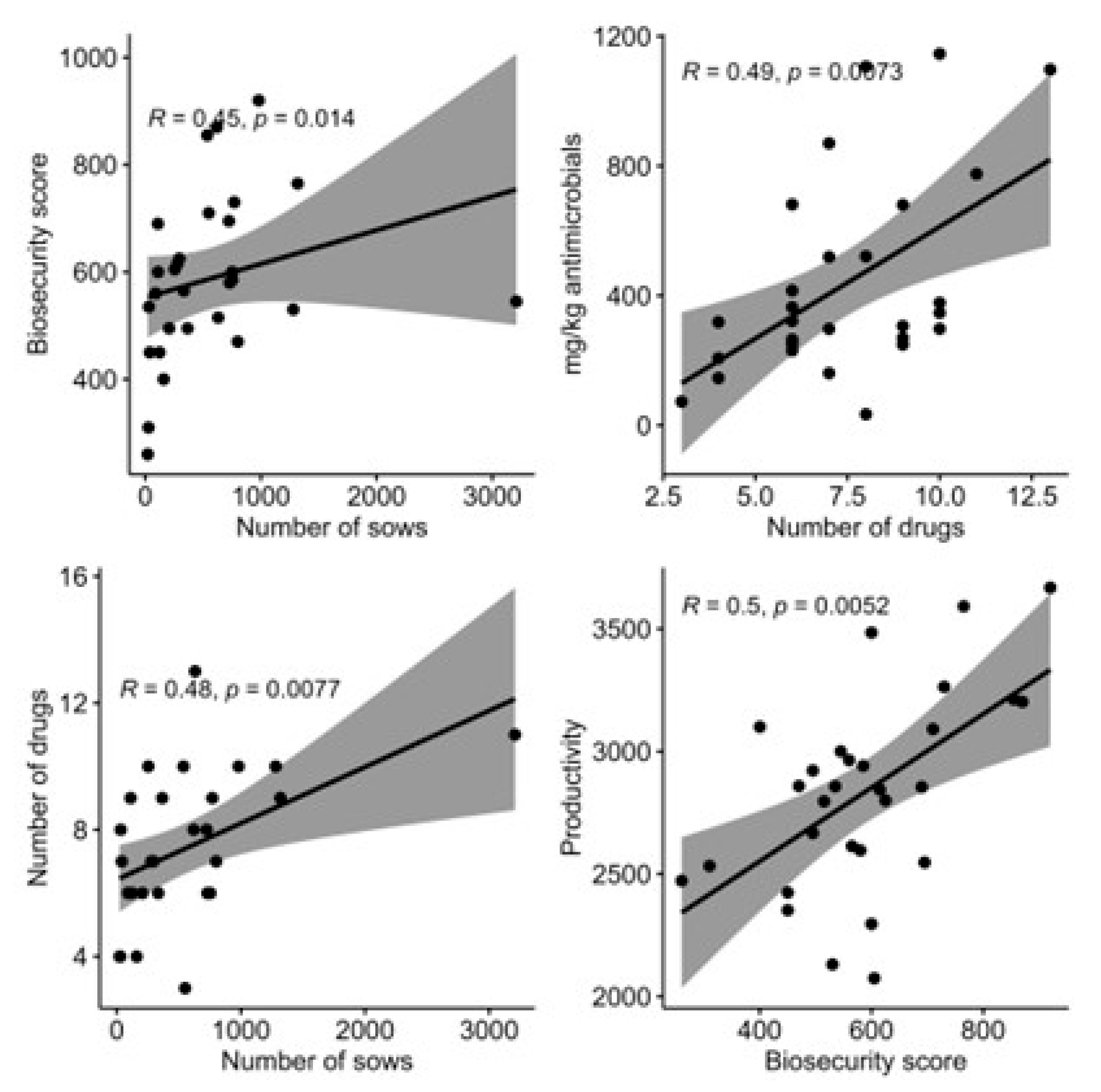
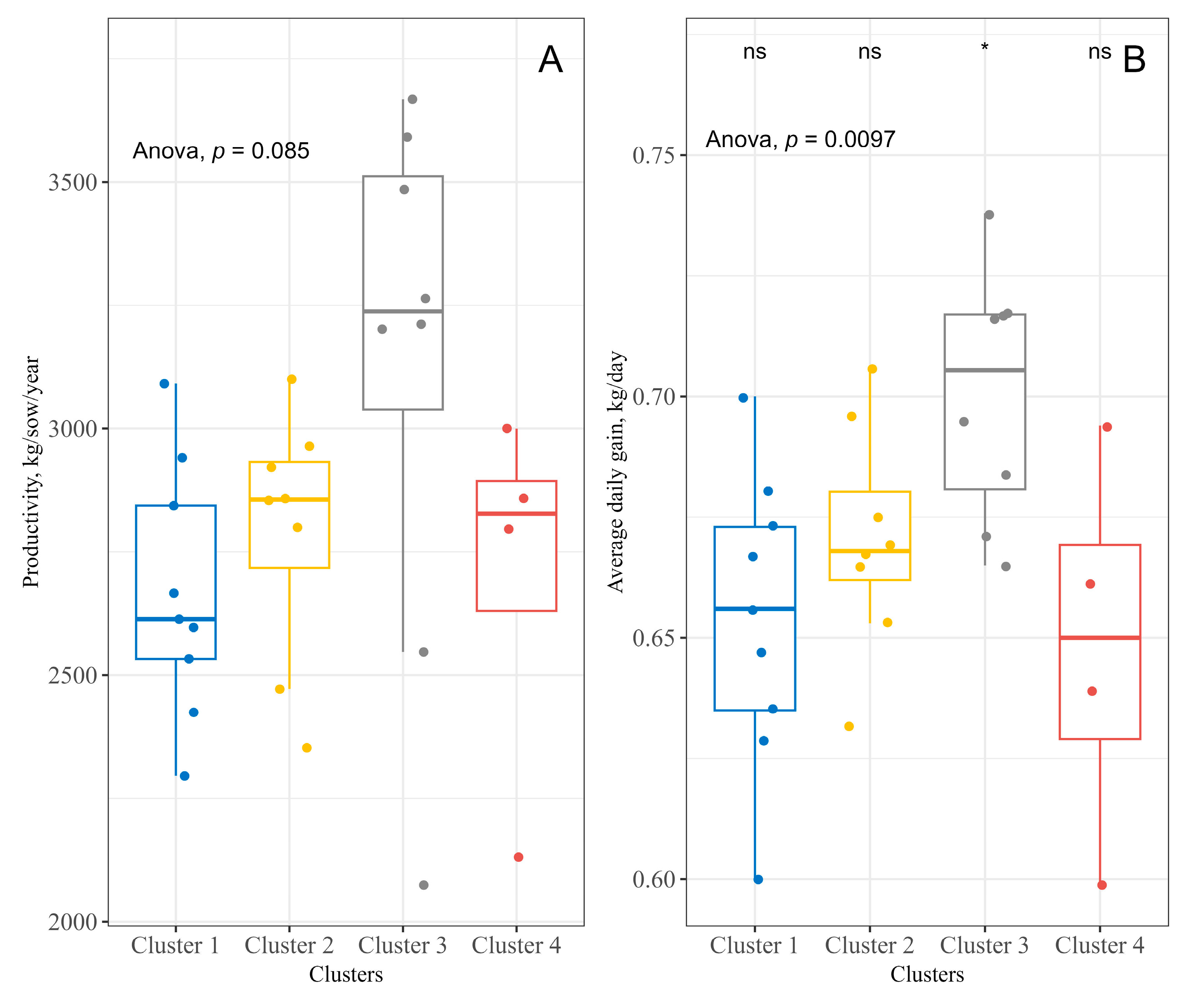
2.3. Relationships between the Studied Variables
3. Discussion
4. Materials and Methods
4.1. Farms
4.2. Data Collection on the Farms
4.3. Data Processing
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brazilian Association of Animal Protein (ABPA) Annual Report. 2022. Available online: https://abpa-br.org/wp-content/uploads/2023/01/ABPA-Annual-Report-2022.pdf (accessed on 12 February 2024).
- World Health Organization (WHO). WHO Global Strategy on Health, Environment, and Climate Change: The Transformation Needed to Improve Lives and Wellbeing Sustainably through Healthy Environments. Available online: https://www.who.int/publications/i/item/9789240000377 (accessed on 12 February 2024).
- Brazilian Ministry of Agriculture, Cattle and Suppying (MAPA). Plano de Ação Nacional de Prevenção e Controle da Resistência aos Antimicrobianos no Âmbito da Agropecuária 2018–2022 (PAN-BR AGRO). Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-pecuarios/resistencia-aos-antimicrobianos/pan-br-agro (accessed on 12 December 2023).
