Safety and Immunogenicity of the Attenuated Yellow Fever Vaccine in Several Neotropical Primate Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals and Restraint Procedures
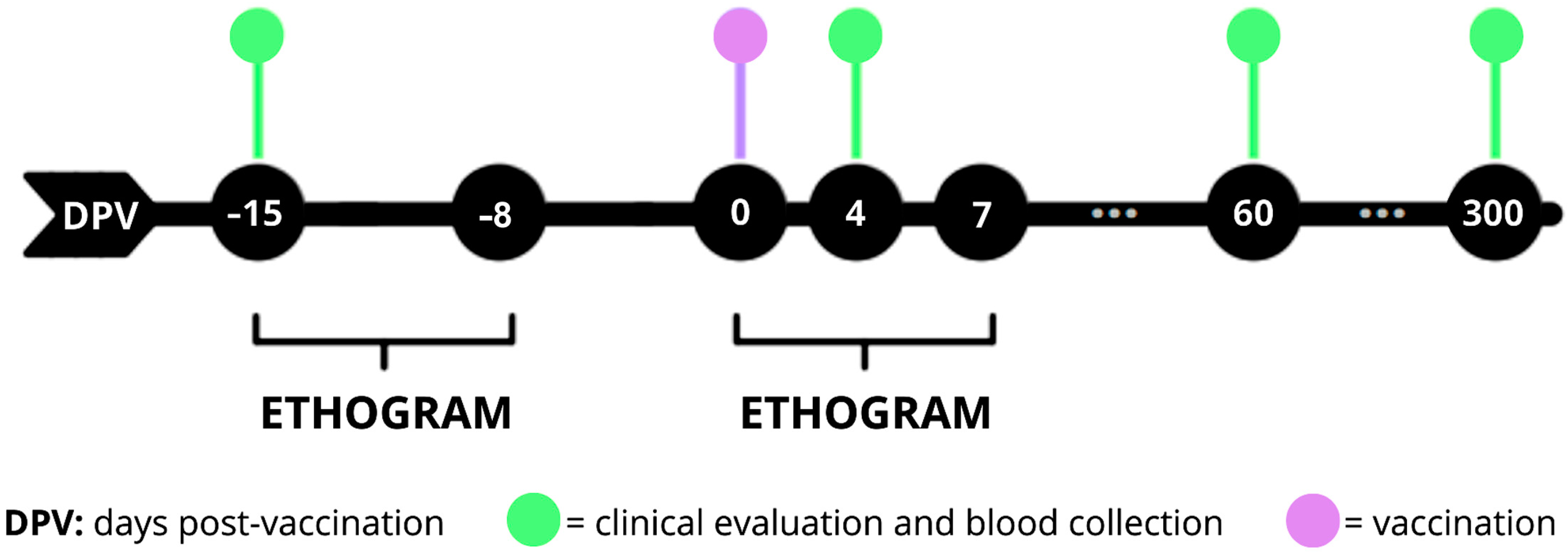
2.3. Experimental Design
2.4. Assessment of RNAemia
2.5. Detection of Neutralizing Antibody Titers Against Yellow Fever Virus
2.6. Detection of IgG Against Yellow Fever Virus
2.7. Statistical Analysis
3. Results
3.1. Ethogram
3.2. Clinical and Health Evaluation
3.3. Post-Vaccination RNAemia
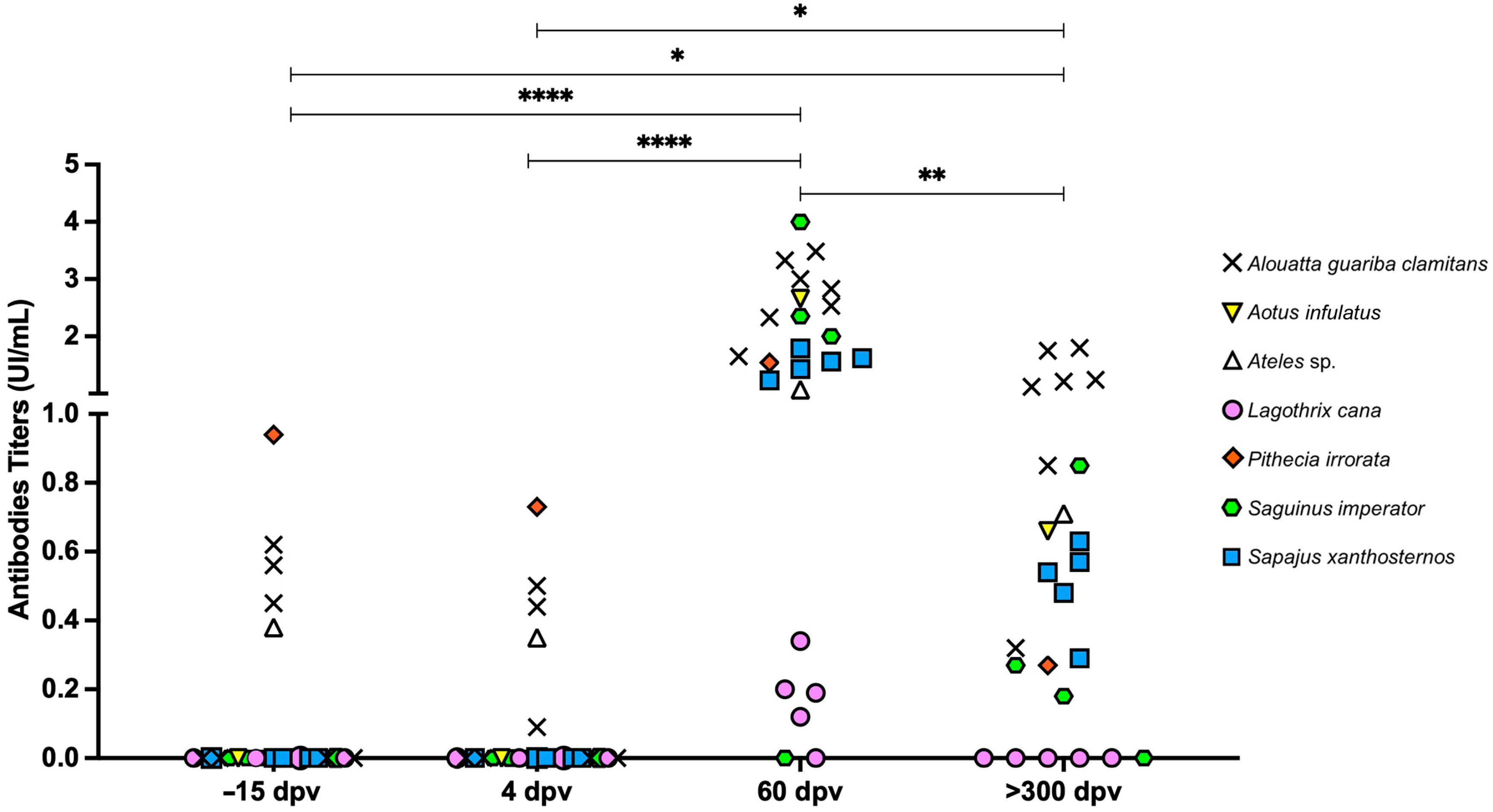
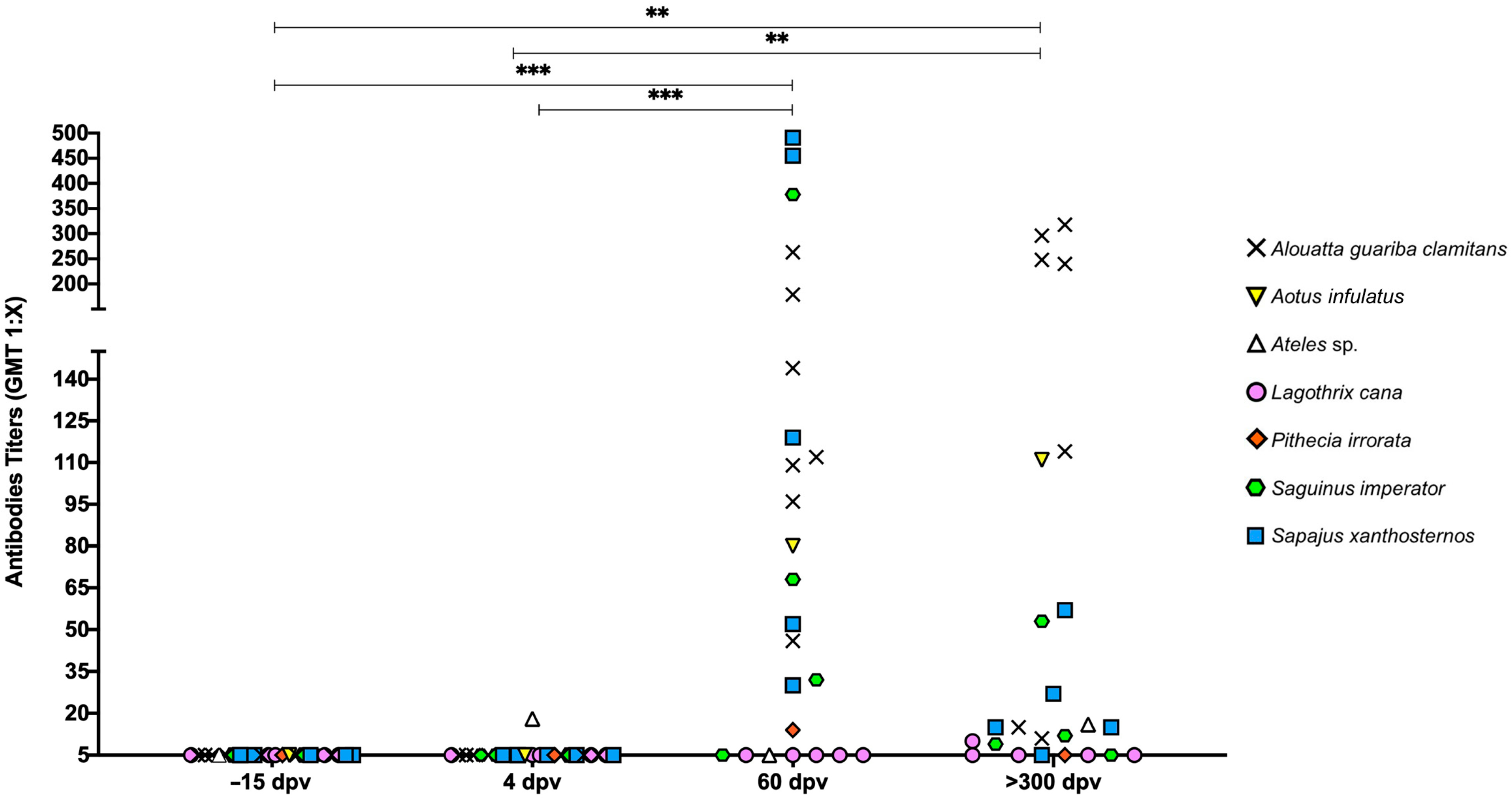
3.4. Post-Vaccination Immunogenicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| YF | Yellow Fever |
| YFV | Yellow Fever Virus |
| NHP | Non-human Primates |
| CITES | Convention on International Trade in Endangered Species |
| ICMBio | Instituto Chico Mendes de Conservação da Biodiversidade |
| DPV | Days Post-vaccination |
| RT–qPCR | Reverse Transcription followed by Polymerase Chain Reaction |
| SC | Subcutaneously |
| PRNT | Plaque Reduction Neutralization Test |
| PFU | Plaque Forming Units |
| CTL | Cellular Technology Limited |
| ELISA | Enzyme Linked Immunosorbent Assay |
| SBD | Blocking/Diluent Solution |
| BSA | Bovine Serum Albumin |
Appendix A
| Microchip | Species | −15 DPV | 0 DPV | 4 DPV | 60 DPV | 300 DPV |
|---|---|---|---|---|---|---|
| 232180 | Saguinus imperator | 0.540 | 0.615 | 0.550 | 0.615 | 0.655 |
| 00064CF8DA | Saguinus imperator | 0.480 | 0.455 | 0.455 | 0.395 | 0.500 |
