Abstract
Background/Objectives: Human papillomavirus (HPV) causes multiple types of cancer, and demographic-based inequities in HPV-related cancers persist. Behavioral interventions have increased HPV vaccination uptake, yet it is unclear how intervention effects vary by demographics. The purpose of this study was to examine whether existing HPV vaccine interventions for adolescents have unequal effects on HPV vaccine uptake. Methods: We searched MEDLINE via PubMed, PsycINFO, CINAHL, Scopus, and Cochrane CENTRAL in October 2023. The search strategy combined keywords and subject terms for HPV vaccine, interventions/health promotion, and adolescents. Studies were included in final analyses if they were peer-reviewed, published in the US between 2006 and 2023, included outcome measures from an evidence-based HPV vaccination intervention, included adolescents aged 9–17, and demographic variables for age, race/ethnicity, income/SES, or geographic region. Studies were excluded if they were review articles, abstract-only, dissertations or theses, non-English language, non-US-based, or outside the age range of 9–17. Studies were also excluded if they did not include an intervention, outcome evaluation measures, or demographic measures. The screening and extraction processes were independently performed by multiple reviewers using Covidence software. Results: Ultimately, 74 articles were included for full extraction. Sex was the most common demographic variable analyzed by the HPV vaccine (n = 38), followed by race/ethnicity (n = 15), income/SES (n = 6), and geographic region (n = 6). Conclusions: Few interventions assess whether intervention results differ by demographics, making it unclear whether these interventions reduce health inequities. This review included a wide variation in study designs, limiting our ability to uniformly assess study conclusions.
1. Introduction
Human papillomavirus (HPV) causes six types of cancer—anal, cervical, oropharyngeal, penile, vaginal, and vulvar [1]—and is responsible for nearly 38,000 new cancer cases per year [2]. Among those diagnosed with HPV-related cancers, cervical cancer is the most common in women, while oropharyngeal cancer is the most common HPV-related cancer in men. Certain populations are disproportionately affected by these cancers. For example, American Indian/Alaskan Native, Hispanic, and Black women have higher cervical cancer incidence rates compared to white women, while cervical cancer mortality is higher among Native Hawaiian or Other Pacific Islander women [3]. Similarly, mortality rates due to oropharyngeal cancer are higher among Native Hawaiian or Other Pacific Islander men and women. Furthermore, women in rural areas experience higher cervical cancer incidence rates, and both men and women in rural areas have higher oropharyngeal cancer incidence rates compared to those in urban areas [4].
Although the HPV vaccine can prevent most of these cancers, uptake remains a challenge. HPV vaccination is recommended for 11–12-year-olds and can be administered as early as 9 [5]. However, as of 2022, only 58.6% of 13–15-year-olds in the United States were up-to-date on the HPV vaccination series [6], which is well below the national target of 80% [7]. Disparities in vaccination rates are particularly evident among uninsured children and children living in non-metropolitan areas, who have lower rates of vaccine uptake and are less likely to complete the HPV vaccine series [8]. These disparities are likely to have long-term implications, further increasing HPV-related cancer incidence and mortality rates among affected populations.
Over the past two decades, behavioral interventions have been a primary strategy to increase HPV vaccination uptake and completion. The 2018 President’s Cancer Panel report emphasized the importance of provider recommendations, clinical system changes, and communication campaigns as critical strategies to accelerate HPV vaccination uptake [9]. A recent systematic review identified several modifiable factors at the individual, provider, and clinic levels that influence vaccination outcomes among adolescents [10]. The authors developed a multilevel framework illustrating how these factors interact and can be targeted to improve vaccination rates. While this framework offers a comprehensive approach to intervention development, it highlights the need for further research to understand how intervention effects may differ across health disparity populations. Avni-Singer et al. [11] conducted a systematic review of HPV vaccine impact studies (i.e., change in population-level burden of disease) to examine how extensively researchers include racial, ethnic, and socioeconomic characteristics to assess for disparities. However, only two studies out of 23 stratified the results by sociodemographic characteristics, which limits our ability to understand whether prevention strategies are reaching populations with the largest burden of disease. Thus, if we are to consider the pathway to HPV vaccine uptake through the lens of behavioral interventions, we must also interrogate the diversity and inclusivity of samples used to establish evidence for these interventions.
Given that HPV vaccination among adolescents in the United States continues to be suboptimal, as well as the presence of health disparities for HPV vaccination and HPV-related cancers, we must investigate the quality and equity of the evidence for HPV vaccine behavioral interventions. By prioritizing certain strategies for HPV vaccination, there is the potential risk of perpetuating and/or creating health disparities if the evidence generated is based on homogeneous samples. Thus, the purpose of this study was to examine whether existing HPV vaccine interventions for adolescents have unequal effects on HPV vaccine uptake.
2. Methods
2.1. Overview
Our systematic review of HPV vaccination interventions required a focus on evidence-based interventions. Thus, with guidance from the National HPV Vaccination Roundtable’s Best Practices Learning Collaborative [12], we assembled a list of interventions to include within our systematic review (Table 1). This systematic review was registered with Open Science Framework [13] and followed PRISMA guidelines [14].

Table 1.
HPV Round Table Intervention Types and Descriptions [12].
2.2. Systematic Search
Adhering to the guidelines described by the Cochrane Handbook for Systematic Reviews of Interventions, we iteratively developed a search for use in the following databases: MEDLINE via PubMed (using the advanced search), PsycINFO, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Complete, Scopus, and Cochrane CENTRAL (searched via Ovid interface). No filters or limits were applied to any of the searches. The initial search was run on 27 September 2023. One of the authors (CH), who is a health sciences librarian, developed the search strategy by combining keywords and subject terms for the main concept domains: HPV vaccine, interventions/health promotion, and adolescents. We iteratively developed a comprehensive list of search terms then developed a search string in PubMed. The PubMed search was peer-reviewed by a second research librarian using the Peer Review of Electronic Search Strategies, or PRESS checklist before it was translated to the other four databases. The full search string can be found in Appendix A.
2.3. Selection Criteria
Studies were included in the final analyses if they met the following criteria:
Peer-reviewed, published in the US between January 2006 and September 2023, include outcome measures from an evidence-based HPV vaccination intervention. Evidence-based HPV vaccination intervention was determined by inclusion on the HPV Round Table: HPV Vaccination Best Practices Learning Collaborative. The interventions had to focus on HPV vaccination among adolescents 9–17 and may include system-level, provider, and parental-focused activities. Demographics had to include HPV vaccination initiation by race, ethnicity, sex, gender, and/or region.
Exclusion criteria were studies without primary data (review articles), non-English language, non-US-based, focused on HPV vaccination outside the age range of 9–17, and did not include evidence-based intervention, outcome evaluation measures, or demographic measures. Additional exclusions were if the article was abstract only, a dissertation, or a thesis.
