Abstract
The title compound was prepared by the nucleophilic addition of hydroxylamine over 1-cyanoadamantane. The poor reactivity of the nitrile substrate, due to its scarcely electrophilic nature, prompted the need to employ several activating conditions. Energy supply via conventional heating, ultrasound, and microwave irradiation did not lead to product formation. Therefore, Lewis acid catalysis was attempted. Initial tests with ZnCl2 led to product formation in poor yields. Conversely, the use of AlCl3 led to the formation of the desired amidoxime in the moderate yield, which was further increased to an excellent yield by performing the reaction in a more concentrated medium. The structural identity of the title compound was proven by spectroscopic methods (IR, NMR). This compound was later employed as a starting material for the synthesis of 3,5-disubstituted 1,2,4-oxadiazole derivatives as potential 11β-HSD1 inhibitors.
1. Introduction
11β-Hydroxysteroid dehydrogenase type 1 (11β-HSD1) is a microsomal enzyme abundantly expressed in brain, liver, and fat tissue [1]. It catalyzes the conversion of cortisone into the active hormone cortisol, thus serving as an intracellular enhancer of glucocorticoid activity [2]. Several researches have pointed to this enzyme as a major pathophysiological actor in diabetes and metabolic syndrome, in which its overexpression has been shown to contribute to metabolic imbalances common in those ailments, thus playing an important etiological role [3,4]. Therefore, the inhibition of 11β-HSD1 is currently regarded as a promising therapeutic target [5].
Recent research by Lagos et al. identified novel 11β-HSD1 inhibitors employing a screening procedure [6]. In our efforts to prepare potent and selective 11β-HSD1 inhibitors based on a pharmacophore structure designed in silico by the Lagos group, we set out to synthesize several 3,5-disubstituted 1,2,4-oxadiazole derivatives. A review of the literature shows that 1,2,4-oxadiazoles are prepared by the condensation of amidoximes with an activated carboxylic acid derivative, a process followed by cyclodehydration in high temperature, affording the desired heterocycle [7].