| 82644296 | Saguinus imperator | 0.385 | 0.385 | 0.385 | 0.395 | 0.400 |
| 252601 | Saguinus imperator | 0.590 | 0.539 | 0.535 | 0.530 | 0.520 |
| 00064D07D0 | Sapajus xanthosternos | 2.810 | 2.780 | 2.785 | 2.970 | 2.980 |
| 901293 | Sapajus xanthosternos | 1.905 | 1.800 | 1.790 | 1.860 | 1.975 |
| 022197 | Sapajus xanthosternos | 4.040 | 3.900 | 3.870 | 3.860 | 3.875 |
| F59F | Sapajus xanthosternos | 3.270 | 3.180 | 3.170 | 3.010 | 3.755 |
| 493175 | Sapajus xanthosternos | 4.205 | 4.120 | 4.125 | 4.185 | 4.165 |
| 234113 | Lagothrix cana | 6.000 | 5.760 | 5.765 | 5.760 | 5.700 |
| 231264 | Lagothrix cana | 6.120 | 5.900 | 5.860 | 5.460 | 5.680 |
| 224298 | Lagothrix cana | 6.320 | 6.065 | 6.060 | 5.540 | 5.675 |
| 2643996 | Lagothrix cana | 3.755 | 3.750 | 3.750 | 3.830 | 4.275 |
| 203805 | Lagothrix cana | 8.680 | 8.480 | 8.480 | 8.765 | 8.900 |
| 00064D1804 | Pithecia irrorata | 3.000 | 2.790 | 2.780 | 2.885 | 3.130 |
| 22194 | Aotus infulatus | 0.930 | 0.905 | 0.915 | 1.000 | 0.985 |
| 203689 | Alouatta guariba clamitans | 3.825 | 3.525 | 3.520 | 3.760 | 4.020 |
| 023759 | Alouatta guariba clamitans | 5.655 | 5.255 | 5.250 | 5.250 | 5.480 |
| 600ED23 | Alouatta guariba clamitans | 2.975 | 3.295 | 3.305 | 3.555 | 3.795 |
| 023756 | Alouatta guariba clamitans | 4.220 | 4.180 | 4.190 | 4.175 | 4.645 |
| 232191 | Alouatta guariba clamitans | 4.140 | 3.715 | 3.700 | 3.230 | 4.150 |
| 64D1D3E | Alouatta guariba clamitans | 8.185 | 7.765 | 7.830 | 8.630 | 8.605 |
| 2649743 | Alouatta guariba clamitans | 1.200 | 1.215 | 1.265 | 1.600 | 2.595 |
