Natural Antimicrobials in Dairy Products: Benefits, Challenges, and Future Trends
Abstract
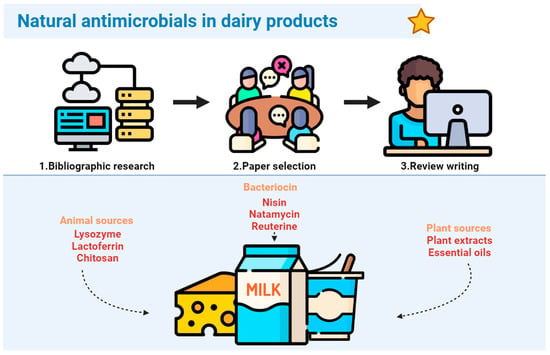
1. Introduction
2. Bacteriocins
2.1. Nisin
2.2. Natamycin
2.3. Reuterine
3. Antimicrobial Compounds from Animal Sources
3.1. Lysozyme
3.2. Lactoferrin
3.3. Chitosan
4. Antimicrobials from Plant Sources
4.1. Plant extracts
4.2. Essential Oils
5. Encapsulation of Natural Antimicrobials
6. Methodology
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leedom, M.; Larson, M.; Marks, H. Dairy product safety: A review of current practices and future trends. J. Dairy Sci. 2020, 103, 10895–10913. [Google Scholar]
- World Health Organization. Global Burden of Diseas: Disease and Injury Incidence, Prevalence, Mortality, and Disability; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 22 January 2024).
- Keba, A.; Rolon, M.L.; Tamene, A.; Dessie, K.; Vipham, J.; Kovac, J.; Zewdu, A. Review of the prevalence of foodborne pathogens in milk and dairy products in Ethiopia. Int. Dairy J. 2020, 109, 104762. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kong, F. Measuring chemical deterioration of foods. In Chemical Changes during Processing and Storage of Foods; Academic Press: Cambridge, MA, USA, 2021; pp. 637–679. [Google Scholar]
- Hussain, A.; Pu, H.; Sun, D.W. SERS detection of sodium thiocyanate and benzoic acid preservatives in liquid milk using cysteamine functionalized core-shelled nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117994. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Antioxidants versus food antioxidant additives and food preservatives. Antioxidants 2019, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Flores, M. Occurrence of Nitrate, Nitrite and Perchlorate in Foods. Encycl. Food Saf. 2023, 2, 510–517. [Google Scholar]
- Hsu, J.L.; Sung, C.C.; Tseng, J.T. Willingness-to-pay for ready-to-eat clean label food products at convenient stores. Future Foods 2023, 7, 100237. [Google Scholar] [CrossRef]
- Delshadi, R.; Bahrami, A.; Assadpour, E.; Williams, L.; Jafari, S.M. Nano/microencapsulated natural antimicrobials to control the spoilage microorganisms and pathogens in different food products. Food Control 2021, 128, 108180. [Google Scholar] [CrossRef]
- Daba, G.M.; Elkhateeb, W.A. Ribosomally synthesized bacteriocins of lactic acid bacteria: Simplicity yet having wide potentials—A review. Int. J. Biol. Macromol. 2023, 256, 128325. [Google Scholar] [CrossRef]
- Murat, D.; Hakkı, T.I. Food stabilizing potential of nisin Z produced by wild Lactococcus lactis subsp. lactis from raw milk and some fermented products. LWT 2021, 150, 112065. [Google Scholar] [CrossRef]
- Wee, S.; Chua, S.L.; Yu, D.; Koh, S.P.; Lee, K.M.; Wu, Y.; Chan, S.H. The detection, characterization, and quantification of dominant degradation products of nisin A and Z in selected dairy products by liquid chromatography–high-resolution mass spectrometry technique. JDS Commun. 2024, 5, 7–12. [Google Scholar] [CrossRef]
- Van Tassell, M.L.; Ibarra-Sánchez, L.A.; Takhar, S.R.; Amaya-Llano, S.L.; Miller, M.J. Use of a miniature laboratory fresh cheese model for investigating antimicrobial activities. J. Dairy Sci. 2015, 98, 8515–8524. [Google Scholar] [CrossRef] [PubMed]
- Taherian, A.R.; Lacasse, P.; Bisakowski, B.; Lanctot, S.; Fustier, P. A comparative study on the rheological and thermogelling properties of chitosan/polyvinyl alcohol blends in dairy products. LWT 2019, 113, 108305. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Koushki, M.R.; Sohrabvandi, S.; Yousefi, M. An overview of antimicrobial activity of lysozyme and its functionality in cheese. Front. Nutr. 2022, 9, 833618. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, F.; Tang, Y.; Ali, M.M.; Shen, Z.; Debrah, A.A.; Yuan, Q.; Du, Z. Boron-doped titania for separation and purification of lactoferrin in dairy products. J. Chromatogr. B. 2022, 1212, 123501. [Google Scholar] [CrossRef]
- Awad, D.A.; El-Hadary, A.; Osman, A.; Alsuhaibani, A.M.; Al-Shawi, A.H.; Elkelish, A.; Hamad, A. Yogurt fortified with enzyme-modified egg white lysozyme impact on sensory, physicochemical, and microbiological properties and potential for functional product development. J. Agric. Food Res. 2023, 14, 100670. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, G.; Zhang, D. Cloning, characterization, and production of a novel lysozyme by different expression hosts. J. Microbiol. Biotechnol. 2014, 24, 1405–1412. [Google Scholar] [CrossRef]
