Flow Cytometry as a Rapid and Valuable Method in Investigation of Colistin Resistance in Carbapenem-Resistant Klebsiella pneumoniae Isolates
Abstract
1. Introduction
2. Results
2.1. Antibiotic Susceptibility Results for K. pneumoniae Strains
2.2. Colistin MIC Results Determined by the Broth Microdilution Method
2.3. Colistin Susceptibility Results Determined by the Flow Cytometry Method
3. Discussion
4. Materials and Methods
4.1. Sample Size and Selection of Strains
4.2. Identification by the VITEK 2 Automated System and Determination of Carbapenem Susceptibility
4.3. Determination of Minimum Inhibitory Concentration (MIC) Values for Colistin by Broth Microdilution (BMD)
4.4. Optimization Studies for FCM
4.5. Determination of Colistin Susceptibility by FCM
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO—World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. Available online: https://www.who.int/publications/i/item/9789241564748 (accessed on 26 May 2023).
- ECDC—European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net): Annual Epidemiological Report for 2021. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER-EARS-Net-2021_2022-final.pdf (accessed on 26 May 2023).
- WHO—World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections Including Tuberculosis. Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12 (accessed on 26 May 2023).
- Giamarellou, H.; Poulakou, G. Multidrug-Resistant Gram-Negative Infections: What are the Treatment Options? Drugs 2009, 69, 1879–1901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, Y.; Wang, L.; Kong, J.; Pan, W.; Zhang, X.; Chen, L.; Yao, Z.; Zhou, T.; Cao, J. Flufenamic acid, a promising agent for the sensitization of colistin-resistant gram-negative bacteria to colistin. Microbiol. Spectr. 2023, 11, e04052-22. [Google Scholar] [CrossRef] [PubMed]
- Satlin, M.J. The search for a practical method for colistin susceptibility testing: Have we found it by going back to the future? J. Clin. Microbiol. 2019, 57, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- CLSI—Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed. M07. Available online: https://clsi.org/standards/products/microbiology/documents/m07/ (accessed on 24 May 2023).
- CLSI—Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed. M100. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 24 May 2023).
- EUCAST—European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 13.0. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 27 May 2023).
- Li, J.; Nation, R.; Milne, R.W.; Turnidge, J.D.; Coulthard, K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 25, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Maciorowski, Z.; Chattopadhyay, P.K.; Jain, P. Basic multicolor flow cytometry. Curr. Protoc. Immunol. 2017, 117, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Steen, H.B.; Boye, E. Escherichia coli growth studied by dual-parameter flow cytophotometry. J. Bacteriol. 1981, 145, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.J.; Allman, R.; Stark, J.M.; Lloyd, D. Rapid estimation of bacterial antibiotic susceptibility with flow cytometry. J. Microsc. 1994, 176, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Suller, M.T.; Stark, J.M.; Lloyd, D. A flow cytometric study of antibiotic-induced damage and evaluation as a rapid antibiotic susceptibility test for methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 1997, 40, 77–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ekelund, O.; Klokkhammer Hetland, M.A.; Hayland Löhr, I.; Schön, T.; Somajo, S. Rapid high-resolution detection of colistin resistance in Gram-negative bacteria using flow cytometry: A comparison with broth microdilution, a commercial screening test and WGS. J. Antimicrob. Chemother. 2021, 76, 3183–3191. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, H.; Erman, G.; Toptan, H.; Koroglu, M.; Altindis, M. Determination of antibiotic susceptibility of bacteria by flow cytometric method. World J. Microbiol. Biotechnol. 2022, 38, 151. [Google Scholar] [CrossRef] [PubMed]