2.4. Screening and Eligibility
Using EndNote Citation Manager (version 20) and Covidence Systematic Review software (covidence.org), duplicates were removed. After that, using the latter software, study titles, and abstracts from the searches were screened against the eligibility criteria. Following that, the qualified studies were screened for full-text review, recording the reasons for which studies were subsequently excluded. The screening process was independently performed by multiple reviewers (IA, SBM, ET, LC, NSJ, SA, DRC). Conflicts were resolved by assessment from a third reviewer, a senior member of the research team (SBM, ET, LC).
2.5. Data Extraction
After the screening process, data were extracted in the following areas: general information (title; lead author; year of publication), characteristics of included studies (aim of study; setting; start and end date; study design; population description; inclusion and exclusion criteria; method of recruitment; description of intervention; type of intervention; measurement of race/ethnicity; sex; income/socioeconomic status; geographic region; other demographics; main HPV outcome variable), and outcomes (number of participants; change in HPV vaccine uptake measurement and statistics; stratification by sex, race, income/socioeconomic status, geographic region, other demographics; limitations; main conclusions). We assessed the risk of bias, including pre-post intervention data, control or comparison group, and random assignment (Figure 1).
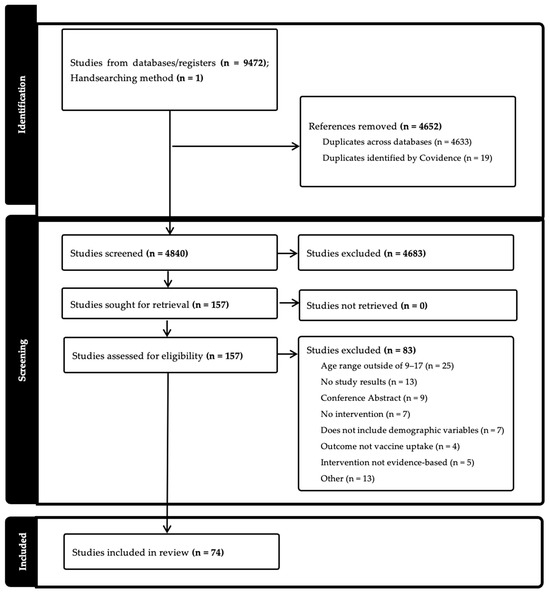
Figure 1.
PRISMA Flowchart [14].
Data from the study were independently extracted by two team members (IA, SBM, ET, LC, NSJ, SA, DRC) using Covidence software [15].
3. Results
3.1. Summary of Findings
A total of 9472 results were found across all five databases (CINAHL 1144; PsycINFO 280; PubMed 3542; Scopus 4050; Cochrane CENTRAL 456), and 4633 duplicates were removed. An additional 1 article was identified through handsearching. After entering into Covidence, 19 additional articles were moved. A total of 4840 items were included in the title and abstract screening. After initial screening, team members completed a full-text review of 157 titles. Ultimately, 74 articles were included for full extraction. (Table 2). The studies reviewed measured either HPV vaccine initiation (n = 16), completion (n = 8), both (n = 44), or other (n = 6) (e.g., whether the visit included a vaccine or not). Study settings were primarily in clinics (n = 52) but also included community (n = 8), school (n = 5), online (n = 1), other (n = 1), or multiple settings (n = 5).

Table 2.
Description of Studies Included in Review.
3.2. Methodology
Less than half of the included studies used an experimental design (n = 33) (Table 2). Sixteen studies were quasi-experimental, and the remaining studies used a non-experimental design (n = 25). Sample sizes for adolescents ranged from 39 to 312,227. Clinic studies ranged from 22 clinics to 267 clinics. Samples included a focus on adolescents (31 studies), adolescents and parents (17 studies), parents (10 studies), and other combinations of parents, adolescents, and/or providers (16 studies). Examining each study by National HPV Vaccination Roundtable: HPV Vaccination Best Practices Learning Collaborative intervention type [12], 51 studies were multi-component (i.e., had more than one intervention type), while 23 studies only used one component. Studies that focused on only one intervention type were most commonly Patient Education (n = 9), followed by Patient Outreach (n = 8), Provider Education (n = 5), and having an EHR feature (n = 1). Among studies that combined intervention types, combinations ranged from two to all six types of interventions. Among the intervention types, Patient Education was the most common with 46 studies, followed by Provider Education and Patient Outreach (38 studies), EHR feature (25), Standing Orders (11), Patient Scheduling (10), and Provider Incentives (5). Fifty studies analyzed HPV vaccine uptake by at least one demographic variable. Information about specific demographic variables is described in the sections below.
3.3. Sex
Thirty-nine studies analyzed HPV vaccine uptake by sex. All 39 studies measured sex as a category of either male or female. Of these studies, 27 examined between-group differences, and 9 found significant differences by sex [27,36,49,63,66,81]. Only one study found that males were more likely to initiate HPV vaccination than females [30]; the rest found females have greater vaccine uptake (Table 3).

Table 3.
Findings of studies that examined between-group differences for at least one demographic variable by HPV vaccine uptake.
3.4. Race/Ethnicity
Fifteen studies measured race/ethnicity and HPV vaccine uptake. Of these studies, 12 examined between-group analyses by race/ethnicity and differences in HPV vaccine uptake. Five studies found at least one significant association between a variable of race/ethnicity and HPV vaccine uptake or completion (Table 3). Rickert et al. [69] found that Hispanic participants were more likely than non-Hispanic participants to initiate a first dose. Results from Caskey et al. [30] indicated that Hispanic, White, and Other race/ethnicity participants were more likely to initiate, receive two doses, or complete the HPV vaccination series than Black participants. Additional studies found that non-White adolescents were more likely to initiate and complete the HPV vaccine series than White participants [21,36,63,88].
3.5. Income/Socioeconomic Status
Five studies analyzed between-group differences by income or socioeconomic status and HPV vaccination. Using poisson regression to estimate relative risk, one study found that when adjusting analyses for household income, unvaccinated daughters had a higher likelihood of vaccine initiation (RR = 2.6, 95% CI:1.4–4.9) and completion (RR = 4.0, 95% CI: 1.2–13.1) in the intervention versus control participants [86] (Table 3). Another study using prevalence ratios found lower vaccine uptake among households earning less than USD 75,000 per year (PR = 6.33, 95% CI 5.51–7.26) and whose mothers had less than a high school education (PR = 3.91, 95% CI 3.05–5.02) [16]. The remaining studies did not have substantial differences in uptake by income or socioeconomic status [68,71,78].
