Journal Description
Vaccines
Vaccines
is an international, peer-reviewed, open access journal published monthly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q1 (Immunology) / CiteScore - Q1 (Pharmacology (medical))
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 18.6 days after submission; acceptance to publication is undertaken in 3.3 days (median values for papers published in this journal in the second half of 2024).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
5.2 (2023);
5-Year Impact Factor:
4.9 (2023)
Latest Articles
Unlocking mRNA Vaccine Potential in Liver Cancer Treatment via Synergistic Bile Acid Modulation
Vaccines 2025, 13(5), 502; https://doi.org/10.3390/vaccines13050502 (registering DOI) - 9 May 2025
Abstract
This Letter to the Editor explores synergistic mechanisms enhancing mRNA cancer vaccine efficacy through bile acid metabolism modulation in liver cancer treatment. The latest evidence indicates that bile acids significantly impair T cell function within the liver cancer microenvironment, creating an immunosuppressive milieu
[...] Read more.
This Letter to the Editor explores synergistic mechanisms enhancing mRNA cancer vaccine efficacy through bile acid metabolism modulation in liver cancer treatment. The latest evidence indicates that bile acids significantly impair T cell function within the liver cancer microenvironment, creating an immunosuppressive milieu that hampers anti-tumor responses. Modulating bile acid composition, particularly increasing ursodeoxycholic acid (UDCA), could reshape the tumor microenvironment (TME) to favor mRNA vaccine-induced T cell activity—a promising strategy to overcome current immunotherapy limitations in liver cancer.
Full article
(This article belongs to the Special Issue Vaccines Targeting the Tumor Microenvironment: Challenges and Future Prospects)
Open AccessArticle
Safety and Influenza Infections in Children Aged 6–35 Months Receiving Cell Culture-Derived Inactivated Quadrivalent Influenza Vaccine During the 2023–2024 Influenza Season in South Korea
by
Hye Eun Lee, Seong-Beom Park, Hye-Young Kim, Sun Heom Baik, Kyungyeon Jung, Juhwan Kim and Ji Young Park
Vaccines 2025, 13(5), 501; https://doi.org/10.3390/vaccines13050501 - 8 May 2025
Abstract
Background/Objectives: Influenza poses a significant risk for young children, particularly those under five. Cell culture-derived influenza vaccines offer advantages in reducing adaptive changes and mitigating egg allergy concerns. SKYCellflu® quadrivalent has been in use since 2015, and this study aimed to assess
[...] Read more.
Background/Objectives: Influenza poses a significant risk for young children, particularly those under five. Cell culture-derived influenza vaccines offer advantages in reducing adaptive changes and mitigating egg allergy concerns. SKYCellflu® quadrivalent has been in use since 2015, and this study aimed to assess its safety and influenza infections in children aged 6–35 months in South Korea. Methods: A prospective cohort, non-interventional, multi-center post-marketing surveillance study was conducted from 2020 to 2024. This study presents data from the 2023–2024 influenza season on safety and influenza infections in children aged 6–35 months following SKYCellflu® vaccination. Safety was assessed based on adverse events (AEs) within 28 days post-vaccination, and influenza infections were assessed via phone calls or medical record screening. Results: Among 333 safety set participants, 54.4% reported at least one AE, with most being mild to moderate. The cumulative incidence of influenza infections among 247 ad hoc subsets was 4.5%, and the incidence rate was 1.3 per 100 person-months (95% CI, 0.7–2.4) during the 2023–2024 influenza season. The two-dose regimen in vaccine-naïve infants aged 6–11 months showed a lower cumulative incidence of influenza infection rate (0.8% vs. 3.8%) and incidence rate (0.3 vs. 0.9 per 100 person-months) than the one-dose group (3.8%). No influenza-related hospitalizations occurred within the ad hoc subset. Conclusions: This study demonstrated a tolerable safety profile and the pattern of influenza infections following SKYCellflu® vaccination. Additionally, the two-dose regimen was associated with a lower incidence of influenza infections, suggesting potential benefits in enhancing protection among infants aged 6–11 months.
Full article
(This article belongs to the Special Issue Vaccine Development for Influenza Virus)
►▼
Show Figures
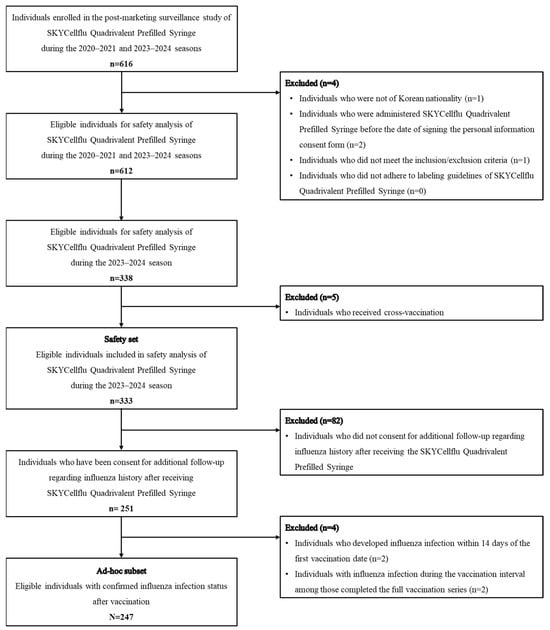
Figure 1
Open AccessArticle
Single-Domain Antibodies That Specifically Recognize Intact Capsids of Multiple Foot-and-Mouth Disease Serotype O Strains
by
Michiel M. Harmsen, Nishi Gupta, Quillan Dijkstra, Sandra van de Water, Marga van Setten and Aldo Dekker
Vaccines 2025, 13(5), 500; https://doi.org/10.3390/vaccines13050500 - 8 May 2025
Abstract
Background/Objectives: Intact (146S) foot-and-mouth disease virus (FMDV) particles easily dissociate into 12S particles with a concomitant decreased immunogenicity. Vaccine quality control with 146S-specific single-domain antibodies (VHHs) is hampered by the high strain specificity of most 146S-specific VHHs. This study aimed to isolate 146S-specific
[...] Read more.
Background/Objectives: Intact (146S) foot-and-mouth disease virus (FMDV) particles easily dissociate into 12S particles with a concomitant decreased immunogenicity. Vaccine quality control with 146S-specific single-domain antibodies (VHHs) is hampered by the high strain specificity of most 146S-specific VHHs. This study aimed to isolate 146S-specific VHHs that recognize all serotype O strains. Methods: Biopanning was performed with the FMDV strain O/SKR/7/2010 146S, using a secondary library of mutagenized M170F VHH that did not recognize O/SKR/7/2010 or using phage-display libraries from llamas immunized with other serotype O strains. Novel VHHs were yeast-produced and their strain-, particle-, and antigenic-site specificities were determined by ELISA. Results: M170F mutagenesis did not improve the cross-reaction with O/SKR/7/2010. However, selection from immune libraries resulted in four VHHs that exhibited high 146S specificity for all five serotype O strains analyzed. These VHHs presumably recognize all serotype O strains since the five strains analyzed represent different phylogenetic clades. They bind the same antigenic site as M170F, which was previously shown to be a conserved site in serotypes A and O, and which has an altered 3D structure when 146S dissociates into 12S particles. M916F had the lowest limit of detection, which varied from 0.7 to 5.9 ng/mL 146S particles for three serotype O strains. Conclusions: We identified four VHHs (M907F, M910F, M912F, and M916F) that specifically bind 146S particles of probably all serotype O strains. They enable further improved FMDV vaccine quality control.