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfel, B.T.; Levin, S.A.; Robinson, T.P.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Morés, N. É possível produzir suínos sem o uso de antimicrobianos melhoradores de desempenho? In Proceedings of the VI Latin-American Congress of Animal Nutrition (CBNA), São Paulo, Brazil, 23–25 September 2014. [Google Scholar]
- Dutra, M.C. Uso de Antimicrobianos em Suinocultura no Brasil: Análise Crítica e Impacto Sobre Marcadores Epidemiológicos de Resistência. Ph.D. Dissertation, Faculty of Veterinary Medicine of the University of São Paulo, São Paulo, Brazil, 2017. Available online: https://www.teses.usp.br/teses/disponiveis/10/10134/tde-31012018-121740/publico/MAURICIO_CABRAL_DUTRA_original.pdf (accessed on 11 December 2023).
- Dutra, M.C.; Moreno, L.Z.; Dias, R.A.; Moreno, A.M. Antimicrobial Use in Brazilian Swine Herds: Assessment of Use and Reduction Examples. Microorganisms 2021, 9, 881. [Google Scholar] [CrossRef] [PubMed]
- Laanen, M.; Persoons, D.; Ribbens, S.; De Jong, E.; Callens, B.; Strubbe, M.; Maes, D.; Dewulf, J. Relationship between biosecurity and production/antimicrobial treatment characteristics in pig herds. Vet J. 2013, 198, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Postma, M.; Backhans, A.; Collineau, L.; Loesken, S.; Sjölund, M.; Belloc, C.; Emanuelson, U.; Beilage, E.G.; Nielsen, E.O.; Stärk, K.D.C.; et al. Evaluation of the relationship between the biosecurity status, production parameters, herd characteristics and antimicrobial usage in farrow-to-finish pig production in four EU countries. Porc. Health Manag. 2016, 2, 9. [Google Scholar] [CrossRef]
- Filippitzi, M.E.; Brinch Kruse, A.; Postma, M.; Sarrazin, S.; Maes, D.; Alban, L.; Nielsen, L.R.; Dewulf, J. Review of transmission routes of 24 infectious diseases preventable by biosecurity measures and comparison of the implementation of these measures in pig herds in six European countries. Transbound. Emerg. Dis. 2018, 65, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Sali, V. Biosecurity and Antimicrobial Use in Pig Production. Licentiate Thesis, Faculty of Veterinary Medicine, University of Helsinki, Helsinki, Finland, 2020. Available online: https://helda.helsinki.fi/server/api/core/bitstreams/3c26958c-5d3e-4775-a6e7-d02c59b324bc/content (accessed on 11 December 2023).
- Alacrón, L.V.; Allepuz, A.; Mateus, E. Biosecurity in pig farms: A review. Porc. Health Manag. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Dhaka, P.; Chantziaras, I.; Vijay, D.; Bedi, J.S.; Makovska, I.; Biebaut, E.; Dewulf, J. Can Improved Farm Biosecurity Reduce the Need for Antimicrobials in Food Animals? A Scoping Review. Antibiotics 2023, 12, 893. [Google Scholar] [CrossRef]
- Talebi Bezmin Abadi, A.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th ed.; WHO Publications: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/9789241515528 (accessed on 12 December 2023).
- Albernaz-Gonçalves, R.; Olmos, G.; Hötzel, M.J. Exploring Farmers’ Reasons for Antibiotic Use and Misuse in Pig Farms in Brazil. Antibiotics 2021, 10, 331. [Google Scholar] [CrossRef]
- MAPA. Ministério da Agricultura Pecuária e Abastecimento. Instrução Normativa No. 45, de 22-de-novembro-de-2016. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-pecuarios/alimentacao-animal/arquivos-alimentacao-animal/legislacao/instrucao-normativa-no-45-de-22-de-novembro-de-2016.pdf (accessed on 20 December 2023).
- Callens, B.; Persoons, D.; Maes, D.; Laanen, M.; Postma, M.; Boyen, F.; Haesebrouck, F.; Butaye, P.; Catry, B.; Dewulf, J. Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Prev. Vet. Med. 2012, 106, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.D. Impact of antibiotic use in the swine industry. Curr. Opin. Microbiol. 2014, 19, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Dutra, M.; de Barcellos, D.E.S.N.; Moreno, A.M. Suinocultura: Uma Saúde e um Bem-Estar, 1st ed.; MAPA: Brasilia, Brazil, 2020; Chapter 9 (Uso racional de antimicrobianos na produção de suínos); pp. 158–176. ISBN 978-65-86803-30-3. [Google Scholar]
- Canadian Antimicrobial Resistance Surveillance System Report. 2017. Available online: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/drugs-health-products/canadian-antimicrobial-resistance-surveillance-system-2017-report-executive-summary/CARSS-Report-2017-En.pdf (accessed on 12 December 2023).