3.6. Geographic Region
Seven studies analyzed geographic regions and HPV vaccine uptake. Among these studies, geography was defined by either urban/suburban area, zip code, rurality, state, or region. Four of the studies statistically compared group differences in HPV vaccine uptake based on geographic region. In two studies, no significant differences were found between Southwestern and Northwestern States [46] or based on rural and urban areas of upstate New York [78]. One found significantly more immunization opportunities in urban versus suburban clinics [57], and another found prevalence ratios indicating that participants in the US South (PR = 6.01, 95% CI 5.38–6.72) and West (PR = 5.67, 95% CI 4.56–7.05) were significantly less likely to complete HPV vaccination [16] (Table 3).
3.7. Other Demographic Variables
Multiple studies reported at least one other demographic variable in relation to HPV vaccine uptake. Of these studies, the most common additional demographics were analyzing age by subgroups (n = 26) and Insurance status (n = 12). Parental information was also recorded in multiple studies, including parental education (n = 3), parental marital status (n = 2), and parental age (n = 2). Two additional studies measured HPV vaccination uptake in the English language [63,68] (Table 3).
4. Discussion
This systematic review found that although a multitude of evidence-based interventions exist to increase HPV vaccination, few stratify vaccine uptake by multiple demographic variables. For each demographic assessed, at least one study found significant differences in HPV vaccine uptake or completion. The demographics most commonly represented among the intervention studies were age group and sex. Since our study only assessed a limited age range (ages 9–17), information stratified by age group only included comparing older versus younger adolescents. We found that 27 studies stratified outcome variables by sex, more than by any other demographic category. Recent data from NIS-Teen indicates sex differences persist in HPV vaccination, and in 2023, 42.9% of girls aged 9–17 had received one or more HPV vaccine doses in comparison with 34.6% of boys [90].
A recent systematic review focusing on area-level variation in HPV vaccination among adolescents and young adults in the US found that HPV vaccination was associated with area-level poverty, urbanicity/rurality, and racial/ethnic composition [91]. We know from previous research that these demographic-related inequities exist in HPV vaccine uptake, yet our findings indicate that evaluations of HPV vaccine interventions largely do not assess whether these disparities persist post-intervention [3,4].
Our findings indicate that when removing categorization by age groups, only around half of the studies assessed any other demographic variable. This limits the extent to which we can draw overall conclusions about how interventions may differentially increase vaccine uptake across different populations. Additionally, among studies that included demographic variables in analyses, some reported only pre- and post-intervention data rather than examining between-group differences. This methodological strategy limits the ability to assess whether interventions are more successful based on specific demographic characteristics.
Marked inequities in HPV vaccine uptake by race/ethnicity exist, yet only 16 of the 73 studies included in our review assessed outcomes based on race/ethnicity. Additionally, none of the studies specifically included Native Hawaiians or other Pacific Islanders despite higher rates of both cervical and oropharyngeal cancers among this group [4]. The specific measures of race/ethnicity differed by study, but overall, four studies found that White participants were less likely than those of other races/ethnicities to be vaccinated. This aligns with findings by race/ethnicity from the nationally representative NIS-Teen survey [8].
Research indicates that adolescents from disadvantaged areas, living in poverty, or otherwise socially vulnerable are less likely to initiate or complete HPV vaccination [92,93]. However, our review found only six studies that examined HPV vaccine intervention outcomes by income or socioeconomic status, with limited significant findings. This leaves us unable to examine whether evidence-based HPV vaccination interventions are differentially effective based on factors related to income. Similarly, previous studies have also found links between geography and HPV vaccination, including lower uptake in rural areas in comparison with urban areas [94]. This review found six studies that measured geographic region, and each was measured differently. Due to the limited number of studies and variation in measurement, we cannot draw conclusions about findings across these studies. Both demographic areas emphasize the need to be able to assess the effectiveness of HPV vaccination stratified by key demographic factors.
This study has many strengths, including that it was framework-based, and each study was extracted by multiple reviewers. However, this study is not without limitations. The review included study designs that were both experimental and non-experimental, limiting our ability to uniformly assess study conclusions. Additionally, this study was specific to adolescents, and findings cannot be extrapolated to other HPV-vaccine-eligible age groups. This systematic review also only included published studies, which may have excluded studies in which the findings were not significant or otherwise not published.
5. Conclusions
HPV vaccination uptake and HPV-related diseases differ based on demographic factors, yet interventions to improve vaccine uptake largely do not assess whether interventions are successful among populations differentially impacted. To achieve health equity in HPV vaccination, we must implement programs that improve vaccination uptake across demographic groups. Future evidence-based HPV vaccination uptake interventions should assess outcomes stratified by demographic variables, especially demographics with documented evidence of inequitable outcomes in HPV-related disease. This will help ensure HPV interventions are not only effective but also equitable, reaching populations most impacted by HPV-related diseases.
Author Contributions
Conceptualization, E.L.T. and S.B.M.; methodology, G.J.C.H., E.L.T. and S.B.M.; software, G.J.C.H., formal analysis, S.B.M., L.C.C., I.A., N.S.J., D.R.-C. and E.L.T.; writing—original draft preparation, S.B.M., E.L.T., I.A. and M.C.; writing—review and editing, S.B.M., E.L.T., L.C.C. and M.C.; supervision, S.B.M., L.C.C. and E.L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This systematic review did not require IRB approval as it only included data from previously published manuscripts.
Data Availability Statement
Extracted data and template data collection form may be available based upon reasonable request of the corresponding author and approval of the research team.
Conflicts of Interest
E.L.T. has served as a consultant for Merck, Inc. for HPV vaccination work and speaking unrelated to this project. She has also received a Merck Investigator Studies Program award administered through the university.