Full article
(This article belongs to the Special Issue Vaccine and Vaccination in Veterinary Medicine)
►▼
Show Figures
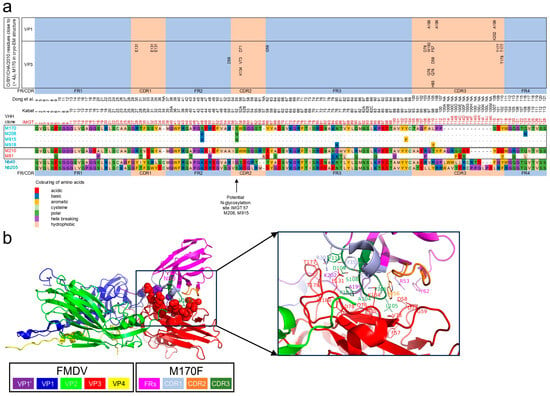
Figure 1
Open AccessRetraction
RETRACTED: Umakanthan et al. COVID-19 Vaccine Hesitancy and Resistance in India Explored through a Population-Based Longitudinal Survey. Vaccines 2021, 9, 1064
by
Srikanth Umakanthan, Sonal Patil, Naveen Subramaniam and Ria Sharma
Vaccines 2025, 13(5), 499; https://doi.org/10.3390/vaccines13050499 - 8 May 2025
Abstract
The journal retracts the article, titled “COVID-19 Vaccine Hesitancy and Resistance in India Explored through a Population-Based Longitudinal Survey” [...]
Full article
Open AccessReview
The New Era of Pneumococcal Vaccination in Adults: What Is Next?
by
Lale Ozisik
Vaccines 2025, 13(5), 498; https://doi.org/10.3390/vaccines13050498 - 7 May 2025
Abstract
Streptococcus pneumoniae remains the leading cause of community-acquired pneumonia in adults and bacterial meningitis in children worldwide. In addition to pneumonia, invasive pneumococcal diseases (IPDs), such as bacteremia and meningitis, pose a significant burden, particularly among older adults and individuals with underlying comorbidities.
[...] Read more.
Streptococcus pneumoniae remains the leading cause of community-acquired pneumonia in adults and bacterial meningitis in children worldwide. In addition to pneumonia, invasive pneumococcal diseases (IPDs), such as bacteremia and meningitis, pose a significant burden, particularly among older adults and individuals with underlying comorbidities. These diseases lead to substantial morbidity and mortality. Pneumococcal vaccination has been a cornerstone of disease prevention, reducing incidence and antimicrobial resistance. Recent advances in understanding S. pneumoniae epidemiology, genomic diversity, and the real-world impact of conjugate vaccines have driven the development and licensure of new-generation pneumococcal vaccines with expanded serotype coverage. Introducing 15-valent (PCV15), 20-valent (PCV20), and 21-valent (PCV21) conjugate vaccines has reshaped pneumococcal immunization strategies, particularly in adults, replacing previous sequential vaccine recommendations in many settings. In parallel, emerging epidemiological data and shifts in pneumococcal serotype distribution continue to influence vaccine policy decisions and immunization guidelines worldwide. In light of these advancements, adult pneumococcal vaccination recommendations continuously evolve to enhance protection in high-risk populations and optimize long-term immunity. This review provides an updated overview of the pneumococcal disease burden, the evolution of pneumococcal vaccines, and the latest immunization strategies in an expanding vaccine landscape. Additionally, we discuss future directions in pneumococcal vaccine development and the potential impact of novel vaccination approaches on public health outcomes.
Full article
(This article belongs to the Special Issue Vaccines and Vaccine Preventable Diseases)
►▼
Show Figures
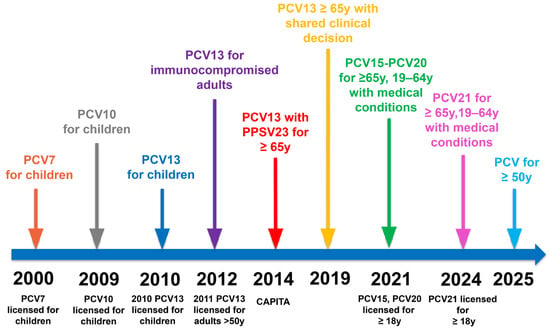
Figure 1
Open AccessArticle
A QS21 + CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice
by
Han Cao, Xiaolong Zhang, Jishuai Cheng, Yang Li, Ning Luan, Jingping Hu, Bingyan Liang, Haihao Zhang, Dandan Gao, Zhentao Lei, Yufeng Yao and Cunbao Liu
Vaccines 2025, 13(5), 497; https://doi.org/10.3390/vaccines13050497 - 6 May 2025
Abstract
Background: HSV-2 infection continues to be a significant global health concern, as there are no approved vaccines despite numerous attempts at development. Methods: This study explored the immunogenicity and protective efficacy of aluminum- or QS21 + CpG-adjuvanted trivalent HSV-2 vaccines and a trivalent
[...] Read more.
Background: HSV-2 infection continues to be a significant global health concern, as there are no approved vaccines despite numerous attempts at development. Methods: This study explored the immunogenicity and protective efficacy of aluminum- or QS21 + CpG-adjuvanted trivalent HSV-2 vaccines and a trivalent HSV-2 mRNA vaccine incorporating the gC2, gD2, and gE2 antigens. Results: Our results demonstrated that the QS21 + CpG-adjuvanted subunit vaccine and mRNA vaccines successfully induced robust antigen-specific humoral and cellular immune responses and provided significant protection against both HSV-2 and HSV-1 infection. These vaccines showed remarkable efficiency in reducing the viral load and preventing clinical symptoms in mice, highlighting their potential for clinical application. Conversely, the aluminum-adjuvanted vaccine exhibited limited effectiveness, emphasizing the superiority of the QS21 + CpG-adjuvanted and mRNA vaccines. Conclusions: These findings provide valuable insights for the continued development of effective HSV vaccines and suggest promising strategies for preventing both HSV-2 and HSV-1 infection.