- Inglis, T.J.; Paton, T.F.; Kopczyk, M.K.; Mulroney, K.T.; Carson, C.F. Same-day antimicrobial susceptibility test using acoustic-enhanced flow cytometry visualized with supervised machine learning. J. Med. Microbiol. 2020, 69, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Somajo, S.; Nilsson, F.; Ekelund, O.; Unemo, M. Detection of antimicrobial resistance in <5 h in Neisseria gonorrhoeae isolates using flow cytometry—Proof of concept for seven clinically relevant antimicrobials. J. Antimicrob. Chemother. 2024, 79, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Marutescu, L.G. Current and future flow cytometry applications contributing to antimicrobial resistance control. Microorganisms 2023, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Mulroney, K.T.; Hall, J.M.; Huang, X.; Turnbull, E.; Bzdyl, N.M.; Chakera, A.; Naseer, U.; Corea, E.M.; Ellington, M.J.; Hopkins, K.L.; et al. Rapid susceptibility profiling of carbapenem-resistant Klebsiella pneumoniae. Sci. Rep. 2018, 8, 6697. [Google Scholar] [CrossRef] [PubMed]
- Saint-Ruf, C.; Crussard, S.; Franceschi, C.; Oranga, S.; Ouattara, J.; Ramjeet, M.; Surre, J.; Matic, I. Antibiotic susceptibility testing of the Gram-negative bacteria based on flow cytometry. Front. Microbiol. 2016, 7, 1121. [Google Scholar] [CrossRef] [PubMed]
- Elder, B.L. Verification and validation of procedures in the clinical microbiology laboratory. Clin. Microbiol. Newsl. 1997, 19, 153–156. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Dortet, L.; Bonnin, R.A.; Pennisi, I.; Gauthier, L.; Jousset, A.B.; Dabos, L.; Furniss, R.C.D.; Mavridou, D.A.I.; Bogaerts, P.; Glupczynski, Y.; et al. Rapid detection and discrimination of chromosome- and MCR-plasmid-mediated resistance to polymyxins by MALDI-TOF MS in Escherichia coli: The MALDIxin test. J. Antimicrob. Chemother. 2018, 73, 3359–3367. [Google Scholar] [CrossRef] [PubMed]
- Jerke, K.H.; Lee, M.J.; Humphries, R.M. Polymyxin susceptibility testing: A cold case reopened. Clin. Microbiol. Newsl. 2016, 38, 69–77. [Google Scholar] [CrossRef]
- Chew, K.L.; La, M.V.; Lin, R.T.P.; Teo, J.W.P. Colistin and polymyxin B susceptibility testing for carbapenem-resistant and mcr-positive Enterobacteriaceae: Comparison of Sensititre, Microscan, Vitek 2, and Etest with broth microdilution. J. Clin. Microbiol. 2017, 55, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.Y.; Ng, S.Y. Comparison of E-test, Vitek and agar dilution for susceptibility testing of colistin. Clin. Microbiol. Infect. 2007, 13, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Turlej-Rogacka, A.; Xavier, B.B.; Janssens, L.; Lammens, C.; Zarkotou, O.; Pournaras, S.; Goossens, H.; Malhotra-Kumar, S. Evaluation of colistin stability in agar and comparison of four methods for MIC testing of colistin. J. Clin. Microbiol. Infect. Dis. 2018, 37, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Pfennigwerth, N.; Kaminski, A.; Korte-Berwanger, M.; Jantsch, J.; Marlinghaus, L.; Gatermann, S.G. Evaluation of six commercial products for colistin susceptibility testing in Enterobacterales. Clin. Microbiol. Infect. 2019, 25, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Puttaswamy, S.; Gupta, S.K.; Regunath, H.; Smith, L.P.; Sengupta, S. A comprehensive review of the present and future antibiotic susceptibility testing (AST) systems. Arch. Clin. Microbiol. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- EUCAST—European Committee on Antimicrobial Susceptibility Testing. Colistin Breakpoints Guidance Document. Available online: https://www.eucast.org/eucastguidancedocuments (accessed on 28 May 2023).