Appendix A. Full Search String for Systematic Review
((“Papillomavirus Vaccines”[Mesh] OR “HPV vaccine”[tiab] OR “HPV vac-cines”[tiab] OR “HPV vaccination”[tiab] OR “HPV vaccinations”[tiab] OR “Papillo-mavirus Vaccination”[tiab] OR “Papillomavirus Vaccinations”[tiab] OR “Human Papilloma Virus vaccination”[tiab] OR “Human Papilloma Virus Vaccinations”[tiab] OR “Papillomavirus Vaccine”[tiab] OR “Papillomavirus Vaccines”[tiab] OR “Human Papilloma Virus Vaccine”[tiab] OR “Human Papilloma Virus Vaccines”[tiab] OR “Human Papillomavirus Vaccine”[tiab] OR ((“Human Papillomavirus Viruses"[Mesh] OR "Papillomavirus Infections"[Mesh] OR “Human Papillomavirus Virus”[tiab] OR “Human Papillomavirus Viruses”[tiab] OR “Human Papillomavirus”[tiab] OR “Hu-man Papillomaviruses”[tiab] OR “Human Papilloma Virus”[tiab] OR “Human Papil-loma Viruses”[tiab] OR “HPV Human Papillomavirus”[tiab] OR “HPV Human Papil-lomaviruses”[tiab] OR HPV[tiab] OR “Papillomavirus Infection”[tiab] OR “Papillo-mavirus Infections”[tiab] OR “Human Papillomavirus Infection”[tiab] OR “Human Papillomavirus Infections”[tiab] AND ("Vaccines"[Mesh] OR "Vaccination"[Mesh] OR "Vaccination Refusal"[Mesh] OR vaccin*[tiab] OR preventive[tiab] OR preven-tion[tiab] OR immunization*[tiab] OR innoculat*[tiab]))) AND (“Patient Education as Topic”[Mesh] OR “Health Promotion”[Mesh] OR “Health Knowledge, Attitudes, Prac-tice”[Mesh] OR “Patient Acceptance of Health Care”[Mesh] OR Intervention[tiab] OR Interventions[tiab] OR “Patient Education”[tiab] OR “Education of Patients”[tiab] OR “Promotion of Health”[tiab] OR “Health Promotion”[tiab] OR “Health Promo-tions”[tiab] OR “Wellness Programs”[tiab] OR “Wellness Program”[tiab] OR “Health Campaigns”[tiab] OR “Health Campaign”[tiab] OR “Health knowledge”[tiab] OR “Health Attitude”[tiab] OR “Health Attitudes”[tiab] OR “Health Care Utiliza-tion”[tiab] OR “Patient Acceptance of Healthcare”[tiab] OR “Health Care Seeking Be-havior”[tiab] OR “Healthcare Seeking Behavior”[tiab] OR Policy[tiab] OR Poli-cies[tiab])) OR "vaccination campaign"[tiab] OR "consumer health"[tiab] OR "healthcare utilization"[tiab])) AND (“Adolescent”[Mesh] OR “Child”[Mesh] OR Ad-olescen*[tiab] OR Teens[tiab] OR Teen[tiab] OR Teenag*[tiab] OR Youth[tiab] OR Youths[tiab] OR Child[tiab] OR Children[tiab] OR Boy[tiab] OR Boys[tiab] OR Girl[tiab] OR Girls[tiab] OR “Young Man”[tiab] OR “Young Men”[tiab] OR “Young Woman”[tiab] OR “Young Women”[tiab] OR childhood[tiab])
References
- Saraiya, M.; Unger, E.R.; Thompson, T.D.; Lynch, C.F.; Hernandez, B.Y.; Lyu, C.W.; Steinau, M.; Watson, M.; Wilkinson, E.J.; Hopenhayn, C.; et al. US Assessment of HPV Types in Cancers: Implications for Current and 9-Valent HPV Vaccines. J. Natl. Cancer Inst. 2015, 107, djv086. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Cancers Linked with HPV Each Year. Available online: https://www.cdc.gov/cancer/hpv/cases.html#cdc_generic_section_2-number-of-hpv-associated-cancer-cases-per-year (accessed on 10 March 2025).
- Centers for Disease Control and Prevention. Cancers by Age, Sex, Race, Ethnicity. Available online: https://gis.cdc.gov/Cancer/USCS/#/Demographics/ (accessed on 10 March 2025).
- Semprini, J.; Zahnd, W.; Brandt, H.M. What Cancers Explain the Growing Rural-Urban Gap in Human Papillomavirus-Associated Cancer Incidence? J. Rural Health 2025, 41, e12915. [Google Scholar] [CrossRef] [PubMed]
- Meites, E. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination—Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Trends Progress Report. Available online: https://progressreport.cancer.gov/prevention/hpv_immunization (accessed on 10 March 2025).
- Office of Disease Prevention and Health Promotion. Increase the Proportion of Adolescents Who Get Recommended Doses of the HPV Vaccine—IID-08. Healthy People 2030. Available online: https://odphp.health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-08 (accessed on 10 March 2025).
- Centers for Disease Control and Prevention. Vaccination Coverage Among Adolescents (13–17). Available online: https://www.cdc.gov/teenvaxview/interactive/index.html (accessed on 10 March 2025).
- Rimer, B.K. HPV Vaccination for Cancer Prevention: Progress, Opportunities, and a Renewed Call to Action; Report to the President of the United States; President’s Cancer Panel: Bethesda, MD, USA, 2018. [Google Scholar]
- Rodriguez, S.A.; Mullen, P.D.; Lopez, D.M.; Savas, L.S.; Fernández, M.E. Factors Associated with Adolescent HPV Vaccination in the U.S.: A Systematic Review of Reviews and Multilevel Framework to Inform Intervention Development. Prev. Med. 2020, 131, 105968. [Google Scholar] [CrossRef]
- Avni-Singer, L.R.; Yakely, A.; Sheth, S.S.; Shapiro, E.D.; Niccolai, L.M.; Oliveira, C.R. Assessing Sociodemographic Differences in Human Papillomavirus Vaccine Impact Studies in the United States: A Systematic Review Using Narrative Synthesis. Public Health 2020, 178, 137–150. [Google Scholar] [CrossRef]
- American Medical Group Association. National HPV Vaccination Roundtable. HPV Vaccination Best Practices Learning Collaborative Summary Report and Lessons Learned. Available online: https://hpvroundtable.org/wp-content/uploads/2023/05/AMGA-HPV-Learning-Collaborative-Summary-Report-2021_FINAL-1.pdf (accessed on 10 March 2025).
- Open Science Framework Registries. Health Equity and HPV Vaccine Interventions Systematic Review. Public Registration, 9 October 2023. Available online: https://osf.io/7zk43 (accessed on 10 March 2025).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software; Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 10 March 2025).