Full article
(This article belongs to the Special Issue Novel Vaccine Designs to Enhance the Engagement of Innate and Adaptive Immunity)
►▼
Show Figures
Figure 1
Open AccessReview
Harnessing Dendritic Cell Function in Hepatocellular Carcinoma: Advances in Immunotherapy and Therapeutic Strategies
by
Shiding Ying, Haiyan Liu, Yongliang Zhang and Yu Mei
Vaccines 2025, 13(5), 496; https://doi.org/10.3390/vaccines13050496 - 4 May 2025
Abstract
Hepatocellular carcinoma (HCC) is a major cause of cancer-related mortality worldwide. Conventional therapies are frequently limited by tumor heterogeneity and the immunosuppressive tumor microenvironment (TME). Dendritic cells (DCs), central to orchestrating antitumor immunity, have become key targets for HCC immunotherapy. This review examines
[...] Read more.
Hepatocellular carcinoma (HCC) is a major cause of cancer-related mortality worldwide. Conventional therapies are frequently limited by tumor heterogeneity and the immunosuppressive tumor microenvironment (TME). Dendritic cells (DCs), central to orchestrating antitumor immunity, have become key targets for HCC immunotherapy. This review examines the biological functions of DC subsets (cDC1, cDC2, pDC, and moDC) and their roles in initiating and modulating immune responses against HCC. We detail the mechanisms underlying DC impairment within the TME, including suppression by regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and cancer-associated fibroblasts (CAFs). Additionally, we discuss novel DC-based therapeutic strategies, such as DC-based vaccines designed to enhance antigen presentation and T cell activation. Combining DC vaccines with immune checkpoint inhibitors (ICIs), including PD-1/PD-L1 and CTLA-4 blockers, demonstrates synergistic effects that can overcome immune evasion and improve clinical outcomes. Despite progress, challenges related to DC subset heterogeneity, TME complexity, and patient variability require the further optimization and personalization of DC-based therapies. Future research should focus on refining these strategies, leveraging advanced technologies like genomic profiling and artificial intelligence, to maximize therapeutic efficacy and revolutionize HCC treatment. By restoring DC function and reprogramming the TME, DC-based immunotherapy holds immense potential to transform the management of HCC and improve patient survival.
Full article
(This article belongs to the Special Issue Dendritic Cells (DCs) and Cancer Immunotherapy)
►▼
Show Figures

Figure 1
Open AccessArticle
Attenuating Mutations in Usutu Virus: Towards Understanding Orthoflavivirus Virulence Determinants and Live Attenuated Vaccine Design
by
Johanna M. Duyvestyn, Peter J. Bredenbeek, Marie J. Gruters, Ali Tas, Tessa Nelemans, Marjolein Kikkert and Martijn J. van Hemert
Vaccines 2025, 13(5), 495; https://doi.org/10.3390/vaccines13050495 - 3 May 2025
Abstract
►▼
Show Figures
Background/Objectives: Understanding virulence determinants can inform safer and more efficacious live attenuated vaccine design. However, applying this knowledge across related viruses does not always result in conserved phenotypes from similar mutants. Methods: Using Usutu virus (USUV), an emerging orthoflavivirus spreading through Europe, we
[...] Read more.
Background/Objectives: Understanding virulence determinants can inform safer and more efficacious live attenuated vaccine design. However, applying this knowledge across related viruses does not always result in conserved phenotypes from similar mutants. Methods: Using Usutu virus (USUV), an emerging orthoflavivirus spreading through Europe, we assessed whether the attenuating effect of the mutations described for related orthoflaviviruses is conserved. Candidate attenuating mutations were selected based on previous studies in other orthoflaviviruses and incorporated into USUV. Results: Nine variants, with mutations in the USUV envelope, non-structural (NS) proteins NS1, NS2A, or NS4B were stable and selected for further characterisation. The variants with an attenuating phenotype in cell culture were then compared to the wild-type virus in an Ifnar−/− mouse model. Mutations of the envelope glycosylation sites and glycosaminoglycan binding sites, which were recognised as more-conserved mechanisms of orthoflavivirus attenuation, were attenuating in USUV as well. However, not all the mutations explored in the USUV non-structural proteins exhibited an attenuated phenotype. Instead, the attenuation was either less pronounced, or there was no change in phenotype relative to the wild-type virus at all. Conclusions: In addition to improving our understanding of USUV virulence determinants, these results add to a growing body of literature highlighting the most promising mechanisms to target for the design of safe live attenuated vaccines against emerging orthoflaviviruses.
Full article
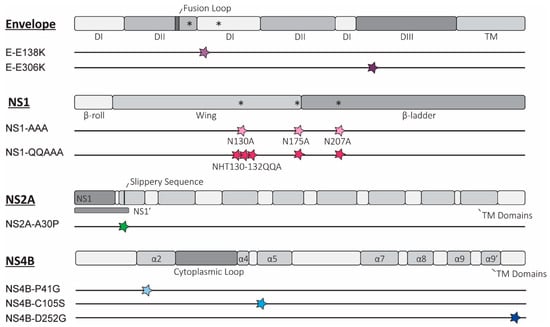
Figure 1
Open AccessReview
Bovine Adenoviral Vector-Based Platform for Vaccine Development
by
Ekramy E. Sayedahmed, Vivek Gairola, Muralimanohara S. T. Murala and Suresh K. Mittal
Vaccines 2025, 13(5), 494; https://doi.org/10.3390/vaccines13050494 - 3 May 2025
Abstract
Adenoviral (AdV) vector-based vaccines employing the human AdV (HAdV) and chimpanzee AdV (ChAdV) vector platforms played a crucial role in combating the COVID-19 pandemic. However, the widespread use of these platforms, the prevalence of various HAdV types, and the resulting preexisting immunity have
[...] Read more.
Adenoviral (AdV) vector-based vaccines employing the human AdV (HAdV) and chimpanzee AdV (ChAdV) vector platforms played a crucial role in combating the COVID-19 pandemic. However, the widespread use of these platforms, the prevalence of various HAdV types, and the resulting preexisting immunity have significantly impacted the vaccines utilizing these vector platforms. Considering these challenges, the bovine AdV type 3 (BAdV-3) vector system has emerged as a versatile and innovative platform for developing next-generation vaccines against infectious diseases. Inherent attributes like a high transduction efficiency, large transgene insertion capacity, broad tissue tropism, and robust induction of innate immunity add significant value to the BAdV vector platform for vaccine design. BAdV-3 vectors effectively elude HAdV-specific preexisting humoral and cellular immune responses. Additionally, BAdV-3 is low in pathogenicity for its host and is anticipated to be safe as a vaccine platform. This systematic review provides an overview of the development of BAdV-3 as a vaccine delivery platform and its application in designing vaccines for infectious agents of human and veterinary importance.
Full article
(This article belongs to the Special Issue Innovations in Vaccine Technology)
►▼
Show Figures

Figure 1
Open AccessConference Report
Proceedings of the 7th Asia Dengue Summit, June 2024
by
Zulkifli Ismail, Duane J. Gubler, Tikki Pangestu, Usa Thisyakorn, Nattachai Srisawat, Daniel Goh, Maria Rosario Capeding, Lulu Bravo, Sutee Yoksan, Terapong Tantawichien, Sri Rezeki Hadinegoro, Kamran Rafiq and Eng Eong Ooi
Vaccines 2025, 13(5), 493; https://doi.org/10.3390/vaccines13050493 - 2 May 2025
Abstract
Background: The 7th Asia Dengue Summit (ADS), titled “Road Map to Zero Dengue Death”, was held in Malaysia from 5 to 7 June 2024. The summit was co-organized by Asia Dengue Voice and Action (ADVA); Global Dengue and Aedes-Transmitted Diseases Consortium
[...] Read more.