- MAPA. Ministério da Agricultura Pecuária e Abastecimento. Instrução Normativa No. 1, de 13 de Janeiro de 2020. Available online: https://www.in.gov.br/web/dou/-/portaria-sda-n-798-de-10-de-maio-de-2023-483054219 (accessed on 20 December 2023).
- Harrell, F.E., Jr. Hmisc: Harrell Miscellaneous. R Package Version 4.5-0. 2021. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 11 December 2023).
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 14 November 2023).
- Hebballi, A. olsrr: Tools for Building OLS Regression Models. R Package Version 0.5.3. 2020. Available online: https://CRAN.R-project.org/package=olsrr (accessed on 14 November 2023).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 14 November 2023).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 14 November 2023).
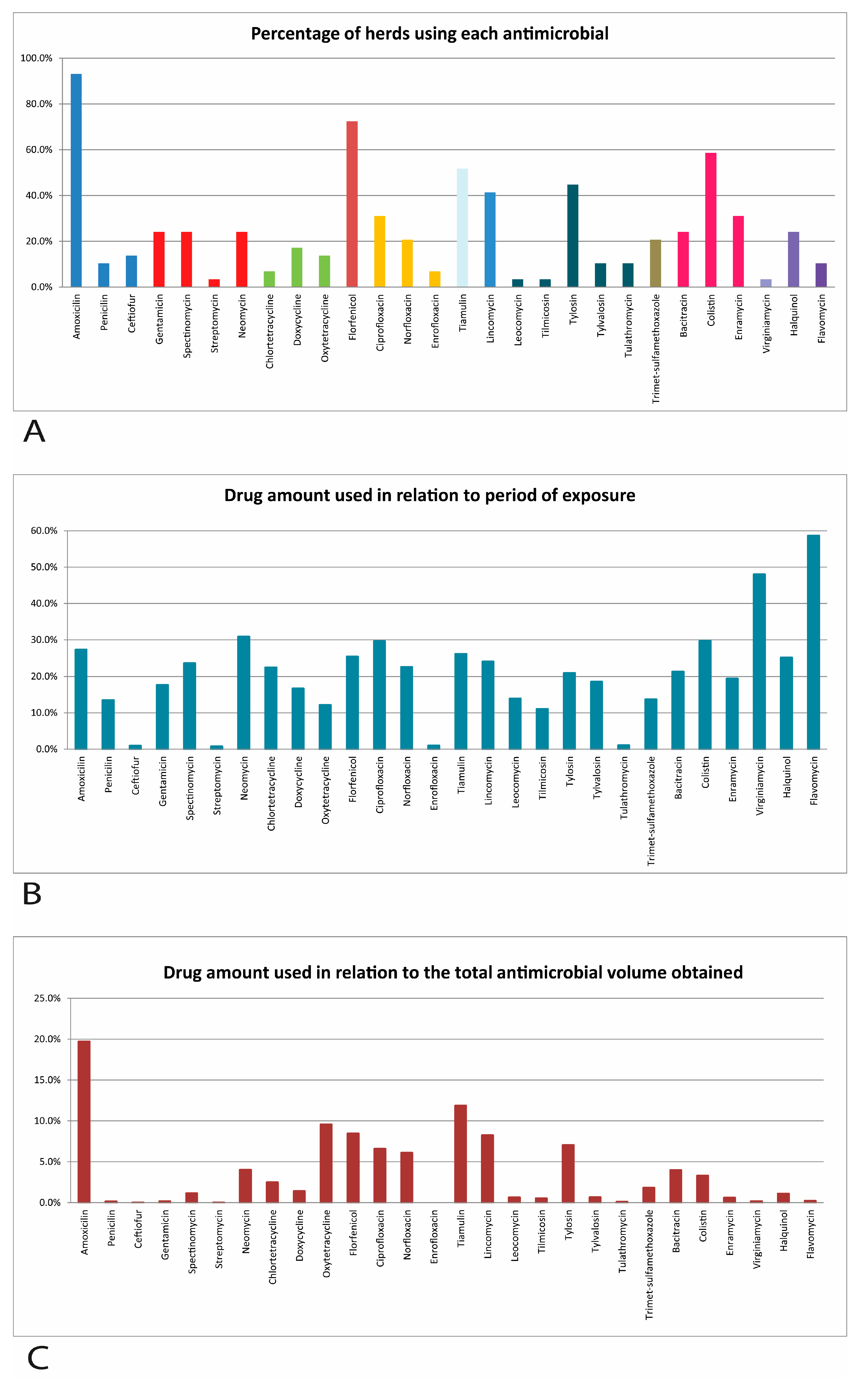

| Farms | Sows (N) | Productivity (kg/sow/year) | DWG * | Biosecurity ** | Use of Antimicrobials | |||
|---|---|---|---|---|---|---|---|---|
| Punctuation | Risk *** | Quantity | Medicated Time | Drugs | ||||
| (kg) | (mg/kg pig) | (days) | (N) | |||||
| TO 1 | 22 | 2472.00 | 0.665 | 260 | EHR | 318.26 | 144 | 4 |
| A3 | 29 | 2532.41 | 0.680 | 310 | RHR | 206.78 | 49 | 4 |
| A5 | 32 | 2857.14 | 0.667 | 535 | RHR | 34.17 | 140 | 8 |
| A6 | 40 | 2352.00 | 0.653 | 450 | RHR | 297.62 | 127 | 7 |
| A10 | 90 | 2964.00 | 0.669 | 560 | RHR | 364.11 | 138 | 6 |
| B1 | 111 | 3484.71 | 0.671 | 600 | RHR | 680.11 | 124 | 9 |
| B2 | 124 | 2424.00 | 0.673 | 450 | RHR | 415.59 | 81 | 6 |
| B13 | 160 | 3100.00 | 0.706 | 400 | RHR | 145.84 | 162 | 4 |
| B5 | 207 | 2666.66 | 0.667 | 495 | RHR | 681.39 | 87 | 6 |
| B9 | 334 | 2613.12 | 0.600 | 565 | RHR | 263.07 | 90 | 6 |
| B10 | 366 | 2921.93 | 0.696 | 495 | RHR | 269.50 | 125 | 9 |
| C2 | 631 | 2796.00 | 0.639 | 515 | RHR | 1097.51 | 126 | 13 |
| C4 | 730 | 2596.04 | 0.635 | 580 | RHR | 264.80 | 90 | 6 |
| C3 | 752 | 2295.85 | 0.629 | 600 | RHR | 249.93 | 90 | 6 |
| C13 | 753 | 2941.03 | 0.647 | 585 | RHR | 230.41 | 80 | 6 |
| C8 | 800 | 2858.45 | 0.694 | 470 | RHR | 870.03 | 123 | 7 |
| C9 | 1280 | 2130.75 | 0.599 | 530 | RHR | 1145.96 | 131 | 10 |
| C12 | 3208 | 3000.00 | 0.661 | 545 | RHR | 775.40 | 144 | 11 |
| A11 | 110 | 2854.44 | 0.632 | 690 | HR | 321.80 | 127 | 6 |