| 204802 | Ateles sp. | 8.140 | 8.305 | 8.380 | 8.915 | 7.885 |
References
- Litvoc, M.N.; Novaes, C.T.G.; Lopes, M.I.B. Yellow fever. Rev. Assoc. Med. Bras. 2018, 64, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.S.; Costa, A.C.; Fernandes, N.C.C.A.; Guerra, J.M.; Santos, F.C.P.; Nogueira, J.S.; D’Agostino, J.G.; Komninakis, S.V.; Witkin, S.S.; Ressio, R.A.; et al. Epizootics due to Yellow Fever Virus in São Paulo State, Brazil: Viral dissemination to new areas (2016–2017). Sci. Rep. 2019, 9, 5474. [Google Scholar] [CrossRef] [PubMed]
- Bacha, H.A.; Johanson, G.H. Yellow fever. Rev. Assoc. Med. Bras. 2017, 63, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Tranquilin, M.V.; Lehmkuhl, R.C.; Maron, A.; Silva, L.R.; Ziliotto, L.; Seki, M.C.; Salomon, G.R. First report of yellow fever virus in non-human primates in the State of Paraná, Brazil. Rev. Soc. Bras. Med. Trop. 2013, 46, 522–524. [Google Scholar] [CrossRef]
- Rylands, A.B.; Schneider, H.; Langguth, A.; Mittermeier, R.A.; Groves, C.P.; Rodríguez-Luna, E. An assessment of the diversity of New World primates. Neotrop. Primates. 2000, 8, 61–93. [Google Scholar] [CrossRef]
- Santos, D.O.D.; Oliveira, A.R.; Lucena, F.P.; Mattos, S.A.; Carvalho, T.P.; Costa, F.B.; Carrasco, A.d.O.T. Histopathologic patterns and susceptibility of neotropical primates naturally infected with yellow fever virus. Vet. Pathol. 2020, 57, 681–686. [Google Scholar] [CrossRef]