Background: The 7th Asia Dengue Summit (ADS), titled “Road Map to Zero Dengue Death”, was held in Malaysia from 5 to 7 June 2024. The summit was co-organized by Asia Dengue Voice and Action (ADVA); Global Dengue and Aedes-Transmitted Diseases Consortium (GDAC); Southeast Asian Ministers of Education Tropical Medicine and Public Health Network (SEAMEO TROPMED); Fondation Mérieux (FMx); and the International Society for Neglected Tropical Diseases (ISNTD). Objectives: Dengue experts from academia and research, as well as representatives from the Ministries of Health, Regional and Global World Health Organization (WHO), and International Vaccine Institute (IVI), came together to highlight the crucial need for an integrated approach for dengue control and achieve the target of zero dengue deaths. Methods: With more than 50 speakers and delegates from over 28 countries, twelve symposiums, and three full days, the 7th ADS highlighted approaches to curb the growing danger of dengue. The summit included topics ranging from emerging dengue trends, insights from dengue human infection models, the immunology of dengue, and vaccine updates to antivirals and host-directed therapeutics. Conclusions: The 7th Asia Dengue Summit reinforced the importance of an integrated, collaborative approach to dengue prevention and control. By bringing together diverse stakeholders and launching innovative initiatives such as the Dengue Slayers Challenge, the summit advanced the regional and global agenda to achieve zero dengue deaths. The exchange of knowledge and strategies at the summit is expected to contribute significantly to improved dengue management and community engagement in affected regions.
Full article
(This article belongs to the Section Vaccines against Tropical and other Infectious Diseases)
Open AccessArticle
Pediatric Rotavirus Hospitalization Rates in the Military Health System Before and During the COVID-19 Pandemic
by
Matthew D. Penfold, Sarah Prabhakar, Apryl Susi, Michael Rajnik, Cade M. Nylund and Matthew D. Eberly
Vaccines 2025, 13(5), 492; https://doi.org/10.3390/vaccines13050492 - 2 May 2025
Abstract
Background/Objectives: Rotavirus gastroenteritis is a vaccine-preventable disease that leads to hospitalization in children less than 5 years of age. Immunizations to prevent rotavirus have greatly altered the epidemiology of significant diarrheal illness. It has been reported that routine immunization rates in children were
[...] Read more.
Background/Objectives: Rotavirus gastroenteritis is a vaccine-preventable disease that leads to hospitalization in children less than 5 years of age. Immunizations to prevent rotavirus have greatly altered the epidemiology of significant diarrheal illness. It has been reported that routine immunization rates in children were impacted during the COVID-19 pandemic. Contrary to this fact, rates of many childhood illnesses also decreased. Methods: The Military Health System Data Repository (MDR) contains the health records of all military beneficiaries. We queried the MDR before and during the COVID-19 pandemic to assess for alterations in immunization rates and hospitalization rates and to assess for risk factors for significant (hospitalizations) rotavirus disease. Results: Our study included a cohort of 1.27 million children under the age of 5 years old. There were 186 unique cases of rotavirus-related hospitalizations over the 5-year study period. During COVID-19 Years 1 and 2, there was a decrease in rotavirus-related hospitalizations compared to the pre-pandemic period. During Year 3, there was a return to the pre-pandemic level of rotavirus hospitalization rates. Patients in the northern United States were less likely to be hospitalized from rotavirus when compared to those in the south. The patients at greatest risk were the youngest beneficiaries. Rotavirus vaccination rates declined in this age group during all three years of the pandemic. Conclusions: As the pandemic resulted in less frequent rotavirus immunizations in the Military Health System (MHS), there was not an increase in rotavirus-related hospitalizations above the pre-pandemic baseline.
Full article
(This article belongs to the Section Vaccines against Infectious Diseases)
►▼
Show Figures
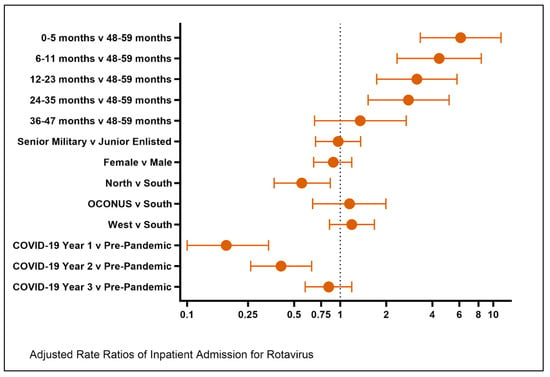
Figure 1
Open AccessArticle
Genetic Sequencing of a Bacterial Pneumonia Vaccine Produced in 1916
by
Yongli Xiao, Sebastian M. Gygli, Tomoko Y. Steen and Jeffery K. Taubenberger
Vaccines 2025, 13(5), 491; https://doi.org/10.3390/vaccines13050491 - 2 May 2025
Abstract
►▼
Show Figures
Background/Objectives: Bacterial vaccines were first developed and used in the late 1800s to prevent chicken cholera and anthrax. Bacterial pneumonia vaccines were widely used during the 1918 influenza pandemic, despite the influenza A/H1N1 virus not yet being identified. Studies showed that bacterial
[...] Read more.
Background/Objectives: Bacterial vaccines were first developed and used in the late 1800s to prevent chicken cholera and anthrax. Bacterial pneumonia vaccines were widely used during the 1918 influenza pandemic, despite the influenza A/H1N1 virus not yet being identified. Studies showed that bacterial pathogens, including Haemophilus influenzae, Streptococcus pneumoniae, and Streptococcus pyogenes, contributed significantly to fatal secondary bacterial pneumonias during the pandemic. In this study, we aimed to characterize the microbial composition of two ampules of a mixed bacterial influenza vaccine produced in 1916, which were labeled as containing killed Bacillus influenzae, Pneumococci, and Streptococcus pyogenes. Methods: DNA was extracted from two 1916-era vaccine ampules, and due to low DNA yields, whole genome amplification (WGA) was performed prior to construction of Illumina sequencing libraries. Deep sequencing was conducted, followed by bioinformatic analysis to identify bacterial DNA content. Consensus genomes were assembled for predominant species, and further analyzed for serotype, phylogeny, and antibiotic resistance genes. Results: The amount of recoverable DNA from these century-old vaccine ampules was limited. The sequencing results revealed minimal detectable S. pneumoniae DNA. The first ampule contained predominantly H. influenzae DNA, while the second vial primarily contained Enterococcus faecium DNA, in addition to S. pyogenes DNA. Consensus genomes for H. influenzae, S. pyogenes, and E. faecium were assembled and analyzed for serotype, phylogeny, and antibiotic resistance genes. Conclusions: This study presents the first genomic analysis of century-old bacterial pneumonia vaccine ampules from the 1918 influenza pandemic era. The findings provide a unique historical perspective on early vaccine formulations and highlight the limitations of early vaccine production.