| B6 | 254 | 2074.27 | 0.684 | 605 | HR | 378.05 | 138 | 10 |
| B8 | 278 | 2843.62 | 0.656 | 615 | HR | 160.83 | 84 | 7 |
| B7 | 295 | 2799.46 | 0.675 | 625 | HR | 519.31 | 136 | 7 |
| B11 | 537 | 3212.11 | 0.717 | 855 | HR | 346.84 | 144 | 10 |
| B12 | 550 | 3091.00 | 0.700 | 710 | HR | 72.98 | 50 | 3 |
| C5 | 620 | 3202.00 | 0.695 | 870 | HR | 1106.55 | 144 | 8 |
| C7 | 724 | 2547.00 | 0.665 | 695 | HR | 521.12 | 137 | 8 |
| C6 | 770 | 3263.29 | 0.717 | 730 | HR | 248.78 | 128 | 9 |
| C11 | 1318 | 3592.00 | 0.738 | 765 | HR | 306.44 | 120 | 9 |
| C10 | 981 | 3668.00 | 0.716 | 920 | MHR | 297.82 | 121 | 10 |
| Total | 16,106 | |||||||
| Average | 549.36 | 2832.90 | 0.670 | 587.07 | RHR | 434.17 | 116.55 | 7.41 |
| Antimicrobial Classes | Drugs | Forms of Administration | ||
|---|---|---|---|---|
| Abbreviation | Name | Intramuscular | In Feed | |
| Aminoglycosides | GEN | Gentamicin | FA | FA, NP |
| ESP | Spectinomycin | - | FA, NP, GF | |
| EST | Streptomycin | FA | - | |
| NEO | Neomycin | - | FA, NP | |
| β-Lactams | AMO | Amoxicillin | FA | FA, NP, GF |
| PEN | Penicillin | FA | FA, NP | |
| CEF | Ceftiofur | FA | - | |
| Phenicol | FFN | Florfenicol | - | NP, GF |
| Diterpenes | TIA | Tiamulin | - | NP, GF |
| Lincosamides | LIN | Lincomycin | FA | FA, NP, GF |
| Macrolides | LEO | Leucomycin | - | GF |
| TILM | Tilmicosin | - | GF | |
| TIL | Tylosin | - | NP, GF | |
| TYLV | Tylvalosin | - | NP, GF | |
| TUL | Tulathromycin | FA, NP | - | |
| Tetracyclines | CLO | Chlortetracycline | - | NP, GF |
| DOX | Doxycycline | - | NP, GF | |
| OXY | Oxytetracycline | FA | NP, GF | |
| Quinolones | CIPRO | Ciprofloxacin (2nd generation) | - | FA, NP, GF |
| NOR | Norfloxacin (2nd generation) | - | FA, NP, GF | |
| ENO | Enrofloxacin (3rd generation) | FA | MT | |
| Sulfonamides | STX | Trimethoprim–sulfamethoxazole | - | NP, GF |
| Bambermycin | FLA | Flavomycin | - | GF |
| Streptogramins | VIRG | Virginiamycin | - | GF |
| Hydroxyquinoline | HAL | Halquinol | - | FA, NP |
| Polypeptides | BMD/BZN | Bac. Methylene Disalicylate | - | GF |
| COL | Colistin | - | FA, NP, | |
| ENRA | Enramycin | - | GF | |
| Class | Antimicrobials | Farms (N) | Exposure Period (Days) | % Pig Life Exposure | ||
|---|---|---|---|---|---|---|
| Min. | Max. | Average | ||||