- Agana-Norman, D.F.; Berenson, A.B.; Chang, M. Impact Assessment of a Provider-Targeted National Vaccine Messaging Campaign on Human Papillomavirus Vaccination Rates among US Adolescent Males. Prev. Med. 2022, 164, 107228. [Google Scholar] [CrossRef]
- Aragones, A.; Bruno, D.M.; Ehrenberg, M.; Tonda-Salcedo, J.; Gany, F.M. Parental Education and Text Messaging Reminders as Effective Community-Based Tools to Increase HPV Vaccination Rates among Mexican American Children. Prev. Med. Rep. 2015, 2, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S.; Zhu, H.; Rochefort, C.; Marks, E.; Fullington, H.M.; Rodriguez, S.A.; Kassa, S.; Tiro, J.A. Mechanisms of Self-Persuasion Intervention for HPV Vaccination: Testing Memory and Autonomous Motivation. Health Psychol. 2021, 40, 887–896. [Google Scholar] [CrossRef]
- Bastani, R.; Glenn, B.A.; Singhal, R.; Crespi, C.M.; Nonzee, N.J.; Tsui, J.; Chang, L.C.; Herrmann, A.K.; Taylor, V.M. Increasing HPV Vaccination among Low-Income, Ethnic Minority Adolescents: Effects of a Multicomponent System Intervention through a County Health Department Hotline. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 175–182. [Google Scholar] [CrossRef]
- Beck, A.; Bianchi, A.; Showalter, D. Evidence-Based Practice Model to Increase Human Papillomavirus Vaccine Uptake: A Stepwise Approach. Nurs. Womens Health 2021, 25, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Berenson, A.B.; Hirth, J.M.; Kuo, Y.F.; Starkey, J.M.; Rupp, R.E. Use of Patient Navigators to Increase HPV Vaccination Rates in a Pediatric Clinical Population. Prev. Med. Rep. 2020, 20, 101194. [Google Scholar] [CrossRef] [PubMed]
- Berenson, A.B.; Rupp, R.; Dinehart, E.E.; Cofie, L.E.; Kuo, Y.F.; Hirth, J.M. Achieving High HPV Vaccine Completion Rates in a Pediatric Clinic Population. Hum. Vaccin. Immunother. 2019, 15, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, T.A.; Broome, M.; Millman, J.; Epstein, J.; Derouin, A. Promoting Strategies to Increase HPV Vaccination in the Pediatric Primary Care Setting. J. Pediatr. Health Care 2022, 36, e36–e41. [Google Scholar] [CrossRef] [PubMed]
- Biehl, R.; Efre, A. Improving Human Papilloma Virus Vaccination Rates among Adolescents. J. Am. Assoc. Nurse Pract. 2023, 35, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Bonville, C.A.; Domachowske, J.B.; Suryadevara, M. A Quality Improvement Education Initiative to Increase Adolescent Human Papillomavirus (HPV) Vaccine Completion Rates. Hum. Vaccin. Immunother. 2019, 15, 1570–1576. [Google Scholar] [CrossRef]
- Bowden, M.; Yaun, J.; Bagga, B. Improving Human Papilloma Virus Vaccination Rates: Quality Improvement. Pediatr. Qual. Saf. 2017, 2, e048. [Google Scholar] [CrossRef]
- Brewer, N.T.; Hall, M.E.; Malo, T.L.; Gilkey, M.B.; Quinn, B.; Lathren, C. Announcements versus Conversations to Improve HPV Vaccination Coverage: A Randomized Trial. Pediatrics 2017, 139, e20161764. [Google Scholar] [CrossRef]
- Brodie, N.; McPeak, K.E. Improving Human Papilloma Virus Vaccination Rates at an Urban Pediatric Primary Care Center. Pediatr. Qual. Saf. 2018, 3, e098. [Google Scholar] [CrossRef]
- Buller, D.B.; Pagoto, S.; Henry, K.; Berteletti, J.; Walkosz, B.J.; Bibeau, J.; Baker, K.; Hillhouse, J.; Arroyo, K.M. Human Papillomavirus Vaccination and Social Media: Results in a Trial With Mothers of Daughters Aged 14–17. Front. Digit. Health 2021, 3, 683034. [Google Scholar] [CrossRef] [PubMed]
- Caskey, R.; Sherman, E.G.; Beskin, K.; Rapport, R.; Xia, Y.; Schwartz, A. A Behavioral Economic Approach to Improving Human Papillomavirus Vaccination. J. Adolesc. Health 2017, 61, 755–760. [Google Scholar] [CrossRef]
- Cassidy, B.; Braxter, B.; Charron-Prochownik, D.; Schlenk, E.A. A Quality Improvement Initiative to Increase HPV Vaccine Rates Using an Educational and Reminder Strategy With Parents of Preteen Girls. J. Pediatr. Health Care 2014, 28, 155–164. [Google Scholar] [CrossRef]
- Cates, J.R.; Crandell, J.L.; Diehl, S.J.; Coyne-Beasley, T. Immunization Effects of a Communication Intervention to Promote Preteen HPV Vaccination in Primary Care Practices. Vaccine 2018, 36, 122–127. [Google Scholar] [CrossRef]
- Cates, J.R.; Diehl, S.J.; Crandell, J.L.; Coyne-Beasley, T. Intervention Effects From a Social Marketing Campaign to Promote HPV Vaccination in Preteen Boys. Vaccine 2014, 32, 4171–4178. [Google Scholar] [CrossRef]
- Cates, J.R.; Fuemmeler, B.F.; Stockton, L.L.; Diehl, S.J.; Crandell, J.L.; Coyne-Beasley, T. Evaluation of a Serious Video Game to Facilitate Conversations About Human Papillomavirus Vaccination for Preteens: Pilot Randomized Controlled Trial. JMIR Serious Games 2020, 8, e16883. [Google Scholar] [CrossRef]
- Coley, S.; Hoefer, D.; Rausch-Phung, E. A Population-Based Reminder Intervention to Improve Human Papillomavirus Vaccination Rates Among Adolescents at Routine Vaccination Age. Vaccine 2018, 36 Pt B, 4904–4909. [Google Scholar] [CrossRef]
- Cox, J.E.; Bogart, L.M.; Elliott, M.N.; Starmer, A.J.; Meleedy-Rey, P.; Goggin, K.; Banerjee, T.; Samuels, R.C.; Hahn, P.D.; Epee-Bounya, A.; et al. Improving HPV Vaccination Rates in a Racially and Ethnically Diverse Pediatric Population. Pediatrics 2022, 150, e2021054186. [Google Scholar] [CrossRef]
- Daley, M.F.; Kempe, A.; Pyrzanowski, J.; Vogt, T.M.; Dickinson, L.M.; Kile, D.; Fang, H.; Rinehart, D.J.; Shlay, J.C. School-Located Vaccination of Adolescents With Insurance Billing: Cost, Reimbursement, and Vaccination Outcomes. J. Adolesc. Health 2014, 54, 282–288. [Google Scholar] [CrossRef]