- Moreira, R.V.; Costa, M.P.; Castro, V.S.; Paes, C.E.; Mutz, Y.S.; Frasao, B.S.; Mano, S.B.; Conte-Junior, C.A. Antimicrobial activity of pequi (Caryocar brasiliense) waste extract on goat Minas Frescal cheese presenting sodium reduction. J. Dairy Sci. 2019, 102, 2966–2972. [Google Scholar] [CrossRef] [PubMed]
- Wanniatie, V.; Qisthon, A.; Husni, A.; Yuliawan, D. Physicochemical and Microbiological Quality of Goat’s Milk Yogurt Added Red Ginger (Zingiber officinale var. Rubrum) Extract. IOP Conf. Ser. Earth Environ. Sci. 2022, 1020, 012027. [Google Scholar] [CrossRef]
- Kamel, D.G.; Hammam, A.R.; El-Diin, M.A.N.; Awasti, N.; Abdel-Rahman, A.M. Nutritional, antioxidant, and antimicrobial assessment of carrot powder and its application as a functional ingredient in probiotic soft cheese. J. Dairy Sci. 2023, 106, 1672–1686. [Google Scholar] [CrossRef]
- Ávila, M.; Calzada, J.; Muñoz-Tébar, N.; Sánchez, C.; de Elguea-Culebras, G.O.; Carmona, M.; Molina, A.; Berruga, M.I.; Garde, S. Inhibitory activity of aromatic plant extracts against dairy-related Clostridium species and their use to prevent the late blowing defect of cheese. Food Microbiol. 2023, 110, 104185. [Google Scholar] [CrossRef]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Coutinho, H.D.M.; et al. Combination of essential oils in dairy products: A review of their functions and potential benefits. LWT 2020, 133, 110116. [Google Scholar] [CrossRef]
- Chen, X.; He, Z.; He, L.; Li, C.; Tao, H.; Wang, X.; Liu, L.; Zeng, X.; Ran, G. Effects of perilla seed oil addition on the physicochemical properties, sensory, and volatile compounds of potato blueberry flavored yogurt and its shelf-life prediction. LWT 2023, 173, 114383. [Google Scholar] [CrossRef]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Focus: Plant-based medicine and pharmacology: Essential oils and health. Yale J. Biol. Med. 2020, 93, 291. [Google Scholar]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food safety through natural antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, G.C.; Poletto, G.; Menezes, C.R. Microencapsulation techniques to aggregate values in dairy foods formulation. Dairy Foods 2022, 139–157. [Google Scholar] [CrossRef]
- Vido, M.A.G.; Alvim, I.D.; Vinderola, G.; Berto, M.I.; de Sá, P.B.Z.R.; Pinilla, C.M.B.; Alves, A.T.S.E. Microencapsulation of Limosilactobacillus reuteri (DSM 23878) for application in infant formula: Heat resistance and bacterial viability during long-time storage. Food Res. Int. 2023, 173, 113378. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Nogueira, M.; Scolaro, B.; Thomazini, M.; Ferro-Furtado, R.; de Castro, I.A.; Favaro-Trindade, C.S. Enhancing stability of echium seed oil and beta-sitosterol by their coencapsulation by complex coacervation using different combinations of wall materials and crosslinkers. Food Chem. 2018, 252, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.; Silva, S.P.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, E.F.; Cotter, P.D.; Ross, R.P.; Hill, C. Strategies to improve the bacteriocin protection provided by lactic acid bacteria. Curr. Opin. Biotechnol. 2013, 24, 130–134. [Google Scholar] [CrossRef]
- Ibarra-Sánchez, L.A.; El-Haddad, N.; Mahmoud, D.; Miller, M.J.; Karam, L. Invited review: Advances in nisin use for preservation of dairy products. J. Dairy Sci. 2020, 103, 2041–2052. [Google Scholar] [CrossRef]
- Li, Q.; Montalban-Lopez, M.; Kuipers, O.P. Increasing the antimicrobial activity of nisin-based lantibiotics against Gram-negative pathogens. Appl. Environ. Microbiol. 2018, 84, e00052-18. [Google Scholar] [CrossRef]
- Felício, B.A.; Pinto, M.S.; Oliveira, F.S.; Lempk, M.W.; Pires, A.C.S.; Lelis, C.A. Effects of nisin on S. aureus count and physicochemical properties of Minas Frescal cheese. J. Dairy Sci. 2015, 98, 4364–4369. [Google Scholar] [CrossRef]
- Meshref, M.S.; Hassan, M.; Riad, M.; Ashour, A. Effect of nisin on the viability of S. aureus Kareish Cheese. J. Vet. Med. Res. 2019, 26, 174–185. [Google Scholar] [CrossRef]
- Ning, Y.; Hou, L.; Ma, M.; Li, M.; Zhao, Z.; Zhang, D.; Wang, Z.; Jia, Y. Synergistic antibacterial mechanism of sucrose laurate combined with nisin against S. aureus and its application in milk beverage. LWT 2022, 158, 113145. [Google Scholar]