| Aminoglycosides | Gentamicin | 7 | 1 | 59 | 20.43 | 17.67 |
| Spectinomycin | 7 | 1 | 42 | 24.29 | 23.61 | |
| Streptomycin | 2 | 1 | 1 | 1.00 | 0.75 | |
| Neomycin | 7 | 11 | 45 | 30.86 | 30.84 | |
| β-Lactams | Amoxicillin | 27 | 1 | 84 | 30.74 | 27.23 |
| Penicillin | 3 | 1 | 30 | 16.67 | 13.45 | |
| Ceftiofur | 4 | 1 | 2 | 1.25 | 0.95 | |
| Phenicol | Florfenicol | 21 | 14 | 55 | 29.38 | 25.39 |
| Diterpene | Tiamulin | 15 | 10 | 64 | 31.20 | 26.12 |
| Lincosamide | Lincomycin | 12 | 1 | 67 | 27.25 | 24.13 |
| Macrolides | Leucomycin | 1 | 20 | 20 | 20.00 | 13.89 |
| Tilmicosin | 1 | 14 | 14 | 14.00 | 11.02 | |
| Tylosin | 12 | 14 | 46 | 25.33 | 20.92 | |
| Tylvalosin | 5 | 15 | 28 | 23.00 | 18.55 | |
| Tulathromycin | 3 | 1 | 2 | 1.33 | 1.06 | |
| Quinolones | Ciprofloxacin | 10 | 15 | 45 | 30.60 | 29.60 |
| Norfloxacin | 6 | 19 | 74 | 31.33 | 22.58 | |
| Enrofloxacin | 2 | 1 | 1 | 1.00 | 0.98 | |
| Tetracyclines | Chlortetracycline | 3 | 18 | 30 | 23.33 | 22.46 |
| Doxycycline | 5 | 10 | 35 | 20.40 | 16.64 | |
| Oxytetracycline | 4 | 1 | 21 | 14.25 | 12.12 | |
| Sulfonamides | Trimethoprim–sulfamethoxazole | 6 | 10 | 18 | 13.67 | 13.67 |
| Bambermycin | Flavomycin | 2 | 80 | 100 | 90.00 | 58.64 |
| Streptogramins | Virginiamycin | 1 | 59 | 59 | 59.00 | 47.97 |
| Hydroxyquinoline | Halquinol | 7 | 7 | 64 | 36.29 | 25.12 |
| Polypeptides | Bacitracin BMD/BZN | 7 | 10 | 43 | 28.00 | 21.31 |
| Colistin | 16 | 12 | 59 | 32.27 | 29.69 | |
| Enramycin | 9 | 11 | 29 | 23.67 | 19.41 | |
| Antimicrobials | % of Antimicrobials Used Per Rearing Phases | |||||
|---|---|---|---|---|---|---|
| All Phases | Farrowing | Nursery | Growing and Finishing | Growing | Finishing | |
| Amoxicillin | 19.70 | 19.88 | 31.25 | 15.11 | 13.26 | 17.72 |
| Tiamulin | 11.85 | 0 | 9.97 | 12.82 | 14.76 | 10.06 |
| Oxytetracycline | 9.57 | – | – | 11.55 | 5.27 | 20.44 |
| Florfenicol | 8.44 | 0 | 3.50 | 11.84 | 13.36 | 8.26 |
| Lincomycin | 8.23 | – | – | 10.06 | 13.46 | 5.26 |
| Tylosin | 7.03 | 0 | – | 8.59 | 5.97 | 14.58 |
| Ciprofloxacin | 6.59 | 11.70 | 8.33 | 5.86 | 7.47 | 3.58 |
| Norfloxacin | 6.10 | 3.56 | 6.43 | 6.02 | 10.28 | 0 |
| Others All stages | 22.70 | |||||
| Neomycin | 44.27 | 12.17 | 0 | 0 | 0 | |
| Colistin | 10.45 | 11.28 | 0 | 0 | 0 | |
| Halquinol | 5.20 | 3.68 | 0 | 0 | 0 | |
