Molecules from Catalytic Processes
A topical collection in Molbank (ISSN 1422-8599). This collection belongs to the section "Organic Synthesis and Biosynthesis".
Viewed by 69693Editor
Interests: catalytic sequential reactions for the synthesis of high value-added compounds from readily available reagents; transition metal-catalyzed C-H activation methodologies; carbonylation reactions; CO2 activation chemistry
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
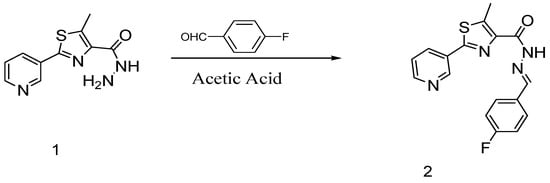
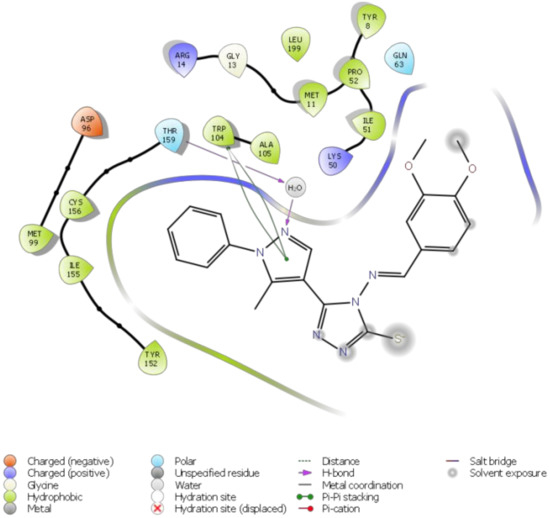
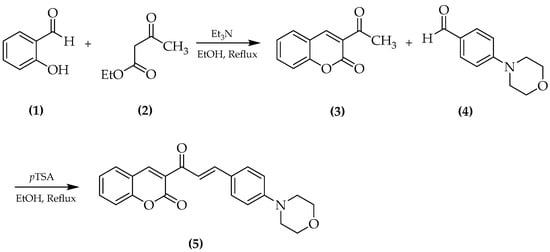
Catalytic reactions represent an outstanding tool to access structurally-complex molecules of relevant interest for academia and industry. Often, sequential catalytic transformations lead to a high level of molecular sophistication that is hard to reach through the traditional organic chemistry. The use of readily available starting materials avoiding at the same time tedious separation steps increases the synthetic importance and the potentiality of these catalytic processes. The focus of this Special Issue is to present papers that cover the synthesis of a single compound or a family of molecules through a catalytic transformation, including metal- or organocatalyzed syntheses. All contributions on this topic are encouraged.
Prof. Dr. Nicola Della Ca’Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Molbank is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 500 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- catalytic processes
- metal-catalyzed organic synthesis
- organocatalyzed transformations
- high-value added molecules
- heterocycle synthesis