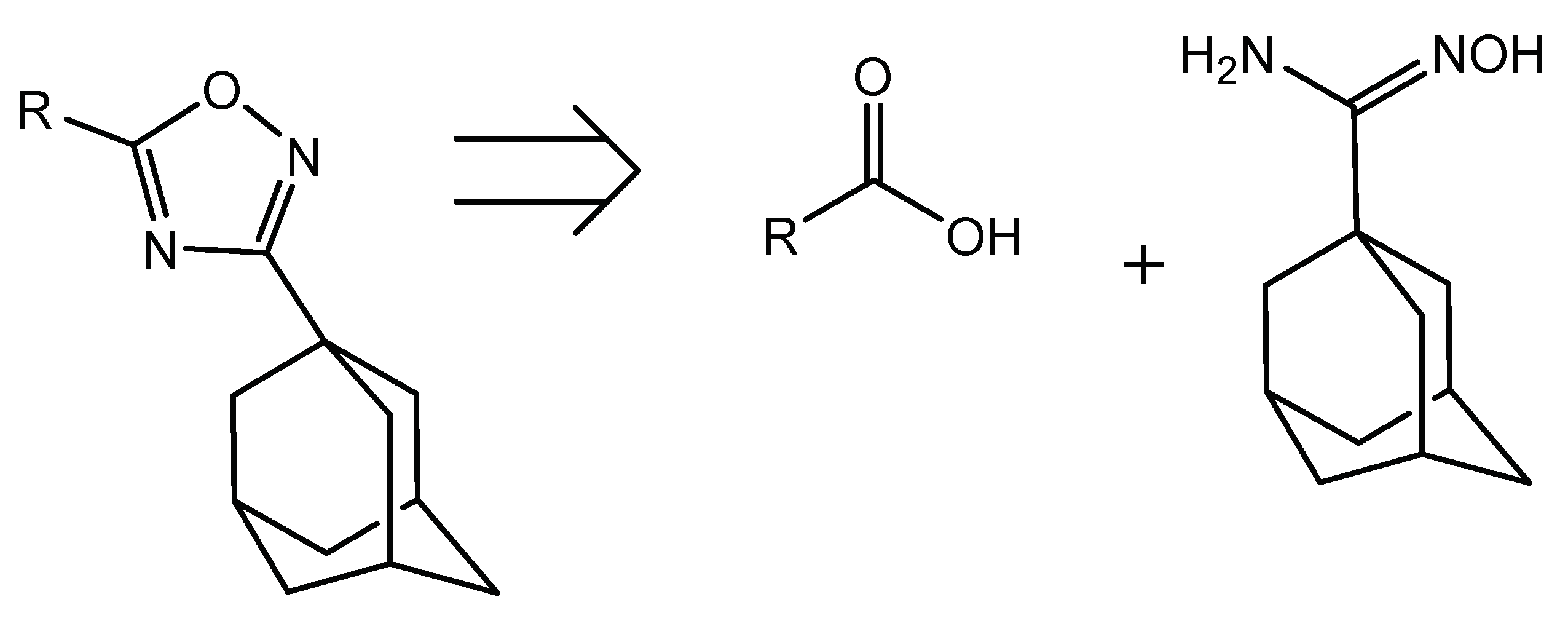
Thus, our efforts were consequently turned to the preparation of the required amidoxime substrate. A retrosynthetic analysis of the desired oxadiazole series shows that all of them come from 1-adamantylamidoxime, given that the pharmacophore structure includes an adamantane ring as a key component necessary for binding to the enzyme (Scheme 1).
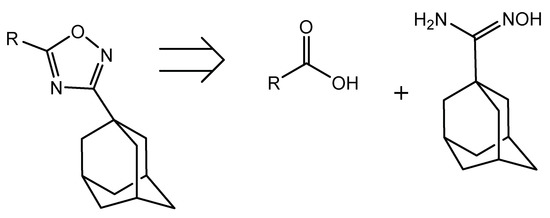
Scheme 1.
Retrosynthetic analysis of the desired oxadiazole derivative series.
Therefore, we proceeded to plan the synthesis of 1-adamantylamidoxime. It is reported in the literature that amidoximes are prepared by the nucleophilic addition of hydroxylamine over a nitrile, usually employing ethanol in reflux conditions [8]. It is interesting to note that this reaction depends on the nitrile substrate being sufficiently electrophilic to be attacked by the nucleophile. Thus, most reported amidoxime syntheses have been carried on employing aromatic nitrile substrates, which are markedly electrophilic, whereas aliphatic nitriles are regarded as largely inert towards this reaction, to the point where the yields of aliphatic amidoxime syntheses are seldom reported [8]. Despite this, we found literature articles that report the synthesis of 1-adamantylamidoxime as part of a broader synthetic route, but which only provide a general method for the preparation of amidoximes, without stating differences in terms of the procedure between aliphatic and aromatic ones. Likewise, such reports do not detail yield or physical constant information for this specific product [9,10]. These antecedents highlight an interest both for the development of specific synthetic procedures, allowing for the preparation of aliphatic amidoximes in high yield and for their spectroscopic characterization.
In this context, we proceeded to prepare 1-adamantylamidoxime from 1-cyanoadamantane and hydroxylamine. The predictably low reactivity of the nitrile substrate, given its poor electrophile character, led us to pursue several approaches to increase its reactivity towards the nucleophile, such as heating, energy irradiation, and, finally and most successfully, Lewis acid catalysis.