- Dang, J.H.T.; McClure, S.; Gori, A.C.T.; Martens, T.; Mojadedi, A.; Smith, U.; Austin, C.J.; Chen, M.S., Jr. Implementation and Evaluation of a Multilevel Intervention to Increase Uptake of the Human Papillomavirus Vaccine Among Rural Adolescents. J. Rural Health 2023, 39, 136–141. [Google Scholar] [CrossRef]
- Davis, K.R.; Norman, S.L.; Olson, B.G.; Demirel, S.; Taha, A.A. A Clinical Educational Intervention to Increase HPV Vaccination Rates Among Pediatric Patients Through Enhanced Recommendations. J. Pediatr. Health Care 2022, 36, 589–597. [Google Scholar] [CrossRef]
- Dempsey, A.F.; Pyrznawoski, J.; Lockhart, S.; Barnard, J.; Campagna, E.J.; Garrett, K.; Fisher, A.; Dickinson, L.M.; O’Leary, S.T. Effect of a Health Care Professional Communication Training Intervention on Adolescent Human Papillomavirus Vaccination: A Cluster Randomized Clinical Trial. JAMA Pediatr. 2018, 172, e180016. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.E.; Zimet, G.D.; Xiao, S.; Tu, W.; Lindsay, B.; Church, A.; Downs, S.M. An Educational Intervention to Improve HPV Vaccination: A Cluster Randomized Trial. Pediatrics 2019, 143, e20181457. [Google Scholar] [CrossRef] [PubMed]
- Eldred, S.V.; Hamid, H.S.; Snider, J.C.; Weinberg, S.H.; Speck, N.; Reed, B.D.; Riley, M. A Medical Student-Driven “Vaccine Blitz” at a School-Based Health Center as an Effective Way to Improve Adolescent Vaccination Rates. Fam. Med. 2015, 47, 546–548. [Google Scholar] [PubMed]
- Fiks, A.G.; Grundmeier, R.W.; Mayne, S.; Song, L.; Feemster, K.; Karavite, D.; Hughes, C.C.; Massey, J.; Keren, R.; Bell, L.M.; et al. Effectiveness of Decision Support for Families, Clinicians, or Both on HPV Vaccine Receipt. Pediatrics 2013, 131, 1114–1124. [Google Scholar] [CrossRef]
- Fisher-Borne, M.; Preiss, A.J.; Black, M.; Roberts, K.; Saslow, D. Early Outcomes of a Multilevel Human Papillomavirus Vaccination Pilot Intervention in Federally Qualified Health Centers. Acad. Pediatr. 2018, 18, S79–S84. [Google Scholar] [CrossRef]
- Gilkey, M.B.; Grabert, B.K.; Heisler-MacKinnon, J.; Bjork, A.; Boynton, M.H.; Kim, K.; Alton Dailey, S.; Liu, A.; Todd, K.G.; Schauer, S.L.; et al. Coaching and Communication Training for HPV Vaccination: A Cluster Randomized Trial. Pediatrics 2022, 150, e2021052351. [Google Scholar] [CrossRef]
- Gilkey, M.B.; Heisler-MacKinnon, J.; Boynton, M.H.; Calo, W.A.; Moss, J.L.; Brewer, N.T. Impact of Brief Quality Improvement Coaching on Adolescent HPV Vaccination Coverage: A Pragmatic Cluster Randomized Trial. Cancer Epidemiol. Biomarkers Prev. 2023, 32, 957–962. [Google Scholar] [CrossRef]
- Glenn, B.A.; Crespi, C.M.; Herrmann, A.K.; Nonzee, N.J.; Rosen, D.L.; Park, C.L.; Johnson, G.; Chang, L.C.; Singhal, R.; Taylor, V.M.; et al. Effectiveness and Feasibility of Three Types of Parent Reminders to Increase Adolescent Human Papillomavirus (HPV) Vaccination. Prev. Med. 2023, 169, 107448. [Google Scholar] [CrossRef]
- Glenn, B.A.; Nonzee, N.J.; Herrmann, A.K.; Crespi, C.M.; Haroutunian, G.G.; Sundin, P.; Chang, L.C.; Singhal, R.; Taylor, V.M.; Bastani, R. Impact of a Multi-Level, Multi-Component, System Intervention on HPV Vaccination in a Federally Qualified Health Center. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 1952–1958. [Google Scholar] [CrossRef]
- Gurfinkel, D.; Kempe, A.; Albertin, C.; Breck, A.; Zhou, X.; Vangala, S.; Beaty, B.; Rice, J.; Tseng, C.H.; Campbell, J.D.; et al. Centralized Reminder/Recall for Human Papillomavirus Vaccination: Findings From Two States-A Randomized Clinical Trial. J. Adolesc. Health 2021, 69, 579–587. [Google Scholar] [CrossRef]
- Henrikson, N.B.; Zhu, W.; Baba, L.; Nguyen, M.; Berthoud, H.; Gundersen, G.; Hofstetter, A.M. Outreach and Reminders to Improve Human Papillomavirus Vaccination in an Integrated Primary Care System. Clin. Pediatr. 2018, 57, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.P.; Bernstein, J.; Pelton, S.; Belizaire, M.; Goff, G.; Horanieh, N.; Freund, K.M. Brief Client-Centered Motivational and Behavioral Intervention to Promote HPV Vaccination in a Hard-to-Reach Population: A Pilot Randomized Controlled Trial. Clin. Pediatr. 2016, 55, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Do, T.Q.N.; Hsu, E.; Schmeler, K.M.; Montealegre, J.R.; Rodriguez, A.M. School-Based Human Papillomavirus Vaccination Program for Increasing Vaccine Uptake in an Underserved Area in Texas. Papillomavirus Res. 2019, 8, 100189. [Google Scholar] [CrossRef] [PubMed]
- Kempe, A.; O’Leary, S.T.; Shoup, J.A.; Stokley, S.; Lockhart, S.; Furniss, A.; Dickinson, L.M.; Barnard, J.; Daley, M.F. Parental Choice of Recall Method for HPV Vaccination: A Pragmatic Trial. Pediatrics 2016, 137, e20152857. [Google Scholar] [CrossRef]
- Krantz, L.; Ollberding, N.J.; Beck, A.F.; Carol Burkhardt, M. Increasing HPV Vaccination Coverage Through Provider-Based Interventions. Clin. Pediatr. 2018, 57, 319–326. [Google Scholar] [CrossRef]
- Lennon, T.; Gundacker, C.; Nugent, M.; Simpson, P.; Magallanes, N.K.; West, C.; Willis, E. Ancillary Benefit of Increased HPV Immunization Rates Following a CBPR Approach to Address Immunization Disparities in Younger Siblings. J. Community Health 2019, 44, 544–551. [Google Scholar] [CrossRef]
- Mackey, J.K.; Thompson, K.; Abdulwahab, A.; Huntington, M.K. A Simple Intervention to Increase Human Papillomavirus Vaccination in a Family Medicine Practice. S. D. Med. 2019, 72, 438–441. [Google Scholar]