Full article

Figure 1
Open AccessArticle
Association Between Human Papillomavirus Vaccination and the Risk of Hashimoto’s Thyroiditis: A Cross-Sectional Study
by
Yifan Yin, Liang Ye, Min Chen, Hao Liu and Jingkun Miao
Vaccines 2025, 13(5), 490; https://doi.org/10.3390/vaccines13050490 - 30 Apr 2025
Abstract
Background/Objectives: Concerns about the occurrence of autoimmune diseases are one of the main reasons influencing the uptake of the human papillomavirus (HPV) vaccine. Limited evidence exists regarding the relationship between HPV vaccination and the risk of Hashimoto’s thyroiditis (HT). Therefore, the purpose
[...] Read more.
Background/Objectives: Concerns about the occurrence of autoimmune diseases are one of the main reasons influencing the uptake of the human papillomavirus (HPV) vaccine. Limited evidence exists regarding the relationship between HPV vaccination and the risk of Hashimoto’s thyroiditis (HT). Therefore, the purpose of this study was to examine the association between HPV vaccination and the risk of HT development in American women. Methods: Using the National Health and Nutrition Examination Survey (NHANES) data from 2007 to 2012, we conducted a cross-sectional study of 2717 women aged 18–59 with comprehensive data on relevant HPV vaccination status, HPV DNA vaginal swab results, and thyroid function. The relationship between HPV vaccination and the risk of HT development was explored by weighted logistic regression, while the association between HPV vaccination and thyroid peroxidase antibodies (TPOAb)/thyroglobulin antibodies (TGAb) levels was analyzed by weighted linear regression. Results: In the fully adjusted model, HPV vaccination was associated with an 87% decrease in the risk of developing HT (OR 0.13; 95% CI 0.02, 0.76). Furthermore, weighted linear regression demonstrated significant negative associations between HPV vaccination and TPOAb levels (−22.27 (−34.86, −9.68), p = 0.001) and TGAb levels (−7.53 (−14.88, −0.18), p = 0.045). HPV vaccination was significantly negatively correlated with the risk of HT development and TPOAb/TGAb levels. Conclusions: We advocate for adherence to vaccination guidelines, which could confer dual protective benefits against HPV and potentially reduce the risk of HT development.
Full article
(This article belongs to the Section Human Papillomavirus Vaccines)
►▼
Show Figures
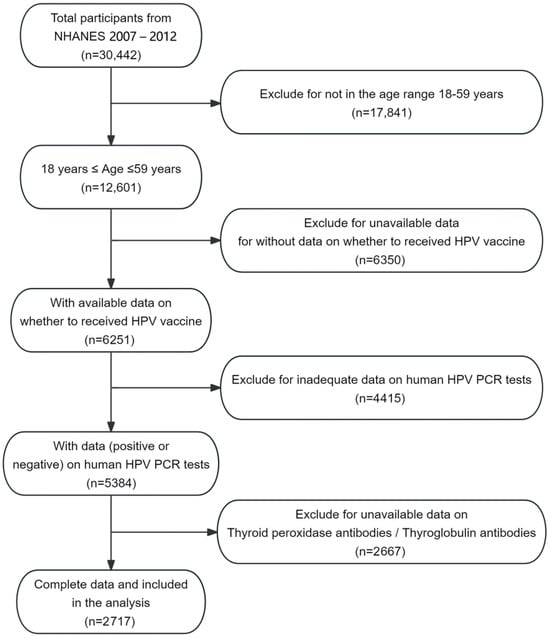
Figure 1
Open AccessArticle
Influenza Vaccine Uptake and Associated Hospitalization Risk in Older Adults with or Without Dementia: Differences Between at Home-Living and Nursing Home Residents in Lombardy, Italy
by
Lorenzo Blandi and Carlo Signorelli
Vaccines 2025, 13(5), 489; https://doi.org/10.3390/vaccines13050489 - 30 Apr 2025
Abstract
Objective: Our population-based cohort study aims to compute the uptake of the influenza vaccine and the associated risk of hospitalization for respiratory diseases of infectious origin based on the residency setting and dementia status of people aged 65 or over. Methods: We conducted
[...] Read more.
Objective: Our population-based cohort study aims to compute the uptake of the influenza vaccine and the associated risk of hospitalization for respiratory diseases of infectious origin based on the residency setting and dementia status of people aged 65 or over. Methods: We conducted a retrospective cohort study on the whole population of residents aged ≥65 in Lombardy, the most populated Italian region. Using region-wide administrative data, we computed the seasonal prevalence of vaccination for influenza from 1 October 2022 to 30 April 2023. To estimate the risk of hospitalization, we applied Fine-Gray sub-distribution hazard models, accounting for the competing risk of death and adjusting for confounders. Results: Our study analyzed 2,420,279 individuals aged 65+ in Lombardy. Overall, 51.4% received an influenza vaccination in 2022–2023. Among residents living at home, 50.8% were vaccinated, while nursing home residents had an uptake of 74.0%. People living with dementia reported a vaccination coverage of 62.6%, and vaccination rates were higher among those residing in nursing homes than those who lived at home. The adjusted sub-hazard ratios (SHRs) showed higher hospitalization risks of 1.88 for unvaccinated individuals with dementia and 1.74 for unvaccinated individuals without dementia living at home. In nursing homes, the SHR for respiratory hospitalization was 2.20 for individuals without dementia and 2.40 for dementia patients. Vaccination reduced risks across all groups, but disparities persisted. Conclusions: People living with dementia were more likely to be hospitalized for respiratory diseases. However, they reported an influenza vaccination coverage that was below expectations and similar to the general population, both in nursing homes and home-living settings. Public health institutions should extend and mention dementia as a higher-risk condition.
Full article
(This article belongs to the Special Issue SARS-CoV-2, Influenza and Other Respiratory Viruses: Preventive Measures Affecting the Determinants of Health and Disease)
►▼
Show Figures
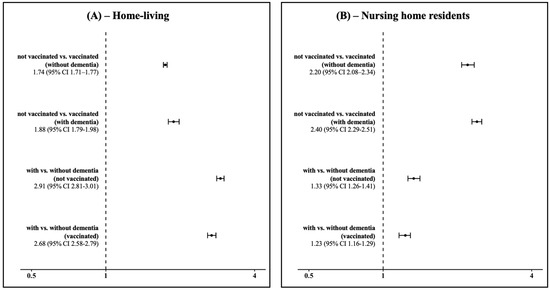
Figure 1
Open AccessArticle
Vaccine-Induced Humoral and Cellular Response to SARS-CoV-2 in Multiple Sclerosis Patients on Ocrelizumab
by
Jelena Drulovic, Olivera Tamas, Neda Nikolovski, Nikola Momcilovic, Vanja Radisic, Marko Andabaka, Bojan Jevtic, Goran Stegnjaic, Milica Lazarevic, Nikola Veselinovic, Maja Budimkic, Sarlota Mesaros, Djordje Miljkovic and Tatjana Pekmezovic
Vaccines 2025, 13(5), 488; https://doi.org/10.3390/vaccines13050488 - 30 Apr 2025
Abstract
Background/Objectives: The aim of our study was to investigate B cell and T cell responses in people with multiple sclerosis (PwMS) treated with ocrelizumab, a humanized anti-CD20 antibody, who were vaccinated with second and/or booster doses of various vaccine brands against COVID-19.