- Oshima, S.; Hirano, A.; Kamikado, H.; Nishimura, J.; Kawai, Y.; Saito, T. Nisin A extends the shelf life of high-fat chilled dairy dessert, a milk-based pudding. J. Appl. Microbiol. 2014, 116, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Modugno, C.; Loupiac, C.; Bernard, A.; Jossier, A.; Neiers, F.; Perrier-Cornet, J.M.; Simonin, H. Effect of high pressure on the antimicrobial activity and secondary structure of the bacteriocin nisin. Innov. Food Sci. Emerg. Technol. 2018, 47, 9–15. [Google Scholar] [CrossRef]
- Cabrini, C.W.; Cunha, A.L.F.S.; Vieira, P.; Durães, G.L.L.S.; Leo, L.L.; Capuchiho, L.C.F.M.; Pinto, M.S. Inhibitory potential of nisin on S. aureus in artisanal Minas cheese from the cerrado. J. Contin. Educ. Vet. Med. Anim. Sci. CRMV-SP 2016, 14, 67. [Google Scholar]
- Wirjantoro, T.I.; Lewis, M.J.; Grandison, A.S.; Williams, G.C.; Delves-Broughton, J. The effect of nisin on the keeping quality of reduced heat-treated milks. J. Food Prot. 2001, 64, 213–219. [Google Scholar] [CrossRef]
- Bhatti, M.; Veeramachaneni, A.; Shelef, L.A. Factors affecting the antilisterial effects of nisin in milk. Int. J. Food Microbiol. 2004, 97, 215–219. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Koo, O.K.; Sostrin, M.L.; Ricke, S.C.; Crandall, P.G.; Johnson, M.G. Characteristics of bacteriocins and use as food antimicrobials in the United States. In Food and Feed Safety Systems and Analysis; Academic Press: Cambridge, MA, USA, 2018; pp. 273–286. [Google Scholar]
- Meena, M.; Prajapati, P.; Ravichandran, C.; Sehrawat, R. Natamycin: A natural preservative for food application—A review. Food Sci. Biotechnol. 2021, 30, 1481–1496. [Google Scholar] [CrossRef]
- Committee of Experts on Food Additives—JECFA; World Health Organization—WHO. Toxicological recommendations and information about specifications. In Food Additives Expert Committee, Safety Assessment of Certain Additives and Contaminants Food, Proceedings of the Sixty-Seventh Meeting of the Joint FAO/WHO Committee of Experts on Food Additives–JEFCA; WHO: Geneva Switzerland, 2007; p. 350. ISBN 978-92-4-166058-7. [Google Scholar]
- González-Forte, L.D.S.; Amalvy, J.I.; Bertola, N. Effect of natamycin on the physicochemical properties of corn starch based films and their effect on Penicillium spp. activity. Int. J. Polym. Anal. Charact. 2019, 24, 63–74. [Google Scholar] [CrossRef]
- Torrijos, R.; Nazareth, T.M.; Calpe, J.; Quiles, J.M.; Manes, J.; Meca, G. Antifungal activity of natamycin and development of an edible film based on hydroxyethylcellulose to avoid Penicillium spp. growth on low-moisture mozzarella cheese. LWT 2022, 154, 112795. [Google Scholar] [CrossRef]
- Ji, X.; Peng, X.; Long, X.; Zhang, Y.; Lin, J.; Yin, J.; Zhang, R.; Zhao, G. Laccase-mediated functionalization of natamycin by gallic acids for the therapeutic effect on Aspergillus fumigatus keratitis. Eur. J. Pharmacol. 2022, 926, 175041. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, J.; Fang, S.; Zuo, X. Manufacture of carboxymethylchitosan films for cheese packaging containing gliadin-na-carboxymethylchitosan nanoparticles co-encapsulating natamycin and theaflavins. J. Int. of Biological Macromolecules 2023, 246, 125685. [Google Scholar] [CrossRef] [PubMed]
- Stock, R.A.; Bonamigo, E.L.; Cadore, E.; Oechsler, R.A. Infectious crystalline keratopathy caused by Cladosporium sp. after penetrating keratoplasty: A case report. Int. Med. Case Rep. J. 2016, 9, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Lule, V.K.; Garg, S.; Gosewade, S.C.; Khedkar, C.D. Natamycin. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016; pp. 56–62. [Google Scholar] [CrossRef]
- Ombarak, R.A.; Shelaby, H.H. The inhibitory Effect of Natamycin and Potassium Sorbate on Mold Growth in Egyptian Fresh Soft Cheese (Tallaga Cheese). Alex. J. Vet. Sci. 2017, 53, 33–37. [Google Scholar] [CrossRef]
- Küçük, G.S.; Çelik, Ö.F.; Mazi, B.G.; Türe, H. Evaluation of alginate and zein films as a carrier of natamycin to increase the shelf life of kashar cheese. Packag. Technol. Sci. 2020, 33, 39–48. [Google Scholar] [CrossRef]
- Anari, H.N.B.; Majdinasab, M.; Shaghaghian, S.; Khalesi, M. Development of a natamycin-based non-migratory antimicrobial active packaging for extending shelf-life of yogurt drink (Doogh). Food Chem. 2022, 366, 130606. [Google Scholar] [CrossRef] [PubMed]