- Karvanen, M.; Malmberg, C.; Lagerbäck, P.; Friberg, L.E.; Carsa, O. Colistin is extensively lost during standard in vitro experimental conditions. Antimicrob. Agents Chemother. 2017, 61, e00857-17. [Google Scholar] [CrossRef] [PubMed]
- Osei Sekyere, J. Mcr colistin resistance gene: A systematic review of current diagnostics and detection methods. Microbiologyopen 2018, 8, e00682. [Google Scholar] [CrossRef] [PubMed]
- Duyan, S.; Kilic, A.; Yilmaz, S.; Ardic, N. Rapid detection of extended-spectrum beta-lactamases by flow cytometry method. Mikrobiyol. Bul. 2015, 49, 600–608. [Google Scholar] [CrossRef] [PubMed]
- e Silva, D.F.; Andrade, F.F.; Gomes, R.; Silva-Dias, A.; Martins-Oliveira, I.; Perez-Viso, B.; Ramos, M.H.; Rodrigues, A.G.; Canton, R.; Pina-Vaz, C. Ultra-rapid flow cytometry assay for colistin MIC determination in Enterobacterales, Pseudomonas aeruginosa and Acinetobacter baumannii. Clin. Microbiol. Infect. 2020, 26, 1559.e1–1559.e4. [Google Scholar] [CrossRef] [PubMed]
- Faria-Ramos, I.; Espinar, M.J.; Rocha, R.; Santos-Antunes, J.; Rodrigues, A.G.; Canton, R.; Pina-Vaz, C. A Novel flow cytometric assay for rapid detection of extended-spectrum beta-lactamase. Clin. Microbiol. Infect. 2013, 19, E8–E15. [Google Scholar] [CrossRef] [PubMed]