[...] Read more.
Background/Objectives: The aim of our study was to investigate B cell and T cell responses in people with multiple sclerosis (PwMS) treated with ocrelizumab, a humanized anti-CD20 antibody, who were vaccinated with second and/or booster doses of various vaccine brands against COVID-19. Additionally, we detected the outcomes related to COVID-19 in PwMS after vaccination, based on follow-up for at least 12 months. Methods: We enrolled 91 PwMS on ocrelizumab and 42 healthy controls (HCs) in a prospective, single-center study, conducted at the Clinic of Neurology, UCCS, between January 2022 and October 2024. The serological responses were measured using the spike receptor-binding domain (RBD) Architect SARS-CoV-2 IgG Quant kit (Abbot), and cellular responses were measured by quantifying IFN-γ secretion in blood incubated with SARS-CoV-2 antigens. Results: A total of 58.2% (53/91) of PwMS on ocrelizumab and 100% of the HCs (42/42) were seropositive after a second or booster vaccination (p < 0.001), irrespective of the vaccine brand received. Anti-spike antibody levels were significantly lower in PwMS on ocrelizumab compared to the HCs (p < 0.001), again irrespective of the vaccine type. Interferon-γ responses were detected in 95.6% of the PwMS receiving ocrelizumab therapy and 97.6% of HCs after vaccination (p = 0.570). In our cohort, PCR-confirmed SARS-CoV-2 infections after vaccination occurred in a similar proportion of the PwMS (45/91, 49.5%) and HCs (15/32, 46.9%) (p = 0.139). Most of the PwMS (36/45, 79.2%) and HCs (13/15, 87.8%) had COVID-19 of mild severity. Conclusions: PwMS treated with ocrelizumab developed diminished humoral and robust cellular responses following two and three SARS-CoV-2 vaccinations. The obtained immunity after SARS-CoV-2 vaccination may translate into lower incidence and severity of COVID-19.
Full article
(This article belongs to the Special Issue Effectiveness and Safety of Vaccines in Special Populations)
►▼
Show Figures
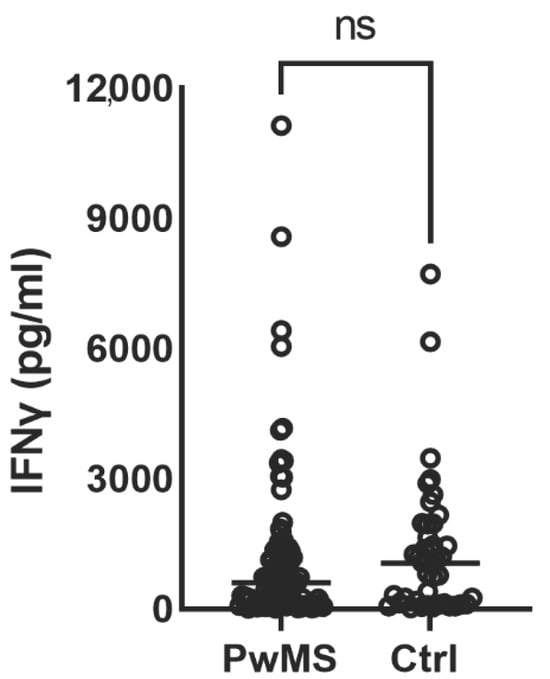
Figure 1
Open AccessArticle
Safety and Immunogenicity of the Attenuated Yellow Fever Vaccine in Several Neotropical Primate Species
by
Nayara Ferreira de Paula, André Duarte Vieira, Daniel Oliveira dos Santos, Lucas dos Reis de Souza, Carlyle Mendes Coelho, Herlandes Penha Tinoco, Paula Cristina Senra Lima, Rafael Otávio Cançado Motta, Valéria do Socorro Pereira, Marcelo Pires Nogueira de Carvalho, Camilla Bayma Fernandes, Adriana de Souza Azevedo, Matheus Soares Arruda, Thais Alkifeles Costa, Betania Paiva Drumond, Fabiola de Oliveira Paes Leme, Marcos da Silva Freire, Tatiane Alves da Paixão, Ayisa Rodrigues Oliveira and Renato Lima Santos
Vaccines 2025, 13(5), 487; https://doi.org/10.3390/vaccines13050487 - 30 Apr 2025
Abstract
Background/Objective: Yellow fever (YF) is an acute infectious disease caused by the yellow fever virus which is transmitted by mosquitoes. Neotropical primates are susceptible to infection, which is often presented as epizootic outbreaks. The aim was to evaluate and characterize the immune response
[...] Read more.
Background/Objective: Yellow fever (YF) is an acute infectious disease caused by the yellow fever virus which is transmitted by mosquitoes. Neotropical primates are susceptible to infection, which is often presented as epizootic outbreaks. The aim was to evaluate and characterize the immune response against YF in different species of neotropical primates from the Belo Horizonte Zoo. Methods: Vaccine 17DD was administered to 24 neotropical primates, with a single subcutaneous dose. Clinical exams, RNAemia, and detection of IgG and neutralizing antibodies against YFV were performed. In addition, an ethogram was performed to assess clinical changes and animal welfare. Results: At 4 days post-vaccination, RNAemia was detected in nine animals. There was seroconversion and persistence of immune response in Alouatta guariba clamitans, Sapajus xanthosternos, Saguinus imperator and Aotus infulatus. However, the vaccine was not immunogenic for Lagothrix cana. In Pithecia irrorata seroconversion did not persist long term, while the Ateles sp. had a transient immune response. No significant clinical manifestations were observed in any of the vaccinated animals. Conclusions: This study demonstrated a safe, immunogenic and persistent immune response induced by the attenuated 17DD vaccine strain in A. guariba clamitans, S. xanthosternos, S. imperator, and A. infulatus.