- Vimont, A.; Fernandez, B.; Ahmed, G.; Fortin, H.P.; Fliss, I. Quantitative antifungal activity of reuterin against food isolates of yeasts and moulds and its potential application in yogurt. Int. J. Food Microbiol. 2019, 289, 182–188. [Google Scholar] [CrossRef]
- Patro-Gołąb, B.; Szajewska, H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for treating acute gastroenteritis in children. An update. Nutrients 2019, 11, 2762. [Google Scholar] [CrossRef]
- Schaefer, L.; Auchtung, T.A.; Hermans, K.E.; Whitehead, D.; Borhan, B.; Britton, R.A. The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology 2010, 156, 1589. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Ortega, S.D.C.; Campos-Múzquiz, L.G.; Charles-Rodriguez, A.V.; Chávez-Gonzaléz, M.L.; Palomo-Ligas, L.; Contreras-Esquivel, J.C.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Biological control of pathogens in artisanal cheeses. Int. Dairy J. 2023, 140, 105612. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Osaili, T.M.; Oqdeh, S.B.; Olaimat, A.N.; Jaradat, Z.W.; Ayyash, M.; Holley, R.A. Antagonistic effects of Lactobacillus reuteri against E. coli O157: H7 in white-brined cheese under different storage conditions. J. Dairy Sci. 2021, 104, 2719–2734. [Google Scholar] [CrossRef] [PubMed]
- Langa, S.; Martín-Cabrejas, I.; Montiel, R.; Peirotén, Á.; Arqués, J.L.; Medina, M. Protective effect of reuterin-producing Lactobacillus reuteri against L. monocytogenes and E. coli O157: H7 in semi-hard cheese. Food Control 2018, 84, 284–289. [Google Scholar] [CrossRef]
- Pilote-Fortin, H.; Said, L.B.; Cashman-Kadri, S.; St-Gelais, D.; Fliss, I. Stability, bioavailability and antifungal activity of reuterin during manufacturing and storage of stirred yoghurt. Int. Dairy J. 2021, 121, 105141. [Google Scholar] [CrossRef]
- Doosh, K.S.; Abdul-Rahman, S.M. Effect of lysozyme isolated from hen egg white in elongation the shelf life of Iraqi soft cheese made from buffalo milk. Pak. J. Nutr. 2014, 13, 635. [Google Scholar] [CrossRef][Green Version]
- Benedé, S.; Molina, E. Chicken egg proteins and derived peptides with antioxidant properties. Foods 2020, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, C.; Labella, C.; Elshafie, H.S.; Camele, I.; Musto, M.; Paolino, R.; D’Adamo, C.; Freschi, P. Effects of different heat treatments on lysozyme quantity and antimicrobial activity of jenny milk. J. Dairy Sci. 2016, 99, 5173–5179. [Google Scholar] [CrossRef] [PubMed]
- D’Incecco, P.; Gatti, M.; Hogenboom, J.A.; Bottari, B.; Rosi, V.; Neviani, E.; Pellegrino, L. Lysozyme affects the microbial catabolism of free arginine in raw-milk hard cheeses. Food Microbiol. 2016, 57, 16–22. [Google Scholar] [CrossRef]
- Ozturkoglu-Budak, S.; Akal, H.C.; Bereli, N.; Cimen, D.; Akgonullu, S. Use of antimicrobial proteins of donkey milk as preservative agents in Kashar cheese production. Int. Dairy J. 2021, 120, 105090. [Google Scholar] [CrossRef]
- Mehyar, G.F.; Al Nabulsi, A.A.; Saleh, M.; Olaimat, A.N.; Holley, R.A. Effects of chitosan coating containing lysozyme or natamycin on shelf-life, microbial quality, and sensory properties of Halloumi cheese brined in normal and reduced salt solutions. J. Food Process. Preserv. 2018, 42, e13324. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Abdelnour, S.A.; Kamal, M.; Khafaga, A.F.; Shakoori, A.M.; Bagadood, R.M.; Naffadi, H.M.; Alyahyawi, A.Y.; Khojah, H.; Alghamdi, S.; et al. Lactoferrin: Antimicrobial impacts, genomic guardian, therapeutic uses and clinical significance for humans and animals. Biomed. Pharmacother. 2023, 164, 114967. [Google Scholar] [CrossRef]
- Cooper, R.E.; Wegner, C.E.; Kügler, S.; Poulin, R.X.; Ueberschaar, N.; Wurlitzer, J.D.; Stettin, D.; Wichard, T.; Pohnert, G.; Küsel, K. Iron is not everything: Unexpected complex metabolic responses between iron-cycling microorganisms. ISME J. 2020, 14, 2675–2690. [Google Scholar] [CrossRef]
- Niaz, B.; Saeed, F.; Ahmed, A.; Imran, M.; Maan, A.A.; Khan, M.K.I.; Tufail, T.; Anjum, F.M.; Hussain, S.; Suleria, H.A.R. Lactoferrin (LF): A natural antimicrobial protein. Int. J. Food Prop. 2019, 22, 1626–1641. [Google Scholar] [CrossRef]
- Brandelli, A.; Lopes, N.A.; Pinilla, C.M.B. Nanostructured Antimicrobials for Quality and Safety Improvement in Dairy Products. Foods 2023, 12, 2549. [Google Scholar] [CrossRef]
- Caputo, L.; Quintieri, L.; Bianchi, D.M.; Decastelli, L.; Monaci, L.; Visconti, A.; Baruzzi, F. Pepsin-digested bovine lactoferrin prevents Mozzarella cheese blue discoloration caused by Pseudomonas fluorescens. Food Microbiol. 2015, 46, 15–24. [Google Scholar] [CrossRef]