- Fernandes, N.C.C.A.; Guerra, J.M.; Díaz-Delgado, J.; Cunha, M.S.; Saad, L.D.; Iglezias, S.D.; Ressio, R.A.; Cirqueira, C.d.S.; Kanamura, C.T.; Jesus, I.P.; et al. Differential yellow fever susceptibility in New World nonhuman primates, comparison with humans, and implications for surveillance. Emerg. Infect. Dis. 2021, 27, 47–56. [Google Scholar] [CrossRef]
- Vasconcelos, P.F. Yellow fever. Rev. Soc. Bras. Med. Trop. 2003, 36, 275–293. [Google Scholar] [CrossRef]
- Barrett, A.D.T. Yellow fever vaccine: The conundrum of 2 doses, one dose, or one-fifth dose to induce and maintain protective immunity. J. Infect. Dis. 2020, 221, 1922–1924. [Google Scholar] [CrossRef]
- Fernandes, A.T.S.; Moreira, S.B.; Gaspar, L.P.; Simões, M.; Cajaraville, A.C.D.R.A.; Pereira, R.C.; de Barros Gomes, M.P.; Linhares, J.H.R.; de Oliveira Santos, V.; Santos, R.T.; et al. Safety and immunogenicity of 17DD attenuated yellow fever vaccine in howler monkeys (Alouatta spp.). J. Med. Primatol. 2021, 50, 36–45. [Google Scholar] [CrossRef]
- Fernandes, A.T.S.; Moreira, S.B.; Gaspar, L.P.; Cajaraville, A.C.D.R.A.; Simões, M.; Pereira, R.C.; de Barros Gomes, M.P.; de Oliveira Santos, V.; Santos, R.T.; da Silva, A.M.V.; et al. Safety and immunogenicity of different 17DD yellow fever vaccines in golden-headed tamarins (Leontopithecus chrysomelas): Inhibition of viremia and RNAemia after homologous live-attenuated vaccination. Vaccine 2025, 48, 126721. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Kraemer, M.U.G.; Hill, S.C.; De Jesus, J.G.; Aguiar, R.S.; Iani, F.C.M.; Xavier, J.; Quick, J.; Du Plessis, L.; Dellicour, S.; et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science 2018, 361, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde. Emergência Epidemiológica de Febre Amarela no Brasil, no Período de Dezembro de 2016 a Julho de 2017. Boletim Epidemiológico—SVS—Ministério da Saúde. 2017. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_epidemiologica_febre_amarela.pdf (accessed on 25 February 2025).
- Ministério da Saúde Brasil; Secretaria de Vigilância em Saúde. Monitoramento do Período Sazonal da Febre Amarela no Brasil—2017/2018. Available online: https://sbim.org.br/images/files/informe-fa-21-11abr18-c.pdf (accessed on 26 April 2025).
- Figueiredo, P.O.; Silva, A.T.S.; Oliveira, J.S.; Marinho, P.E.; Rocha, F.T.; Domingos, G.P.; Poblete, P.C.P.; Oliveira, L.B.S.; Duarte, D.C.; Bonjardim, C.A.; et al. Detection and molecular characterization of yellow fever virus, 2017, Brazil. Ecohealth 2018, 15, 864–870. [Google Scholar] [CrossRef]
- Moreira-Soto, A.; Torres, M.; de Mendonça, M.L.; Mares-Guia, M.; Rodrigues, C.d.S.; Fabri, A.; dos Santos, C.; Araújo, E.M.; Fischer, C.; Nogueira, R.R.; et al. Evidence for multiple sylvatic transmission cycles during the 2016-2017 yellow fever virus outbreak, Brazil. Clin. Microbiol. Infect. 2018, 9, 1019-e1. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.L. Febre Amarela: Ameaça à Saúde Pública e Tragédia Ambiental. Jornal do Brasil. 2018. Available online: http://www.jb.com.br/artigo/noticias/2018/03/24/febre-amarela-ameaca-a-saude-publica-e-tragedia-ambiental/ (accessed on 25 February 2025).