Full article
(This article belongs to the Special Issue A One-Health Perspective on Immunization Against Infectious Diseases)
►▼
Show Figures
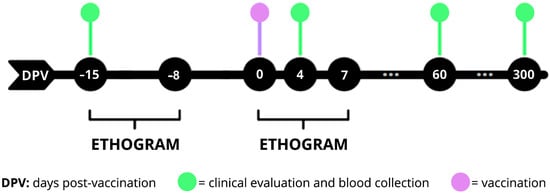
Figure 1
Open AccessArticle
A Similar Nonclinical Safety Evaluation of Prev(e)nar 13 in a Multi-Dose Formulation Containing the Preservative 2-Phenoxyethanol
by
Yana Chervona, Wen Shen, Shambhunath Choudhary, Victoria Markiewicz, Peter C. Giardina and Cynthia M. Rohde
Vaccines 2025, 13(5), 486; https://doi.org/10.3390/vaccines13050486 - 30 Apr 2025
Abstract
Background: 2-Phenoxyethanol (2-PE) has been safely included as a preservative and/or stabilizer in more than thirty vaccine formulations at amounts ranging from 0.5 to 5 mg per dose; however, the nonclinical safety data publicly available for intramuscular (IM) or subcutaneous (SC) administration are
[...] Read more.
Background: 2-Phenoxyethanol (2-PE) has been safely included as a preservative and/or stabilizer in more than thirty vaccine formulations at amounts ranging from 0.5 to 5 mg per dose; however, the nonclinical safety data publicly available for intramuscular (IM) or subcutaneous (SC) administration are relatively limited. Here, in addition to the available clinical and nonclinical data for 2-PE, we summarize the nonclinical safety data of experimental 13vPnC (Prev(e)nar 13) formulations with or without 2-PE. Methods: Two repeat-dose toxicity studies in rabbits, one for a 2-PE-free formulation of 13vPnC and the other for an MDV formulation of 13vPnC with 5 mg/dose 2-PE, were conducted as part of an overall nonclinical safety package for vaccine development. The studies were designed and conducted in compliance with the relevant guidelines and regulations. Results: In repeat-dose toxicity studies in rabbits, five IM administrations of a preservative-free 13vPnC single-dose syringe formulation or a 13vPnC multi-dose vial (MDV) formulation containing 5 mg 2-PE/0.5 mL dose were well tolerated with no systemic toxicity. Robust serotype-specific IgG antibody responses to each of the 13 pneumococcal serotypes were also confirmed for both formulations. The observations for the 13vPnC MDV including local inflammatory reaction, increases in fibrinogen, and increased splenic germinal centers were nonadverse, reversible, and consistent with findings previously observed for the IM administration of vaccines, including the 2-PE-free 13vPnC single-dose syringe formulation. Conclusions: Together with the other available nonclinical and clinical data of 2-PE and vaccine formulations containing 2-PE and following the 3Rs principle, our risk-assessment-based recommendation is that no additional nonclinical safety studies are needed when evaluating a 2-PE-containing presentation of a previously well-characterized vaccine product if the amount of 2-PE is ≤10 mg/dose.
Full article
(This article belongs to the Section Human Vaccines and Public Health)
►▼
Show Figures
Figure 1
Open AccessSystematic Review
Health Equity and Human Papillomavirus Vaccine Interventions for Adolescents: A Systematic Review
by
Sarah B. Maness, Lois Coleman Carpenter, Idara Akpan, Nubwa St. James, Daniela Romero-Cely, G. J. Corey Harmon, Miranda Cano and Erika L. Thompson
Vaccines 2025, 13(5), 485; https://doi.org/10.3390/vaccines13050485 - 30 Apr 2025
Abstract
Background/Objectives: Human papillomavirus (HPV) causes multiple types of cancer, and demographic-based inequities in HPV-related cancers persist. Behavioral interventions have increased HPV vaccination uptake, yet it is unclear how intervention effects vary by demographics. The purpose of this study was to examine whether existing
[...] Read more.
Background/Objectives: Human papillomavirus (HPV) causes multiple types of cancer, and demographic-based inequities in HPV-related cancers persist. Behavioral interventions have increased HPV vaccination uptake, yet it is unclear how intervention effects vary by demographics. The purpose of this study was to examine whether existing HPV vaccine interventions for adolescents have unequal effects on HPV vaccine uptake. Methods: We searched MEDLINE via PubMed, PsycINFO, CINAHL, Scopus, and Cochrane CENTRAL in October 2023. The search strategy combined keywords and subject terms for HPV vaccine, interventions/health promotion, and adolescents. Studies were included in final analyses if they were peer-reviewed, published in the US between 2006 and 2023, included outcome measures from an evidence-based HPV vaccination intervention, included adolescents aged 9–17, and demographic variables for age, race/ethnicity, income/SES, or geographic region. Studies were excluded if they were review articles, abstract-only, dissertations or theses, non-English language, non-US-based, or outside the age range of 9–17. Studies were also excluded if they did not include an intervention, outcome evaluation measures, or demographic measures. The screening and extraction processes were independently performed by multiple reviewers using Covidence software. Results: Ultimately, 74 articles were included for full extraction. Sex was the most common demographic variable analyzed by the HPV vaccine (n = 38), followed by race/ethnicity (n = 15), income/SES (n = 6), and geographic region (n = 6). Conclusions: Few interventions assess whether intervention results differ by demographics, making it unclear whether these interventions reduce health inequities. This review included a wide variation in study designs, limiting our ability to uniformly assess study conclusions.
Full article
(This article belongs to the Special Issue Vaccines and Vaccination: HIV, Hepatitis Viruses, and HPV)
►▼
Show Figures
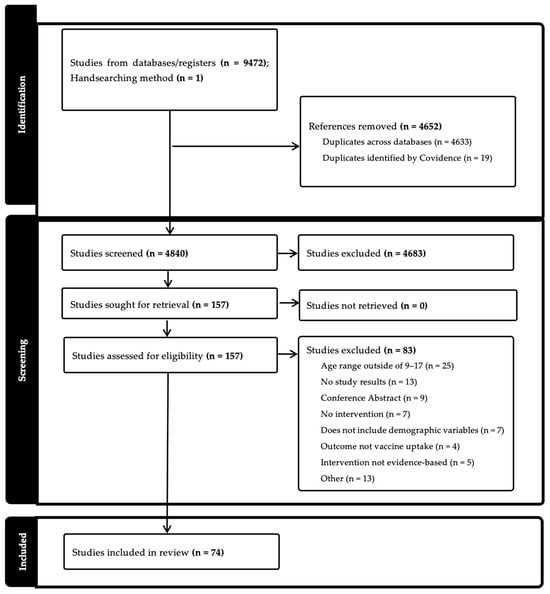
Figure 1
Open AccessArticle
The COVID-19 Vaccination Rollout in Tanzania: The Role of Coordination in Its Success
by
Fredrick Rwegerera, Mwendwa Mwenesi, Belinda J. Njiro, Florian Tinuga, Pricilla Kinyunyi, Mary Rose Giattas, Alice Christensen, Ntuli Kapologwe, Adam Meshack, Joseline Ishengoma, Sophia A. Kagoye, Mwinyi I. Msellem, Mwanahamisi Hassan Magwangwala, Fatma Mohammed Kabole, Daniel Ali and Chizoba Wonodi
Vaccines 2025, 13(5), 484; https://doi.org/10.3390/vaccines13050484 - 30 Apr 2025
Abstract
Background: The national rollout of a vaccine is a complex and significant undertaking, made more challenging when the health system is experiencing shock, such as in a pandemic. Tanzania had relative success in its COVID-19 vaccination rollout compared to other African countries.