- Biernbaum, E.N.; Gnezda, A.; Akbar, S.; Franklin, R.; Venturelli, P.A.; McKillip, J.L. Lactoferrin as an antimicrobial against Salmonella enterica and E. coli O157: H7 in raw milk. JDS Commun. 2021, 2, 92–97. [Google Scholar] [CrossRef]
- Adnan, A.; Nadeem, M.; Ahmad, M.H.; Tayyab, M.; Kamran Khan, M.; Imran, M.; Iqbal, A.; Rahim, M.A.; Awuchi, C.G. Effect of lactoferrin supplementation on composition, fatty acids composition, lipolysis and sensory characteristics of cheddar cheese. Int. J. Food Prop. 2023, 26, 437–452. [Google Scholar] [CrossRef]
- Ombarak, R.A.; Hinenoya, A.; Awasthi, S.P.; Iguchi, A.; Shima, A.; Elbagory, A.R.M. Prevalence and pathogenic potential of E. coli isolates from raw milk and raw milk cheese in Egypt. Int. J. Food Microbiol. 2016, 221, 69–76. [Google Scholar] [CrossRef]
- Hassan, A.Z.H.A.R.; Halim Tawfik Bebawy, J.A.K.L.E.E.N.; Hafaz, R.; S Hasan, W.A.L.A.A. Using Lactoferrin as A Trail to Control E Coli and Staph. Aureus Isolated from Some Types of Cheese. Assiut Vet. Med. J. 2022, 68, 49–57. [Google Scholar] [CrossRef]
- Goulding, D.A.; O’Regan, J.; Bovetto, L.; O’Brien, N.M.; O’Mahony, J.A. Influence of thermal processing on the physicochemical properties of bovine lactoferrin. Int. Dairy J. 2021, 119, 105001. [Google Scholar] [CrossRef]
- Jagdale, S.; Dixit, A.; Gaware, S.; Agarwal, B. Chitosan as excellent bio-macromolecule with myriad of anti-activities in biomedical applications–A review. Int. J. Biol. Macromol. 2023, 257, 128697. [Google Scholar] [CrossRef] [PubMed]
- Research and Markets. Chitosan Market Size, Share & Trends Analysis Report by Application (Pharmaceutical, Water Treatment, Cosmetics, Biomedical, Food & Beverage), by Region, and Segment Forecasts, 2023–2030; Research and Markets: Dublin, Ireland, 2023. [Google Scholar]
- Lingait, D.; Rahagude, R.; Gaharwar, S.S.; Das, R.S.; Verma, M.G.; Srivastava, N.; Kumar, A.; Mandavgane, S. A review on versatile applications of biomaterial/polycationic chitosan: An insight into the structure-property relationship. Int. J. Biol. Macromol. 2023, 257, 128676. [Google Scholar] [CrossRef] [PubMed]
- Alfaifi, M.Y.; Alkabli, J.; Elshaarawy, R.F. Suppressing of milk-borne pathogenic using new water-soluble chitosan-azidopropanoic acid conjugate: Targeting milk-preservation quality improvement. Int. J. Biol. Macromol. 2020, 164, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Zedan, H.; Hosseini, S.M.; Mohammadi, A. The effect of tarragon (Artemisia dracunculus) essential oil and high molecular weight chitosan on sensory properties and shelf life of yogurt. LWT 2021, 147, 111613. [Google Scholar] [CrossRef]
- Dıblan, S.; Kaya, S. Shelf life modelling of kaşar cheese packaged with potassium sorbate, nisin, silver substituted zeolite, or chitosan incorporated active multilayer plastic films. Int. Dairy J. 2023, 140, 105596. [Google Scholar] [CrossRef]
- Dong, J.; Wang, S.; Li, M.; Liu, J.; Sun, Z.; Chen, Z. Application of a Chitosan-based Active Packaging Film Prepared with Cell-free Supernatant of Lacticaseibacillus paracasei ALAC-4 in Mongolian Cheese Preservation. J. Food Prot. 2023, 86, 100158. [Google Scholar] [CrossRef]
- Taha, S.H.; El-Sherbiny, I.M.; Salem, A.S.; Abdel-Hamid, M.; Hamed, A.H.; Ahmed, G.A. Antiviral Activity of Curcumin Loaded Milk Proteins Nanoparticles on Potato Virus Y. Pak. J. Biol. Sci. PJBS 2019, 22, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Bitwell, C.; Sen, I.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Shehata, M.G.; Abd El-Aziz, N.M.; Mehany, T.; Simal-Gandara, J. Taro leaves extract and probiotic lactic acid bacteria: A synergistic approach to improve antioxidant capacity and bioaccessibility in fermented milk beverages. LWT 2023, 187, 115280. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P. The genus Artemisia: A 2012–2017 literature review on chemical composition, antimicrobial, insecticidal and antioxidant activities of essential oils. Medicines 2017, 4, 68. [Google Scholar] [CrossRef]
- Dagli, N.; Dagli, R.; Mahmoud, R.S.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 335–340. [Google Scholar] [CrossRef]