| Others Farrowing | 4.94 | |||||
| Trimethoprim–sulfamethoxazole | 0 | 3.92 | – | – | 0 | |
| Doxycycline | 0 | 3.73 | – | 3.75 | 0 | |
| Other Nursery | 5.74 | |||||
| Bacitracin BMD/BZN | 0 | 0 | 5.65 | – | 12.55 | |
| Chlortetracycline | 0 | 0 | 2.93 | 5.01 | 0 | |
| Other GF | 9.57 | 7.41 | 7.55 | |||
| Total (mg) | 1,306,917.70 | 17,689.84 | 366,403.46 | 922,823.98 | 540,566.74 | 382,257.66 |
| % of the total | 100% | 1.35% | 28.04% | 70.61% | 41.36% | 29.25% |
| Antimicrobial | Pulse | Nursery | Growing | Finishing | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age (d) | Period (d) | Dose/day (mg/kg) | N | Age (d) | Period (d) | Dose/day (mg/kg) | N | Age (d) | Period (d) | Dose/day (mg/kg) | |||||
| Start | End | Start | End | Start | End | |||||||||||
| Amoxicillin | 1st | 24 | 30.29 | 43.24 | 13.25 | 17.66 | 7 | 66.43 | 84.71 | 18.29 | 15.36 | 4 | 120.75 | 136.00 | 15.25 | 15.03 |
| 2nd | 12 | 43.08 | 59.08 | 16.00 | 15.31 | 1 | 96.00 | 111.00 | 15.00 | 12.50 | - | - | - | - | - | |
| 3rd | 2 | 48.00 | 70.50 | 22.50 | 21.38 | - | - | - | - | - | - | - | - | - | - | |
| Tiamulin | 1st | 11 | 46.45 | 62.64 | 16.18 | 11.09 | 8 | 78.00 | 94.38 | 16.38 | 10.29 | 4 | 112.00 | 128.25 | 16.25 | 8.88 |
| 2nd | 1 | 50.00 | 60.00 | 10.00 | 9.00 | 4 | 98.25 | 114.25 | 16.00 | 8.88 | - | - | - | - | - | |
| Oxytetracycline | 1st | 1 | 36.00 | 43.00 | 7.00 | 37.50 | 1 | 93.00 | 107.00 | 14.00 | 42.50 | 2 | 111.00 | 128.50 | 17.50 | 35.00 |
| Florfenicol | 1st | 10 | 40.50 | 55.30 | 14.80 | 4.83 | 13 | 77.38 | 94.54 | 17.15 | 5.62 | 6 | 123.33 | 138.67 | 15.33 | 4.58 |
| 2nd | 2 | 50.00 | 65.00 | 15.00 | 5.25 | 3 | 98.67 | 117.00 | 18.33 | 4.83 | 0 | - | - | - | - | |
| Lincomycin | 1st | 5 | 33.40 | 47.00 | 13.60 | 2.75 | 7 | 73.14 | 90.29 | 17.14 | 7.49 | 3 | 110.33 | 128.67 | 18.33 | 12.10 |
| 2nd | 1 | 36.00 | 50.00 | 14.00 | 1.10 | 1 | 101.00 | 121.00 | 20.00 | 15.51 | - | - | - | - | - | |
| Tylosin | 1st | 1 | 21.00 | 36.00 | 15.00 | 12.50 | 4 | 80.25 | 96.25 | 16.00 | 5.63 | 9 | 111.78 | 132.33 | 20.56 | 5.49 |
| Ciprofloxacin | 1st | 7 | 28.14 | 42.71 | 14.57 | 12.68 | 3 | 80.33 | 95.33 | 15.00 | 14.17 | 1 | 111.00 | 125.00 | 14.00 | 15.00 |
| 2nd | 4 | 50.00 | 69.00 | 19.00 | 11.56 | 1 | 101.00 | 121.00 | 20.00 | 15.00 | - | - | - | - | - | |