[...] Read more.
Background: The national rollout of a vaccine is a complex and significant undertaking, made more challenging when the health system is experiencing shock, such as in a pandemic. Tanzania had relative success in its COVID-19 vaccination rollout compared to other African countries. Objectives: To better understand factors that contributed to this success, we examined the role of coordination (one of the six immunization system building blocks) on the outcomes of the national vaccine rollout. Methods: We obtained qualitative information from the published literature, COVID-19 vaccination program documents for Tanzania Mainland and Zanzibar, and reports from two documentation workshops with national, regional, and district stakeholders from the government, partners, academia, and civil society. Triangulating this information, we describe the COVID-19 vaccination coordination structure, the roles and responsibilities of its members, and the changes in their engagement and activities over the 18 months following the introduction of the COVID-19 vaccine. We also obtained quantitative data from the CHANJOCOVID system to analyze time trends in national COVID-19 vaccine coverage rates for the period August 2021 to December 2022. Results: We found that Tanzania had a multi-level, multi-partner integrated coordination mechanism that provided strategic direction, oversight, and guidance for the vaccination rollout. The coordination structure was initially weak but strengthened over time. Based on the level of coordination activities undertaken, we identified three periods marking different strengths of the coordination mechanisms, these corresponded with different trends in vaccination coverage in the mainland. In the first period (July–December 2021), the coordination mechanism was weak, and vaccine coverage was low, with only 3% of the target population vaccinated on the mainland. In the second period (January–May 2022), when stakeholder engagement was expanded and the coordination mechanism improved, there was a concurrent rise in vaccine coverage from 4% to 25%. In the third period (June–December 2022), coordination was further strengthened, and vaccination strategies were intensified; a corresponding increase in vaccine uptake was observed with coverage reaching 100% of the target population. Conclusions: Qualitative insights from the three time periods suggest a positive association between coordination strength and COVID-19 vaccine coverage. Coordination fostered collaboration, enhanced stakeholder engagement, and facilitated data-driven decision making. This enabled Tanzania to overcome complex challenges and achieve significant progress in vaccination coverage. Strong coordination and effective collaboration among stakeholders are essential mechanisms and processes to optimize vaccine delivery resources and ensure the equitable distribution and uptake of vaccines in Tanzania.
Full article
(This article belongs to the Special Issue Understanding Infectious Disease Vaccinations: Implications for Health and Safety)
►▼
Show Figures
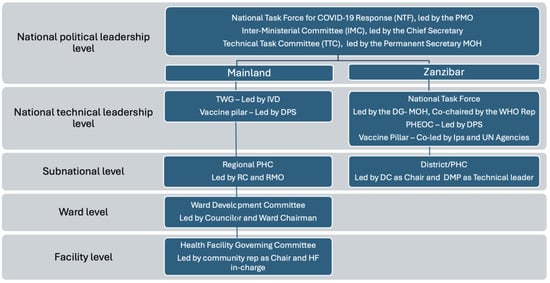
Figure 1
Open AccessReview
Dengue Vaccine Development and Deployment into Routine Immunization
by
Annelies Wilder-Smith, Thomas Cherian and Joachim Hombach
Vaccines 2025, 13(5), 483; https://doi.org/10.3390/vaccines13050483 - 29 Apr 2025
Abstract
Dengue has emerged as a significant global health threat. Despite decades of research, only two dengue vaccines—CYD-TDV (Dengvaxia) and TAK-003 (Qdenga)—have been licensed to date, with limited implementation. This paper explores and outlines strategies for integrating dengue vaccines into routine immunization programs, particularly
[...] Read more.
Dengue has emerged as a significant global health threat. Despite decades of research, only two dengue vaccines—CYD-TDV (Dengvaxia) and TAK-003 (Qdenga)—have been licensed to date, with limited implementation. This paper explores and outlines strategies for integrating dengue vaccines into routine immunization programs, particularly in high-burden regions. TAK-003, a tetravalent live-attenuated vaccine, has demonstrated 61% efficacy against virologically confirmed dengue and 84% efficacy against hospitalizations in endemic settings. However, concerns remain about vaccine-enhanced disease, particularly among seronegative individuals exposed to DENV3 and DENV4. WHO recommends targeted introduction in high-transmission settings without pre-vaccination screening, while ongoing post-introduction studies will further clarify long-term safety and efficacy. Effective vaccine rollout requires a multi-pronged approach, including school-based immunization, integration with adolescent health services, and strong community engagement. Decision-making for vaccine introduction should be guided by National Immunization Technical Advisory Groups (NITAGs), local epidemiological data, and cost-effectiveness assessments. While future vaccines, including mRNA and virus-like particle candidates, are under development, optimizing the use of currently available vaccines is crucial to reducing dengue’s public health impact. Given the continued rise in cases, immediate action—combining vaccination with vector control—is essential to prevent further morbidity and mortality.
Full article
(This article belongs to the Special Issue 50 Years of Immunization—Steps Forward)

Journal Menu
► ▼ Journal Menu-
- Vaccines Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Animals, Arthropoda, Insects, Vaccines, Veterinary Sciences, Pathogens
Ticks and Tick-Borne Pathogens: 2nd Edition
Topic Editors: Alina Rodriguez-Mallon, Alejandro Cabezas-CruzDeadline: 31 March 2026

Conferences
Special Issues
Special Issue in
Vaccines
Exploring Cutaneous Autoimmune Disease Flares in the Wake of COVID-19 Infection and Vaccination
Guest Editors: Angeliki-Victoria Roussaki-Schulze, Efterpi Zafiriou, Emmanouil KarampinisDeadline: 10 May 2025
Special Issue in
Vaccines
Vaccination-Induced Antibody and B Cell Immune Response
Guest Editor: Qiao WangDeadline: 20 May 2025
Special Issue in
Vaccines
Research Progress of New Tuberculosis Vaccines and Vaccine Design
Guest Editors: Wenping Gong, Ashok AspatwarDeadline: 31 May 2025
Special Issue in
Vaccines
Innovations Advancing Vaccine Preparedness and Response Capabilities to Counter Pandemic Influenza
Guest Editors: Ruben Donis, Sam Lee, Christine Oshansky, Leah WatsonDeadline: 31 May 2025
Topical Collections
Topical Collection in
Vaccines
COVID-19 Vaccines and Vaccination
Collection Editors: Ralph Tripp, Scott Anthony
Topical Collection in
Vaccines
COVID-19 Vaccine Hesitancy: Correlates and Interventions
Collection Editors: Manoj Sharma, Kavita Batra
Topical Collection in
Vaccines
Topic Advisory Panel Members’ Collection Series: Immunization and Vaccines for Infectious Diseases
Collection Editors: Shumaila Hanif, Ravinder Kumar