2. Results and Discussion
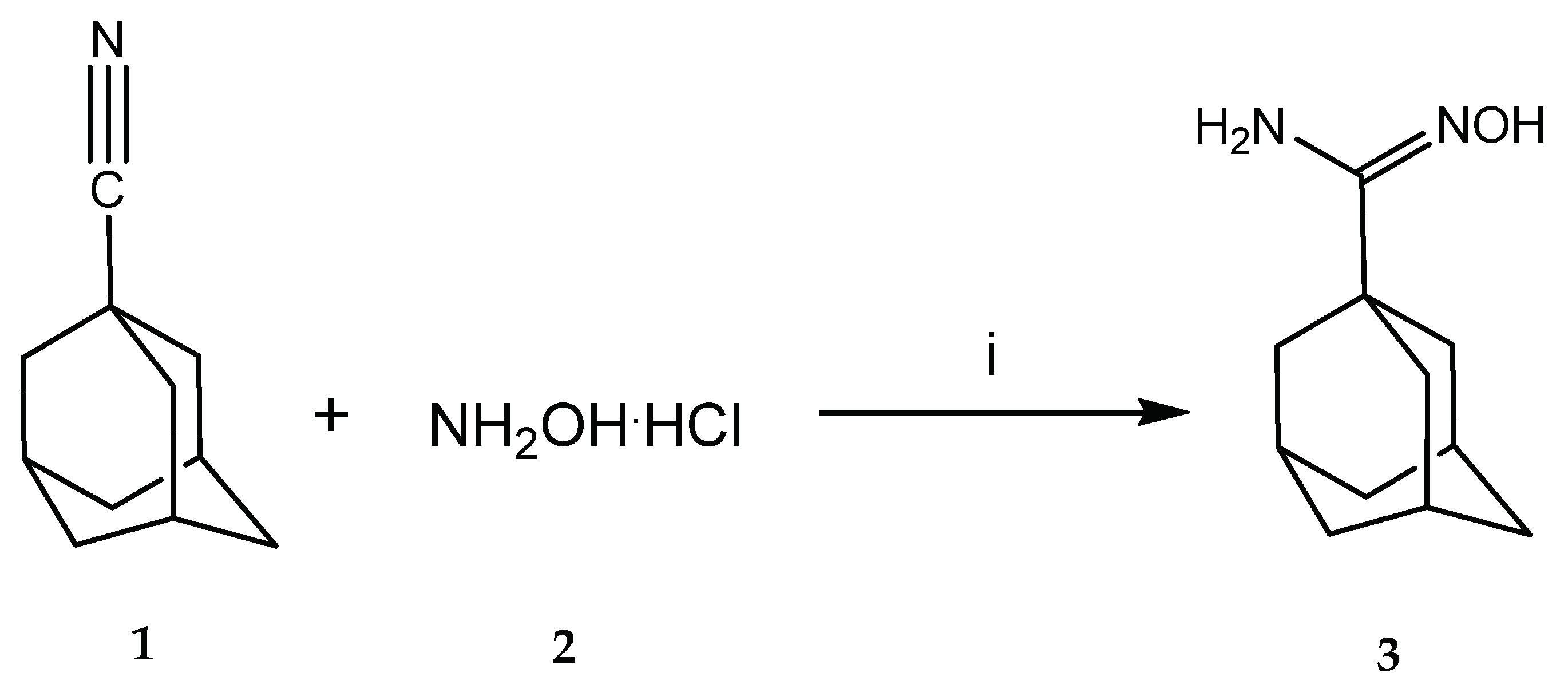
Title compound 3 was synthesized through the nucleophilic attack of hydroxylamine over the nitrile group of 1-cyanoadamantane (Scheme 2). Both reactants are available commercially. Low reactivity of the nitrile function towards the nucleophile prompted us to attempt several activating conditions. In all cases, hydroxylamine was employed as its hydrochloride salt, and generation of the free base was achieved in situ by combining the hydrochloride with a base (sodium carbonate in most attempts).
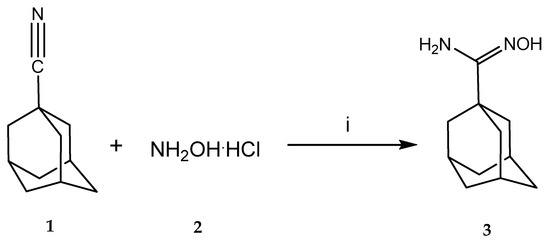
Scheme 2.