- Mayne, S.L.; duRivage, N.E.; Feemster, K.A.; Localio, A.R.; Grundmeier, R.W.; Fiks, A.G. Effect of Decision Support on Missed Opportunities for Human Papillomavirus Vaccination. Am. J. Prev. Med. 2014, 47, 734–744. [Google Scholar] [CrossRef]
- McLean, H.Q.; VanWormer, J.J.; Chow, B.D.W.; Birchmeier, B.; Vickers, E.; DeVries, E.; Meyer, J.; Moore, J.; McNeil, M.M.; Stokley, S.; et al. Improving Human Papillomavirus Vaccine Use in an Integrated Health System: Impact of a Provider and StaffIntervention. J. Adolesc. Health 2017, 61, 252–258. [Google Scholar] [CrossRef]
- O’Leary, S.C.; Frost, H.M. Does HPV Vaccination Initiation at Age 9 Improve HPV Initiation and Vaccine Series Completion Rates by Age 13? Hum. Vaccin. Immunother. 2023, 19, 2180971. [Google Scholar] [CrossRef] [PubMed]
- Parra-Medina, D.; Morales-Campos, D.Y.; Mojica, C.; Ramirez, A.G. Promotora Outreach, Education and Navigation Support for HPV Vaccination to Hispanic Women with Unvaccinated Daughters. J. Cancer Educ. 2015, 30, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Paskett, E.D.; Krok-Schoen, J.L.; Pennell, M.L.; Tatum, C.M.; Reiter, P.L.; Peng, J.; Bernardo, B.M.; Weier, R.C.; Richardson, M.S.; Katz, M.L. Results of a Multilevel Intervention Trial to Increase Human Papillomavirus (HPV) Vaccine Uptake Among Adolescent Girls. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.B.; Foley, S.; Hassan, A.; Jansen, E.; Preiss, S.; Isher-Witt, J.; Fisher-Borne, M. Impact of a Multilevel Quality Improvement Intervention Using National Partnerships on Human Papillomavirus Vaccination Rates. Acad. Pediatr. 2021, 21, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.B.; Legler, A.; Jansen, E.; Bernstein, J.; Pierre-Joseph, N.; Eun, T.J.; Biancarelli, D.L.; Schuch, T.J.; Leschly, K.; Fenton, A.T.H.R.; et al. Improving HPV Vaccination Rates: A Stepped-Wedge Randomized Trial. Pediatrics 2020, 146, e20192737. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.; Southard, E. Teaching It Forward: Educating Parents About HPV/HPV Vaccine. J.Doctoral Nurs. Pract. 2019, 12, 46–58. [Google Scholar] [CrossRef]
- Rand, C.M.; Brill, H.; Albertin, C.; Humiston, S.G.; Schaffer, S.; Shone, L.P.; Blumkin, A.K.; Szilagyi, P.G. Effectiveness of Centralized Text Message Reminders on Human Papillomavirus Immunization Coverage for Publicly Insured Adolescents. J. Adolesc. Health 2015, 56 (Suppl. S5), S17–S20. [Google Scholar] [CrossRef]
- Rand, C.M.; Vincelli, P.; Goldstein, N.P.N.; Blumkin, A.; Szilagyi, P.G. Effects of Phone and Text Message Reminders on Completion of the Human Papillomavirus Vaccine Series. J. Adolesc. Health 2017, 60, 113–119. [Google Scholar] [CrossRef]
- Rand, C.M.; Tyrrell, H.; Wallace-Brodeur, R.; Goldstein, N.P.N.; Darden, P.M.; Humiston, S.G.; Albertin, C.S.; Stratbucker, W.; Schaffer, S.J.; Davis, W.; et al. A Learning Collaborative Model to Improve Human Papillomavirus Vaccination Rates in Primary Care. Acad. Pediatr. 2018, 18, S46–S52. [Google Scholar] [CrossRef] [PubMed]
- Richman, A.R.; Torres, E.; Wu, Q.; Carlston, L.; O’Rorke, S.; Moreno, C.; Olsson, J. Text and Email Messaging for Increasing Human Papillomavirus Vaccine Completion among Uninsured or Medicaid-Insured Adolescents in Rural Eastern North Carolina. J. Health Care Poor Underserved 2019, 30, 1499–1517. [Google Scholar] [CrossRef]
- Rickert, V.I.; Auslander, B.A.; Cox, D.S.; Rosenthal, S.L.; Rupp, R.E.; Zimet, G.D. School-Based HPV Immunization of Young Adolescents: Effects of Two Brief Health Interventions. Hum. Vaccin. Immunother. 2015, 11, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Santa Maria, D.; Markham, C.; Misra, S.M.; Coleman, D.C.; Lyons, M.; Desormeaux, C.; Cron, S.; Guilamo-Ramos, V. Effects of a Randomized Controlled Trial of a Brief, Student-Nurse Led, Parent-Based Sexual Health Intervention on Parental Protective Factors and HPV Vaccination Uptake. BMC Public Health 2021, 21, 585. [Google Scholar] [CrossRef] [PubMed]
- Scarinci, I.C.; Hansen, B.; Kim, Y.I. HPV Vaccine Uptake among Daughters of Latinx Immigrant Mothers: Findings from a Cluster Randomized Controlled Trial of a Community-Based, Culturally Relevant Intervention. Vaccine 2020, 38, 4125–4134. [Google Scholar] [CrossRef] [PubMed]
- Shegog, R.; Savas, L.S.; Healy, C.M.; Frost, E.L.; Coan, S.P.; Gabay, E.K.; Preston, S.M.; Spinner, S.W.; Wilbur, M.; Becker, E.; et al. AVPCancerFree: Impact of a Digital Behavior Change Intervention on Parental HPV Vaccine-Related Perceptions and Behaviors. Hum. Vaccin. Immunother. 2022, 18, 2087430. [Google Scholar] [CrossRef] [PubMed]
- Smajlovic, A.; Toth, C.D. Quality Improvement Project to Increase Human Papillomavirus Two-Dose Vaccine Series Completion by 13 Years in Pediatric Primary Care Clinics. J. Adolesc. Health 2023, 72, 958–963. [Google Scholar] [CrossRef]
- Staras, S.A.; Vadaparampil, S.T.; Livingston, M.D.; Thompson, L.A.; Sanders, A.H.; Shenkman, E.A. Increasing Human Papillomavirus Vaccine Initiation Among Publicly Insured Florida Adolescents. J. Adolesc. Health 2015, 56 (Suppl. S5), S40–S46. [Google Scholar] [CrossRef]
- Staras, S.A.S.; Vadaparampil, S.T.; Thompson, L.A.; Scherr, C.; Gurka, M.J.; Filipp, S.L.; Shenkman, E.A. Postcard Reminders for HPV Vaccination Mainly Primed Parents for Providers’ Recommendations. Prev. Med. Rep. 2020, 20, 101188. [Google Scholar] [CrossRef]