- Sacchetto, L.; Silva, N.I.; De Rezende, I.M.; Arruda, M.S.; Costa, T.A.; De Mello, É.M.; Oliveira, G.F.G.; Alves, P.A.; De Mendonça, V.E.; Stumpp, R.G.A.V.; et al. Neighbor danger: Yellow fever virus epizootics in urban and urban-rural transition areas of Minas Gerais state, during 2017-2018 yellow fever outbreaks in Brazil. PLoS Negl. Trop. Dis. 2020, 14, 0008658. [Google Scholar] [CrossRef]
- Domingo, C.; Charrel, R.N.; Schmidt-Chanasit, J.; Zeller, H.; Reusken, C. Yellow fever in the diagnostics laboratory. Emerg. Microbes Infect. 2018, 7, 129. [Google Scholar] [CrossRef]
- Mason, R.A.; Tauraso, N.M.; Ginn, R.K.; O’Brien, T.C.; Trimmer, R.W. Yellow fever vaccine. V. Antibody response in monkeys inoculated with graded doses of the 17D vaccine. Appl. Microbiol. 1972, 23, 908–913. [Google Scholar] [CrossRef]
- Trindade, G.F.; Marchevsky, R.S.; de Fillipis, A.M.; Nogueira, R.M.; Bonaldo, M.C.; Acero, P.C.; Caride, E.; Freire, M.S.; Galler, R. Limited replication of yellow fever 17DD and 17D-dengue recombinant viruses in rhesus monkeys. An. Acad. Bras. Cienc. 2008, 80, 311–321. [Google Scholar] [CrossRef]
- World Health Organization. Diagnóstico Laboratorial de Infecção Pelo Vírus da Febre Amarela. 2017. Available online: https://www.paho.org/pt/documentos/diagnostico-laboratorial-infeccao-pelo-virus-da-febre-amarela (accessed on 25 February 2025).
- Santos, A.P.; Matos, D.C.S.; Bertho, A.L.; Mendonça, S.C.F.; Marcovistz, R. Detection of Th1/Th2 cytokine signatures in yellow fever 17DD first-time vaccinees through ELISpot assay. Cytokine 2008, 42, 152–155. [Google Scholar] [CrossRef]
- Davis, N.C. The transmission of yellow fever: Experiments with the “woolly monkey” (Lagothrix lagotricha Humboldt), the “spider monkey” (Ateleus ater Cuvier), and the “squirrel monkey” (Saimiri scireus Linnaeus). J. Exp. Med. 1930, 51, 703–720. [Google Scholar] [CrossRef]
- Strode, G.K. Yellow Fever; McGraw-Hill: New York, NY, USA, 1951; pp. 304–384. [Google Scholar]
- Reinhardt, B.; Jaspert, R.; Niedrig, M.; Kostner, C.; L’age-Stehr, J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: A model of human flavivirus infection. J. Med. Virol. 1998, 56, 159–167. [Google Scholar] [CrossRef]
- Rathore, A.P.S.; St John, A.L. Cross-Reactive Immunity Among Flaviviruses. Front. Immunol. 2020, 11, 334. [Google Scholar] [CrossRef] [PubMed]

| Microchip | Species | Common Name | Sex | Age | Weight (kg) |
|---|---|---|---|---|---|
| 232180 | Saguinus imperator | Emperor tamarin | Male | 12 years | 0.615 |
| 00064CF8DA | Saguinus imperator | Emperor tamarin | Male | 14 years | 0.455 |
| 82644296 | Saguinus imperator | Emperor tamarin | Male | 11 months | 0.385 |
| 252601 | Saguinus imperator | Emperor tamarin | Male | 4 years | 0.539 |
| 00064D07D0 | Sapajus xanthosternos | Yellow-breasted capuchin | Female | 16 years | 2.78 |
| 901293 | Sapajus xanthosternos | Yellow-breasted capuchin | Female | 4 years | 1.8 |
| 022197 | Sapajus xanthosternos | Yellow-breasted capuchin | Male | 7 years | 3.9 |
| F59F | Sapajus xanthosternos | Yellow-breasted capuchin | Female | 13 years | 3.18 |
| 493175 | Sapajus xanthosternos | Yellow-breasted capuchin | Male | 8 years | 4.120 |
| 234113 | Lagothrix cana | Woolly monkey | Female | 10 years | 5.76 |