- Yis, R. How Accurately Do We Detect Colistin Sensitivity in Carbapenem-Resistant Enterobacterales (CRE) Isolates? In Proceedings of the XXXVIII International Turkish Microbiology Congress, Antalya, Türkiye, 4–8 November 2018; Available online: https://www.researchgate.net/publication/344743442 (accessed on 24 May 2023).
- Ozkul Kocak, C.; Hazırolan, G.C. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae clinical isolates. Türk Mikrobiyol. Cem. Derg. 2019, 49, 17–23. [Google Scholar] [CrossRef]
- Besli, Y.; Liste, Ü.; Kırbaş, E.; Sancak, B. The use of resapolymyxin NP test in determining the sensitivity of colistin in Escherichia coli, and Klebsiella pneumoniae isolates. Mikrobiyol. Bul. 2022, 56, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Dogan, E.; Kaya, S.; Oren, S.; Tok, D.; Ardic, N.; Baysallar, M. Rapid identification of Klebsiella pneumoniae by matrix-assisted laser desorption/ionization-time of flight mass spectrometry and detection of meropenem resistance by flow cytometric assay. J. Clin. Lab. Anal. 2016, 30, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- e Silva, D.F.; Silva-Dias, A.; Gomes, R.; Martins-Oliveira, I.; Ramos, M.H.; Rodrigues, A.G.; Canton, R.; Pina-Vaz, C. Evaluation of rapid colistin susceptibility directly from positive blood cultures using a flow cytometry assay. Int. J. Antimicrob. Agents 2019, 54, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Makarounis, K.; Leventopoulos, M.; Georgoulias, G.; Nikolopoulos, D.; Zeginiadou, T.; Xountasi, M.; Kotrotsos, P.; Nosi, E.; Gennimata, V.; Venieratos, D.; et al. Detection of Chlamydia trachomatis inside spermatozoa using flow cytometry: Effects of antibiotic treatment (before and after) on sperm count parameters. J. Microbiol. Methods 2022, 203, 106604. [Google Scholar] [CrossRef] [PubMed]
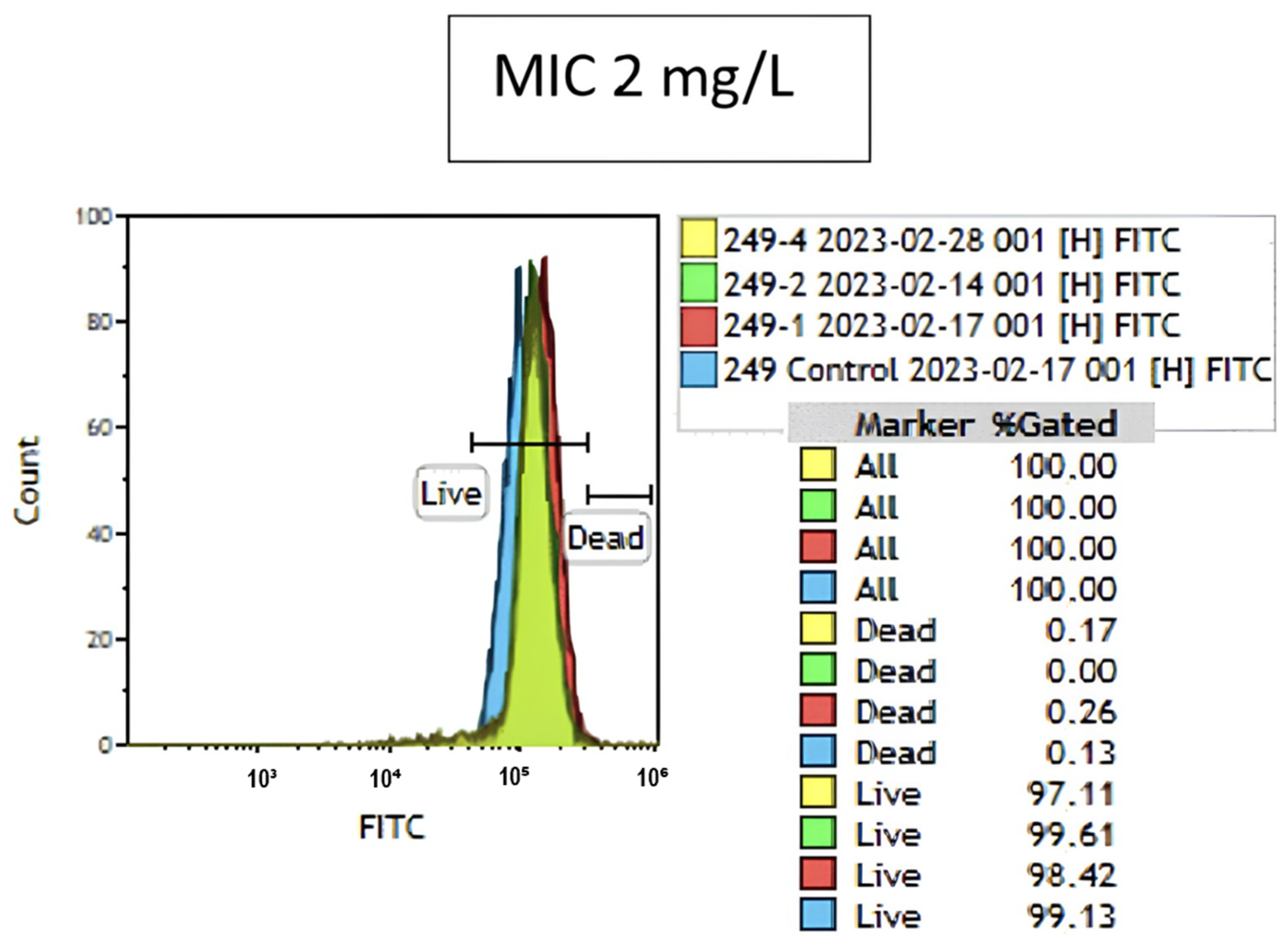
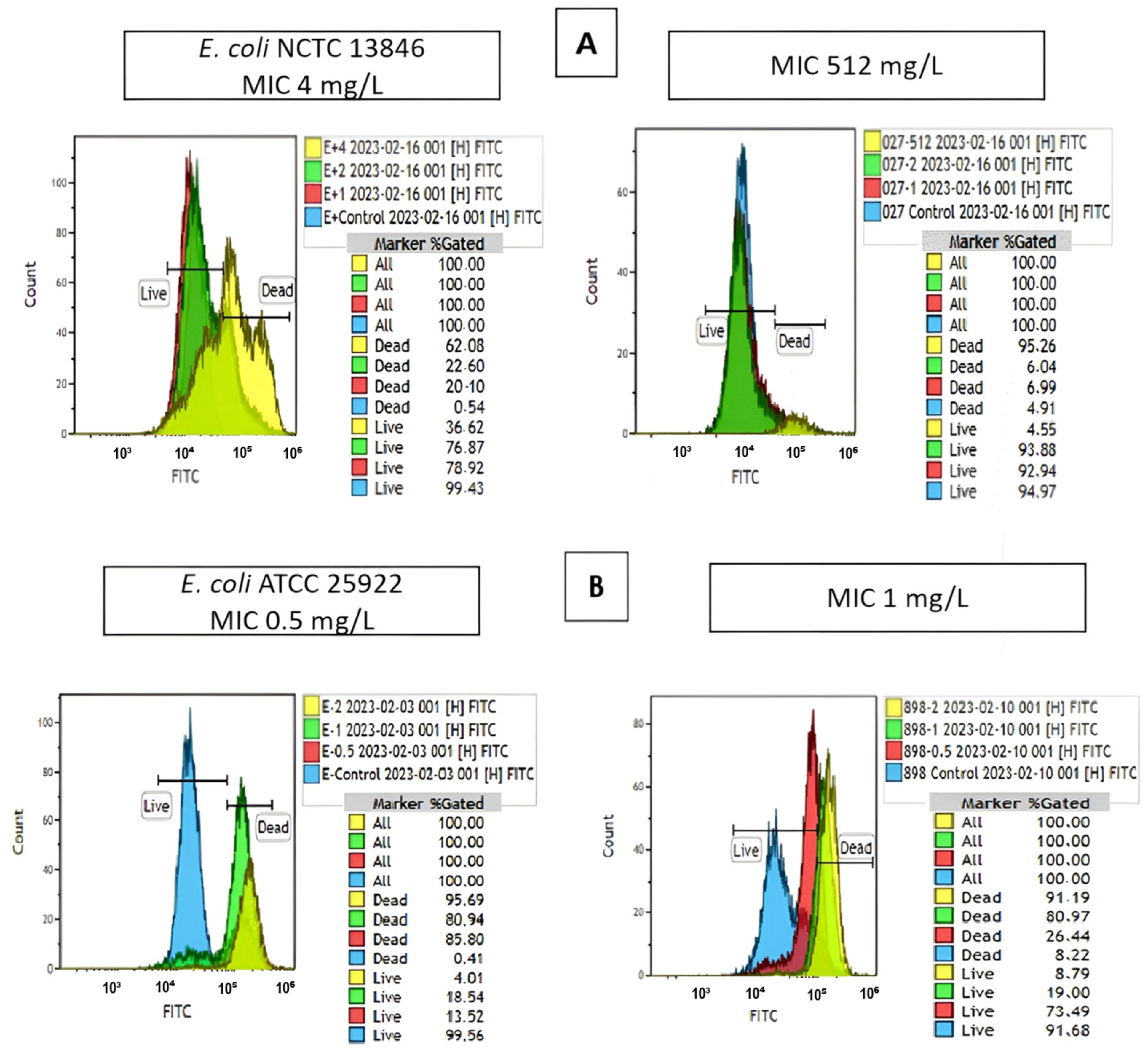
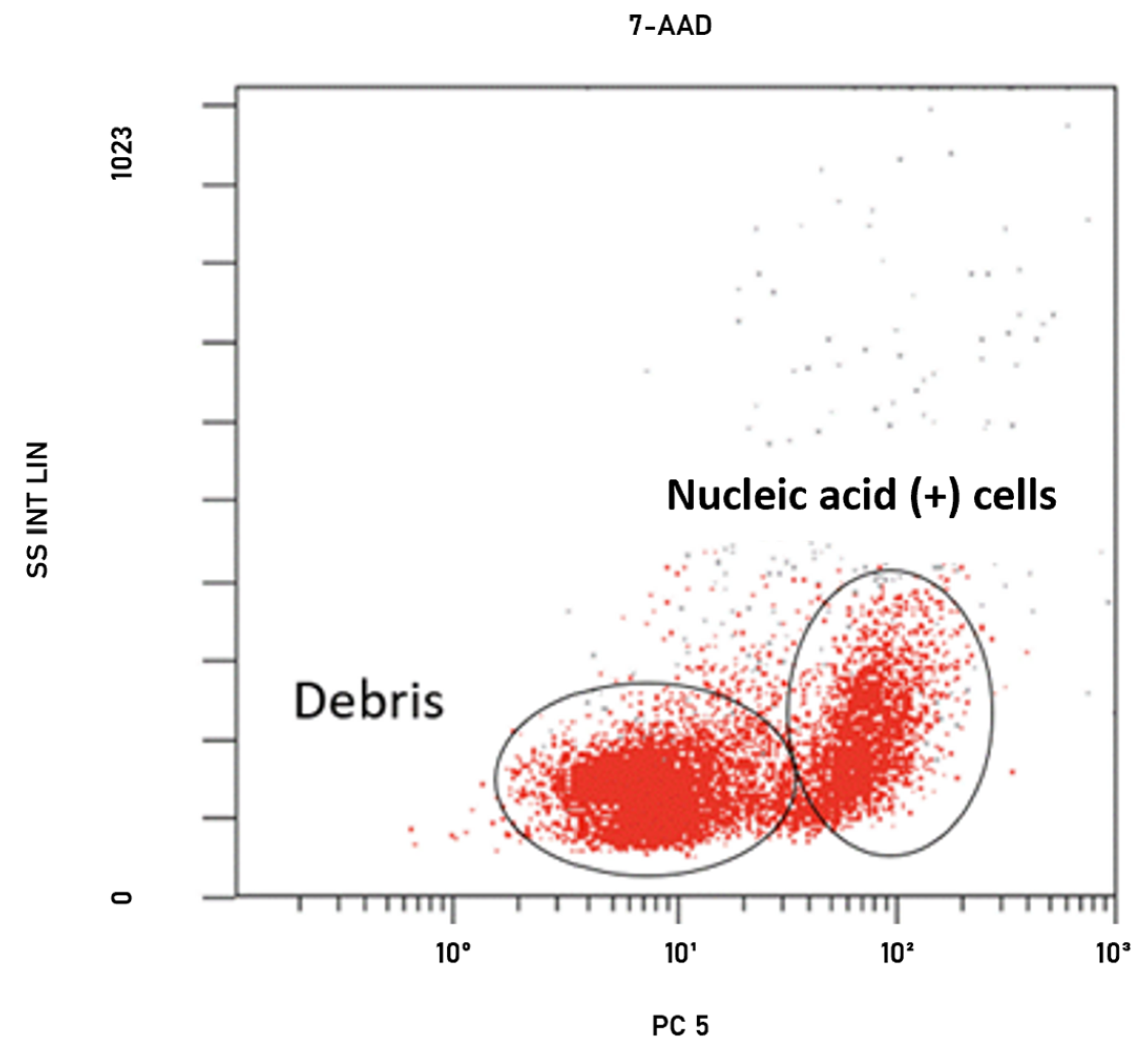
| Susceptible | Resistant | MIC₅₀–MIC₉₀ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colistin MIC (mg/L) | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 128 | 512 | 16 | 32 |
| Number (n) | 1 | 2 | 2 | 2 | 3 | 2 | 5 | 5 | 12 | 29 | 20 | 1 | 1 | ||
| Total | 17 (20%) | 68 (80%) | |||||||||||||
| Broth Microdilution (BMD) | |||