- Steiner, C.R.; Dechant, J.; Brungo, L.; Cassidy, B. An Evidence-Based Protocol to Improve HPV Vaccine Initiation Rates at a County Immunization Clinic. J. Community Health Nurs. 2021, 38, 73–84. [Google Scholar] [CrossRef]
- Strasel, M.; VanLangen, K.M.; Benzer, J.; Geyer, A.; Jameson, A.P.; Dumkow, L.E. HPV Vaccination Rates in 9- and 10-Year-Olds Following a Pharmacist-Led Intervention. J. Am. Pharm. Assoc. (2003) 2023, 64, 278–282. [Google Scholar] [CrossRef]
- Szilagyi, P.; Albertin, C.; Gurfinkel, D.; Beaty, B.; Zhou, X.; Vangala, S.; Rice, J.; Campbell, J.D.; Whittington, M.D.; Valderrama, R.; et al. Effect of State Immunization Information System Centralized Reminder and Recall on HPV Vaccination Rates. Pediatrics 2020, 145, e20192689. [Google Scholar] [CrossRef]
- Szilagyi, P.G.; Albertin, C.; Humiston, S.G.; Rand, C.M.; Schaffer, S.; Brill, H.; Stankaitis, J.; Yoo, B.K.; Blumkin, A.; Stokley, S. A Randomized Trial of the Effect of Centralized Reminder/Recall on Immunizations and Preventive Care Visits for Adolescents. Acad. Pediatr. 2013, 13, 204–213. [Google Scholar] [CrossRef]
- Szilagyi, P.G.; Humiston, S.G.; Stephens-Shields, A.J.; Localio, R.; Breck, A.; Kelly, M.K.; Wright, M.; Grundmeier, R.W.; Albertin, C.; Shone, L.P.; et al. Effect of Training Pediatric Clinicians in Human Papillomavirus Communication Strategies on Human Papillomavirus Vaccination Rates: A Cluster Randomized Clinical Trial. JAMA Pediatr. 2021, 175, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Underwood, N.L.; Gargano, L.M.; Jacobs, S.; Seib, K.; Morfaw, C.; Murray, D.; Hughes, J.M.; Sales, J.M. Influence of Sources of Information and Parental Attitudes on Human Papillomavirus Vaccine Uptake Among Adolescents. J. Pediatr. Adolesc. Gynecol. 2016, 29, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Varman, M.; Sharlin, C.; Fernandez, C.; Vasudevan, J.; Wichman, C. Human Papilloma Virus Vaccination Among Adolescents in a Community Clinic Before and After Intervention. J. Community Health 2018, 43, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Vinci, D.M.; Ryan, J.; Howard, M.; Snider, D.; Strahan, B.; Smith, G.; McClain, R. Increasing Human Papillomavirus Vaccination in a Federally Qualified Health Center Organization Using a Systems-Based Intervention Integrating EHR and Statewide Immunization Information System. J. Community Health 2022, 47, 53–62. [Google Scholar] [CrossRef]
- White, L.S.; Maulucci, E.; Kornides, M.; Aryal, S.; Alix, C.; Sneider, D.; Gagnon, J.; Winfield, E.C.; Fontenot, H.B. HPV Vaccination Rates of 7th Grade Students After a Strong Recommending Statement from the School Nurse. J. Sch. Nurs. 2022, 40, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.A.; Dixon, B.E.; Xiao, S.; Tu, W.; Lindsay, B.; Sheley, M.; Dugan, T.; Church, A.; Downs, S.M.; Zimet, G. Physician Clinical Decision Support System Prompts and Administration of Subsequent Doses of HPV Vaccine: A Randomized Clinical Trial. Vaccine 2019, 37, 4414–4418. [Google Scholar] [CrossRef]
- Winer, R.L.; Gonzales, A.A.; Noonan, C.J.; Buchwald, D.S. A Cluster-Randomized Trial to Evaluate a Mother-Daughter Dyadic Educational Intervention for Increasing HPV Vaccination Coverage in American Indian Girls. J. Community Health 2016, 41, 274–281. [Google Scholar] [CrossRef]
- Woodall, W.G.; Zimet, G.; Kong, A.; Buller, D.; Reither, J.; Chilton, L.; Myers, V.; Starling, R. Vacteens.org: A Mobile Web App to Improve HPV Vaccine Uptake. Front. Digit. Health 2021, 3, 693688. [Google Scholar] [CrossRef]
- Zimmerman, R.K.; Raviotta, J.M.; Nowalk, M.P.; Moehling, K.K.; Reis, E.C.; Humiston, S.G.; Lin, C.J. Using the 4 Pillars™ Practice Transformation Program to Increase Adolescent Human Papillomavirus, Meningococcal, Tetanus-Diphtheria-Pertussis and Influenza Vaccination. Vaccine 2017, 35, 6180–6186. [Google Scholar] [CrossRef]
- Zorn, S.; Darville-Sanders, G.; Vu, T.; Carter, A.; Treend, K.; Raunio, C.; Vasavada, A. Multi-Level Quality Improvement Strategies to Optimize HPV Vaccination Starting at the 9-Year Well Child Visit: Success Stories from Two Private Pediatric Clinics. Hum. Vaccin. Immunother. 2023, 19, 2163807. [Google Scholar] [CrossRef]
- Villarroel, M.A.; Galinsky, A.M.; Lu, P.J.; Pingali, C. Human Papillomavirus Vaccination Coverage in Children Ages 9–17 Years: United States, 2022; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2024. [Google Scholar]
- Do, E.K.; Rossi, B.; Miller, C.A.; Ksinan, A.J.; Wheeler, D.C.; Chukmaitov, A.; Fuemmeler, B.F. Area-Level Variation and Human Papillomavirus Vaccination Among Adolescents and Young Adults in the United States: A Systematic Review. Cancer Epidemiol. Biomarkers Prev. 2021, 30, 13–21. [Google Scholar] [CrossRef]
- Kurani, S.; MacLaughlin, K.L.; Jacobson, R.M.; Sauver, J.L.S.; Jenkins, G.D.; Fan, C.; Rutten, L.J.F. Socioeconomic Disadvantage and Human Papillomavirus (HPV) Vaccination Uptake. Vaccine 2022, 40, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Humble, S.; Barnette, A.; Brandt, H.; Thompson, V.; Klesges, L.M.; Silver, M.I. Associations of Geographic-Based Socioeconomic Factors and HPV Vaccination Among Male and Female Children in Five US States. BMC Public Health 2024, 24, 702. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Gerend, M.A.; Boakye, E.A. Rural–Urban Differences in Human Papillomavirus Vaccination Among Young Adults in 8 US States. Am. J. Prev. Med. 2021, 60, 298–299. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).