- Jemaa, M.B.; Falleh, H.; Neves, M.A.; Isoda, H.; Nakajima, M.; Ksouri, R. Quality preservation of deliberately contaminated milk using thyme free and nanoemulsified essential oils. Food Chem. 2017, 217, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Abbes, C.; Mansouri, A.; Landoulsi, A. Synergistic effect of the lactoperoxidase system and cinnamon essential oil on total flora and salmonella growth inhibition in raw milk. J. Food Quality. 2018, 2018, 8547954. [Google Scholar] [CrossRef]
- Campos, A.C.L.P.; Nandi, R.D.S.; Scandorieiro, S.; Gonçalves, M.C.; Reis, G.F.; Dibo, M.; Medeiros, L.P.; Panagio, L.A.; Fagan, E.P.; Kobayashi, R.K.T.; et al. Antimicrobial effect of Origanum vulgare (L.) essential oil as an alternative for conventional additives in the Minas Cheese Manufacture. LWT 2022, 157, 113063. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, X.; Zhang, W.; Xia, F.; Sun, M.; Li, H.; Bai, H.; Cui, H.; Shi, L. 1H NMR–based metabolomics reveals the antimicrobial action of oregano essential oil against E. coli and S. aureus in broth, milk, and beef. LWT 2023, 176, 114540. [Google Scholar] [CrossRef]
- Dannenberg, G.S.; Funck, G.D.; Mattei, F.J.; da Silva, W.P.; Fiorentini, Â.M. Antimicrobial and antioxidant activity of essential oil from pink pepper tree (Schinus terebinthifolius Raddi) in vitro and in cheese experimentally contaminated with L. monocytogenes. Innov. Food Sci. Emerg. Technol. 2016, 36, 120–127. [Google Scholar] [CrossRef]
- Tomar, O.; Akarca, G. Efeitos de sorvetes produzidos com óleos essenciais de limão, tangerina e casca de laranja sobre algumas propriedades físico-químicas, microbiológicas e sensoriais. Rev. Veterinária Kocatepe 2019, 12, 62–70. [Google Scholar]
- Fancello, F.; Petretto, G.L.; Marceddu, S.; Venditti, T.; Pintore, G.; Zara, G.; Mannazzu, I.; Budroni, M.; Zara, S. Antimicrobial activity of gaseous Citrus limon var pompia leaf essential oil against L. monocytogenes Ricotta Salata Cheese. Food Microbiol. 2020, 87, 103386. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zheng, J.; He, L.; Li, C.; Hu, P.; Tao, H.; Wang, X. Evaluation of the effect of essential oil addition on the quality parameters and predicted shelf life of potato yogurt. J. Food Prot. 2021, 84, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Kamel, D.G.; Mansour, A.I.; El-Diin, M.A.N.; Hammam, A.R.; Mehta, D.; Abdel-Rahman, A.M. Using Rosemary Essential Oil as a Potential Natural Preservative during Stirred-like Yogurt Making. Foods 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulou, G.; Bardouki, H.; Vamvakias, M.; Panas, P.; Paraskevas, P.; Kourkoutas, Y. Assessment of Antimicrobial Efficiency of Pistacia lentiscus and Fortunella margarita Essential Oils against Spoilage and Pathogenic Microbes in Ice Cream and Fruit Juices. Microbiol. Res. 2022, 13, 667–680. [Google Scholar] [CrossRef]
- Badola, R.; Panjagari, N.R.; Singh, R.R.B.; Singh, A.K.; Prasad, W.G. Effect of clove bud and curry leaf essential oils on the anti-oxidative and anti-microbial activity of burfi, a milk-based confection. J. Food Sci. Technol. 2018, 55, 4802–4810. [Google Scholar] [CrossRef]
- Haseli, A.; Pourahmad, R.; Eshaghi, M.R.; Rajaei, P.; Akbari-Adergani, B. Application of nanoencapsulated Mofarrah (Nepeta crispa) essential oil as a natural preservative in yogurt drink (doogh). LWT 2023, 186, 115256. [Google Scholar] [CrossRef]
- Olabiran, T.E.; Awolu, O.O.; Ayo-Omogie, H.N. Quality chracterization of functional soy-based yoghurt incorporated with scent leaf (Ocimum gratissimum) essential oil microcapsules. Food Chem. Adv. 2023, 3, 100336. [Google Scholar] [CrossRef]
- Genovese, A.; Balivo, A.; Salvati, A.; Sacchi, R. Functional ice cream health benefits and sensory implications. Food Res. Int. 2022, 161, 111858. [Google Scholar] [CrossRef]
- Prakash, B.; Singh, P.P.; Gupta, V.; Raghuvanshi, T.S. Essential oils as green promising alternatives to chemical preservatives for agri-food products: New insight into molecular mechanism, toxicity assessment, and safety profile. Food Chem. Toxicol. 2024, 183, 114241. [Google Scholar] [CrossRef]
- Gurkan, H.; Hayaloglu, A.A. Changes in volatiles and essential oil composition of three organs (leaf, stem and flower) of purple basil (Ocimum basilicum L.) by GC–MS combined with multivariate statistical approach. Food Chem. Adv. 2023, 2, 100292. [Google Scholar] [CrossRef]
- Wani, A.K.; Singh, J.; Shukla, S. Therapeutic application and toxicity associated with Crocus sativus (saffron) and its phytochemicals. Pharmacol. Res.-Mod. Chin. Med. 2022, 4, 100136. [Google Scholar]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef]