|---|---|---|---|
| Flow Cytometry (FCM) | R1 | S1 | Total |
| R2 | 68 | 1 | 69 |
| S2 | 0 | 16 | 16 |
| Total | 68 | 17 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uçak, Ş.C.; Öngen, B. Flow Cytometry as a Rapid and Valuable Method in Investigation of Colistin Resistance in Carbapenem-Resistant Klebsiella pneumoniae Isolates. Antibiotics 2024, 13, 418. https://doi.org/10.3390/antibiotics13050418
Uçak ŞC, Öngen B. Flow Cytometry as a Rapid and Valuable Method in Investigation of Colistin Resistance in Carbapenem-Resistant Klebsiella pneumoniae Isolates. Antibiotics. 2024; 13(5):418. https://doi.org/10.3390/antibiotics13050418
Chicago/Turabian StyleUçak, Şafak Ceren, and Betigül Öngen. 2024. "Flow Cytometry as a Rapid and Valuable Method in Investigation of Colistin Resistance in Carbapenem-Resistant Klebsiella pneumoniae Isolates" Antibiotics 13, no. 5: 418. https://doi.org/10.3390/antibiotics13050418
APA StyleUçak, Ş. C., & Öngen, B. (2024). Flow Cytometry as a Rapid and Valuable Method in Investigation of Colistin Resistance in Carbapenem-Resistant Klebsiella pneumoniae Isolates. Antibiotics, 13(5), 418. https://doi.org/10.3390/antibiotics13050418