| Norfloxacin | 1st | 5 | 42.00 | 61.20 | 19.20 | 15.17 | 2 | 82.00 | 104.50 | 22.50 | 15.93 | - | - | - | - | - |
| 2nd | 1 | 29.00 | 36.00 | 7.00 | 15.00 | 1 | 108.00 | 133.00 | 25.00 | 19.60 | - | - | - | - | - | |
| Variables | Cluster 1 (9 Farms) | Cluster 2 (8 Farms) | Cluster 3 (8 Farms) | Cluster 4 (4 Farms) |
|---|---|---|---|---|
| SOWS | 417.44 ± 284.49 (29–753) | 139.38 ± 127.89 (22–366) | 664.38 ± 384.52 (111–1318) | 1479.75 ± 1184.51 (631–3208) |
| SCORE | 545.56 ± 114.63 (310–710) | 501.88 ± 134.51 (260–690) | 755.00 ± 120.30 (600–920) | 515.00 ± 32.40 (470–545) |
| ATM | 282.86 ± 175.26 (72.98–681.39) | 283.83 ± 144.56 (34.17–19.31) | 485.71 ± 287.59 (248.78–1106.55) | 972.22 ± 178.01 (775.40–1145.96) |
| DRUGS | 5.56 ± 1.24 (3–7) | 6.38 ± 1.77 (4–9) | 9.12 ± 0.83 (8–10) | 10.25 ± 2.50 (7–13) |
| DAYS | 77.89 ± 16.53 (49–90) | 137.38 ± 12.12 (125–162) | 132.00 ± 9.96 (120–144) | 131.00 ± 9.27 (123–144) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, B.C.d.; Santa Rosa, I.C.d.A.; Dutra, M.C.; Ferreira, F.N.A.; Moreno, A.M.; Moreno, L.Z.; Silva, J.d.M.G.; Garcia, S.K.; Fontes, D.d.O. Antimicrobial Use in Pig Farms in the Midwestern Region of Minas Gerais, Brazil. Antibiotics 2024, 13, 403. https://doi.org/10.3390/antibiotics13050403
Oliveira BCd, Santa Rosa ICdA, Dutra MC, Ferreira FNA, Moreno AM, Moreno LZ, Silva JdMG, Garcia SK, Fontes DdO. Antimicrobial Use in Pig Farms in the Midwestern Region of Minas Gerais, Brazil. Antibiotics. 2024; 13(5):403. https://doi.org/10.3390/antibiotics13050403
Chicago/Turabian StyleOliveira, Bruno César de, Idael Christiano de Almeida Santa Rosa, Maurício Cabral Dutra, Felipe Norberto Alves Ferreira, Andrea Micke Moreno, Luisa Zanolli Moreno, Júlia da Mata Góes Silva, Simone Koprowski Garcia, and Dalton de Oliveira Fontes. 2024. "Antimicrobial Use in Pig Farms in the Midwestern Region of Minas Gerais, Brazil" Antibiotics 13, no. 5: 403. https://doi.org/10.3390/antibiotics13050403
APA StyleOliveira, B. C. d., Santa Rosa, I. C. d. A., Dutra, M. C., Ferreira, F. N. A., Moreno, A. M., Moreno, L. Z., Silva, J. d. M. G., Garcia, S. K., & Fontes, D. d. O. (2024). Antimicrobial Use in Pig Farms in the Midwestern Region of Minas Gerais, Brazil. Antibiotics, 13(5), 403. https://doi.org/10.3390/antibiotics13050403