- Santhanakrishnan, V.P.; Shoba, N.; Varun, E.; Mohankumar, S.; Raveendran, M. Aromatic profiling of Murraya koenigii leaves by Thermal Desorption Gas chromatography-Mass Spectroscopy (TD-GC-MS). Heliyon 2023, 9, e17832. [Google Scholar]
- Frohlich, P.C.; Santos, K.A.; Ascari, J.; dos Santos Refati, J.R.; Palú, F.; Cardozo-Filho, L.; da Silva, E.A. Antioxidant compounds and eugenol quantification of clove (Syzygium aromaticum) leaves extracts obtained by pressurized liquid extraction and supercritical fluid extraction. J. Supercrit. Fluids 2023, 196, 105865. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Alrosan, M.; Gammoh, S.; Tranchant, C.C.; Alhamad, M.N.; Rababah, T.; Zghoul, R.; Alzoubi, H.; Ghatasheh, S.; Ghozlan, K.; et al. Encapsulation-based technologies for bioactive compounds and their application in the food industry: A roadmap for food-derived functional and health-promoting ingredients. Food Biosci. 2022, 50, 101971. [Google Scholar] [CrossRef]
- Timbe, P.P.R.; da Motta, A.D.S.; Isaía, H.A.; Brandelli, A. Polymeric nanoparticles loaded with Baccharis dracunculifolia DC essential oil: Preparation, characterization, and antibacterial activity in milk. J. Food Process. Preserv. 2020, 44, e14712. [Google Scholar] [CrossRef]
- Tang, P.L.; Cham, X.Y.; Hou, X.; Deng, J. Potential use of cinnamon leaf residues in the fortification of stirred yogurt. Food Biosci. 2022, 48, 101838. [Google Scholar] [CrossRef]
- Locali-Pereira, A.R.; Lopes, N.A.; Menis-Henrique, M.E.C.; Janzantti, N.S.; Nicoletti, V.R. Modulation of volatile release and antimicrobial properties of pink pepper essential oil by microencapsulation in single- and double-layer structured matrices. Int. J. Food Microbiol. 2020, 335, 108890. [Google Scholar] [CrossRef]
- Ismail, S.A.; Hassan, A.A.; Nour, S.A.; El-Sayed, H.S. Production of stirred yogurt fortified with prebiotic xylooligosaccharide, probiotic and symbiotic microcapsules. Biocatal. Agric. Biotechnol. 2023, 50, 102729. [Google Scholar] [CrossRef]
- Pinilha, C.M.B.; Lopes, N.A.; Brandelli, A. Lipid-based nanostructures for the delivery of natural antimicrobials. Molecules 2021, 26, 3587. [Google Scholar] [CrossRef]
- Lopes, N.A.; Pinilla, C.M.B.; Brandelli, A. Antimicrobial activity of lysozyme-nisin co-encapsulated in polysaccharide-coated liposomes. Food Hydrocoll. 2019, 93, 1–9. [Google Scholar] [CrossRef]
- Orhan-Yanıkan, E.; Gülseren, G.; Ayhan, K. Antimicrobial characteristics of Thymus vulgaris and Rosa damascena oils against some bacteria transmitted by milk. Microchem. J. 2022, 183, 108069. [Google Scholar] [CrossRef]
- Azarashkan, Z.; Farahani, S.; Abedinia, A.; Akbarmivehie, M.; Motamedzadegan, A.; Heidarbeigi, J.; Hayaloğlu, A.A. Co-encapsulation of broccoli sprout extract nanoliposomes in broccoli seed gum basil: Effects on antioxidant, antibacterial and anti-Listeria activities in vitro in ricotta cheese. Int. J. Food Microbiol. 2022, 376, 109761. [Google Scholar] [CrossRef] [PubMed]
| Natural Antimicrobial | Dairy Product | Dosage | Main Results and Target Microorganism | References |
|---|---|---|---|---|
| Nisin | Minas Frescal cheese | 500 IU·mL−1 | It increased the lag phase of S. aureus. It resulted in a decrease in S. aureus counts in cheese dough and whey. | [34] |
| Nisin | Dairy beverage | 3 MICs | Associated with lactose laurate, it inhibited the growth of S. aureus for 10 days, stabilizing the pH and relative viscosity. | [36] |
| Natamicin | Mozarella | 0.25, 0.5, and 1 mg/dm2 | The dosage of 1 mg/dm2 of natamycin combined with a hydroxyethylcellulose film and pulverized reduced 5.28 Log CFU/g and 4.19 Log CFU/g (99.9%) of the fungal population of Penicillium spp. It increased the shelf life. | [46] |
| Natamicin | Dairy beverage | 1% pp/p | Films incorporated with 1% natamycin and treated with UV rays for 6 min provided maximum antiyeast activity against Rhodotorula mucilaginosa and Candida parapsilosis. | [53] |
| Reuterin (Lactobacillus reuteri) | Fresh cheese | 6 log10 cfu of Lb. reuteri | Adding L. reuteri significantly decreased (p > 0.05) the E. coli O157:H7 population in fresh cheeses after 28 days in 10% or 15% brine at 10 °C or 25 °C. | [58] |
| Reuterin | Yogurt | 1.38 mM and 6.9 mM | Reuterin at a concentration of 1.38 mM showed a fungistatic effect, and at a concentration of 6.9 mM, it showed a fungicidal effect against a representative panel of contaminating fungi in dairy products. | [54] |