Synthesis of 1-adamantylamidoxime. Conditions listed here were those leading to product formation in the highest yield. Reagents and conditions: (i) Na2CO3, AlCl3, ethanol, and glycerol.
Table 1 summarizes the several assays that were attempted until favorable reaction conditions, leading to formation of 3 in good yield, were found. Our first attempt involved stirring equimolar amounts of reactants 1 and 2 in a 50% v/v ethanol-water mixture employing sodium carbonate as a base, affording no desired product (entry 1). Similar attempts employing ultrasound (entry 2) and microwave irradiation (entry 3) were likewise unsuccessful. Reflux heating employing the same reactants afforded the desired amidoxime product in very low yield (entry 4). In an attempt to more efficiently generate the free hydroxylamine base, potassium hydroxide was employed as the base; under these conditions, no desired product was obtained, as most of the nitrile substrate was hydrolyzed into the corresponding carboxylic acid (entry 5). At this point, we reasoned that Lewis acids might be more successful at increasing the nitrile reactivity towards the nucleophile, since these species, being electron acceptors, polarize substrates, thus enhancing their electrophile character. With this approach in mind, we proceeded to adapt a literature procedure describing the preparation of tetrazoles from nitriles employing ZnCl2 [11]. Use of this compound in catalytic amounts under reflux conditions afforded the desired product with a disappointingly low yield (entry 6). Conversely, employment of ZnCl2 under microwave irradiation afforded no product (entry 7). We then reasoned that a more electron-avid Lewis acid, such as AlCl3, might be necessary to convey adequate electrophilic character to 1. Therefore, we employed this Lewis acid in catalytic amounts under reflux conditions, adapting a literature procedure devised for the synthesis of tetrazoles employing AlCl3 [12]; these conditions afforded the desired product in a modest yield (entry 8). We then noted that increasing the mass of 1 while keeping the solvent amount constant (i.e., concentrating the reactant solution) afforded product 3 in higher yields (entries 10 and 11), while decreasing its mass led to poorer yields (entry 9). This could highlight the importance of a sufficiently concentrated solution to provide a good likelihood for effective collisions between the reactants to occur. It is worth noting that this principle might also lead to increased yields with the previously tested Lewis acid catalyst, ZnCl2. While it was outside of the scope of this research to conduct a detailed synthetic optimization study, it would be advisable to continue exploring this reaction during the course of a future research, employing different Lewis acids and varying amounts of substrate 1 in each case.

Table 1.
Reaction attempts for the synthesis of 1-adamantylamidoxime. Abbreviations used: a: NH2OH·HCl, b: Na2CO3, c: KOH, d: ZnCl2, e: AlCl3.
After these assays, optimal reaction conditions among those tested were identified as those listed in entry 11. Such conditions involved a 10 h reflux employing a very concentrated solution of the nitrile substrate, adding hydroxylamine in excess amount, and employing AlCl3 in a catalytic quantity (Scheme 2). It was noted that best results were obtained when hydroxylamine hydrochloride and sodium carbonate were stirred overnight in an ethanol-glycerol mixture, and then the free-base-containing mixture was added over the previously formed nitrile-AlCl3 complex, which was also generated by stirring both reactants separately.
In conclusion, the present research details a preliminary and exploratory study of a possible method leading to the preparation of aliphatic amidoximes in high yield. It is worth noting that, to our knowledge, even though aliphatic amidoximes have been reported in the literature, no other method tailored for the specific preparation of aliphatic amidoximes has been described to date. Certainly, a complete study would require assaying a wider variety of Lewis acid catalysts in varying amounts and employing several different aliphatic nitrile substrates in a range of concentrations. As this research showed, varying the nitrile mass to alter the concentration of the medium seems to be a key point when searching for the optimum reaction conditions. Such efforts in future research could lead to a simple and efficient method for the preparation of aliphatic amidoximes.