| 231264 | Lagothrix cana | Woolly monkey | Female | 8 years | 5.9 |
| 224298 | Lagothrix cana | Woolly monkey | Female | 7 years | 6.065 |
| 2643996 | Lagothrix cana | Woolly monkey | Female | 2 years | 3.75 |
| 203805 | Lagothrix cana | Woolly monkey | Male | 8 years | 8.48 |
| 00064D1804 | Pithecia irrorata | Gray’s bald-faced saki | Female | 12 years | 2.79 |
| 22194 | Aotus infulatus | Owl monkey | Female | 14 years | 0.905 |
| 203689 | Alouatta guariba clamitans | Brown howler monkey | Female | 9 years | 3.525 |
| 023759 | Alouatta guariba clamitans | Brown howler monkey | Female | 8 years | 5.255 |
| 600ED23 | Alouatta guariba clamitans | Brown howler monkey | Female | 18 years | 3.295 |
| 023756 | Alouatta guariba clamitans | Brown howler monkey | Female | 7 years | 4.18 |
| 232191 | Alouatta guariba clamitans | Brown howler monkey | Female | 3 years | 3.715 |
| 64D1D3E | Alouatta guariba clamitans | Brown howler monkey | Male | 7 years | 7.765 |
| 2649743 | Alouatta guariba clamitans | Brown howler monkey | Female | 9 months | 1.215 |
| 204802 | Ateles sp. | Spider monkey | Male | 10 years | 8.305 |
| Species | Microchip | Ct Value |
|---|---|---|
| Sapajus xanthosternos | 00064D07D0 | 36 |
| Sapajus xanthosternos | 901293 | 32 |
| Sapajus xanthosternos | F59F | 35 |
| Pithecia irrorata | 00064D1804 | 29 |
| Alouatta guariba clamitans | 203689 | 28.1 |
| Alouatta guariba clamitans | 600ED23 | 29.36 |
| Alouatta guariba clamitans | 232191 | 33.59 |
| Alouatta guariba clamitans | 64D1D3E | 29.85 |
| Alouatta guariba clamitans | 26497437 | 36.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paula, N.F.d.; Vieira, A.D.; Santos, D.O.d.; Souza, L.d.R.d.; Coelho, C.M.; Tinoco, H.P.; Lima, P.C.S.; Motta, R.O.C.; Pereira, V.d.S.; Carvalho, M.P.N.d.; et al. Safety and Immunogenicity of the Attenuated Yellow Fever Vaccine in Several Neotropical Primate Species. Vaccines 2025, 13, 487. https://doi.org/10.3390/vaccines13050487
Paula NFd, Vieira AD, Santos DOd, Souza LdRd, Coelho CM, Tinoco HP, Lima PCS, Motta ROC, Pereira VdS, Carvalho MPNd, et al. Safety and Immunogenicity of the Attenuated Yellow Fever Vaccine in Several Neotropical Primate Species. Vaccines. 2025; 13(5):487. https://doi.org/10.3390/vaccines13050487
Chicago/Turabian StylePaula, Nayara Ferreira de, André Duarte Vieira, Daniel Oliveira dos Santos, Lucas dos Reis de Souza, Carlyle Mendes Coelho, Herlandes Penha Tinoco, Paula Cristina Senra Lima, Rafael Otávio Cançado Motta, Valéria do Socorro Pereira, Marcelo Pires Nogueira de Carvalho, and et al. 2025. "Safety and Immunogenicity of the Attenuated Yellow Fever Vaccine in Several Neotropical Primate Species" Vaccines 13, no. 5: 487. https://doi.org/10.3390/vaccines13050487
APA StylePaula, N. F. d., Vieira, A. D., Santos, D. O. d., Souza, L. d. R. d., Coelho, C. M., Tinoco, H. P., Lima, P. C. S., Motta, R. O. C., Pereira, V. d. S., Carvalho, M. P. N. d., Fernandes, C. B., Azevedo, A. d. S., Arruda, M. S., Costa, T. A., Drumond, B. P., Leme, F. d. O. P., Freire, M. d. S., Paixão, T. A. d., Oliveira, A. R., & Santos, R. L. (2025). Safety and Immunogenicity of the Attenuated Yellow Fever Vaccine in Several Neotropical Primate Species. Vaccines, 13(5), 487. https://doi.org/10.3390/vaccines13050487