| Lisozyme | Milk | 1.09 mg/L | It inhibited the growth of Bacillus megaterium, Bacillus mojavensis, Clavibacter michiganensis, Clostridium tyrobutyricum, Xanthomonas campestris, and E. coli, with an action similar to ampicillin and kanamycin. The exception was Bacillus mojavensis, which showed resistance to lysozyme in milk samples subjected to heat treatment. | [63] |
| Lysozyme-hydrolyzed peptides | Yogurt | 0.4% | Inhibition of molds and yeasts during 28 days of storage. It resulted in an increased antioxidant capacity. Higher scores for color, appearance, taste, and overall acceptance. | [17] |
| Lactoferrin | Milk | 14.06 mg/mL and 112.5 mg/mL | Significant decrease (p ≤ 0.05) in the count of E. coli O157:H7 and Salmonella enterica at levels equal to or greater than 14.06 mg/mL and 112.5 mg/mL of lactoferrin, respectively. | [72] |
| Lactoferrin | Cheddar | 5, 10, 15, and 20 mg/100 g | After 45 days, viable bacterial counts significantly decreased at doses of 15 and 20 mg/100 g. There was a 22% decrease in the total viable bacterial count and a 72% increase in the antioxidant capacity at a dosage of 20 mg/100 g. All dosages significantly increased the antioxidant capacity, but there was no change in the proximate and fatty acid composition or in the cheese’s color, flavor, and texture scores. | [73] |
| Conjugate of chitosan and azidopropanoic acid | Milk | 0.25 mg/mL | It resulted in the total inhibition of the E. coli O157:H7 and S. aureus population in raw milk refrigerated at 4 °C after 20 and 24 h, respectively. No species of S. aureaus were detected in milk after 6 days of storage. In total, a 99.7% reduction in coliform counts after 10 days of storage. | [80] |
| Chitosan | Kasar cheese | 2% p/p | Incorporated into the active film, it promoted the total inhibition of yeasts and fungi in the cheese after 20 days of storage at 15 °C, a 1.5 log reduction in S. aureus counts, and an increase in titratable acidity. | [82] |
| Pequi extract (Caryocar brasiliense) | Caprin fresh cheese | 6.25 mL/L | Cheeses with the extract added to the dough or those immersed in the extract exhibited a significant decrease (p < 0.05) in LAB counts after 21 days of storage. Samples added from the extract showed lower luminosity (p < 0.05). The cheese added with pequi extract to the dough showed greater hardness (p < 0.05). | [19] |
| Red ginger extract | Goat’s milk yogurt | 1%, 2%, 3%, and 4% p/p | There was a decrease in the total viable bacterial count after adding 2% of the extract. The addition of 2% extract reduced the viscosity, density, and LAB count, but the addition of 4% extract increased the LAB count. | [20] |
| Oregano OE | Cured cheese | 0.02% p/p | It resulted in the inhibition of the growth of Aspergillus flavus, Fusarium oxysporum, Penicillium citrinum, E. coli, and S. aureus during 30 days of maturation. It resulted in a reduction of seven logs in the S. aureus population in the first hour of ripening and the inhibition of all viable E. coli cells after three days of ripening, without changes in the pH and moisture of the cheeses. | [93] |
| Aroeira OE | Ice cream | 0.2% p/p | It resuled in a decrease of approximately 3 log cfu/g in the counts of E. coli, L. monocytogenes, and P. fragi until the seventh week of storage. There was a low sensory acceptance. | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soutelino, M.E.M.; Silva, A.C.d.O.; Rocha, R.d.S. Natural Antimicrobials in Dairy Products: Benefits, Challenges, and Future Trends. Antibiotics 2024, 13, 415. https://doi.org/10.3390/antibiotics13050415
Soutelino MEM, Silva ACdO, Rocha RdS. Natural Antimicrobials in Dairy Products: Benefits, Challenges, and Future Trends. Antibiotics. 2024; 13(5):415. https://doi.org/10.3390/antibiotics13050415
Chicago/Turabian StyleSoutelino, Maria Eduarda Marques, Adriana Cristina de Oliveira Silva, and Ramon da Silva Rocha. 2024. "Natural Antimicrobials in Dairy Products: Benefits, Challenges, and Future Trends" Antibiotics 13, no. 5: 415. https://doi.org/10.3390/antibiotics13050415
APA StyleSoutelino, M. E. M., Silva, A. C. d. O., & Rocha, R. d. S. (2024). Natural Antimicrobials in Dairy Products: Benefits, Challenges, and Future Trends. Antibiotics, 13(5), 415. https://doi.org/10.3390/antibiotics13050415