Regarding the course of the research project this synthesis was contextualized in, we proceeded to employ product 3 in the subsequent synthesis of 3-adamantyl-5-substituted-1,2,4-oxadiazole derivatives as potential 11β-HSD1 inhibitors.
3. Materials and Methods
3.1. Materials
Reagents were purchased from commercial suppliers, specifically Merck (Darmstadt, Germany) and Sigma-Aldrich (St. Louis, MO, USA), and were used without further purification. Solvents were purchased from commercial suppliers and were purified by distillation prior to their use.
3.2. Instrumentation
Melting points were determined on a Stuart Scientific SMP30 apparatus (Bibby Scientific Limited, Staffordshire, UK), employing open-glass capillaries. Several reactions were performed on a Monowave 300 (Anton Paar, Graz, Austria) microwave reactor. Ultrasonic reactions were performed on a Branson 2210R-MT Ultrasonic Cleaner (Marshall Scientific, Danbury, CT, USA) device. Infrared spectra were recorded on a Bruker Vector 22 spectrometer (Bruker, Billerica, MA, USA) using KBr pellets. NMR spectra were recorded on a Bruker Avance III HD 400 (Bruker) spectrometer, at 400 MHz for 1H and 100 MHz for 13C-NMR. Spectra were recorded in CDCl3, using the solvent signal as reference, with an acquisition temperature of 4 °C. The chemical shifts are expressed in ppm (δ scale) downfield from tetramethylsilane (TMS), and coupling constants values (J) are given in Hertz. High resolution mass spectra were obtained on an Exactive™ Plus Orbitrap (ThermoFisher Scientific, Bremen, Germany) high-resolution mass spectrometer using electron impact ionization.
3.3. Synthesis
1-Adamantylamidoxime (3)
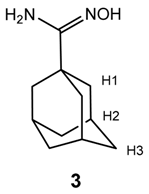
Conditions listed here were those leading to product formation in the highest yields. A mixture of hydroxylamine hydrochloride (2.59 g, 37.2 mmol) and sodium carbonate (3.93 g, 37.1 mmol) in ethanol (10 mL) and glycerol (10 mL) was prepared and stirred overnight at room temperature. A solution of 1-cyanoadamantane (2.00 g, 12.4 mmol) and aluminum chloride (0.25 g, 1.86 mmol) in ethanol (30 mL) was prepared, stirred for 30 min at room temperature, and added dropwise over the hydroxylamine-carbonate mixture. The resulting white suspension was vigorously stirred at reflux conditions for 10 h. Reaction progress was monitored by TLC employing iodine vapor as a revealing agent. After 10 h, the ethanol was removed by vacuum. The residue was partitioned with water (30 mL) and dichloromethane (30 mL), and the aqueous phase was further extracted with dichloromethane (3 × 30 mL). The organic layer was washed with brine (3 × 30 mL) and dried over anhydrous sodium sulfate. Removal of the solvent under vacuum afforded a white-colored crude, which was purified by recrystallization employing acetone/n-hexane 5:3. Yield: 85%; m.p.: 210–215 °C. IR (KBr) cm−1: 3505 (N-H), 3405 (free O-H), 3218 (H-bonded O-H), 2908–2850 (C-H sp3); 1645 (C=N), 1582 (NH2), 1454 (CH2), 1357 (C-N). 1H-NMR (ppm) (400 MHz, CDCl3) δ: 4.51 (s, 2H, -NH2); 1.98 (bs, 3H, H-2); 1.80 (bs, 6H, H-1); 1.67 (m, 6H, H-3). 13C-NMR (ppm) (101 MHz, CDCl3) δ: 159.64 (q); 39.74 (CH2); 36.63 (CH2); 36.51 (q); 28.12 (CH). HRMS (MS) m/z calculated for C11H19N2O (M + H)+: 195.1419, found: 195.1491.
Supplementary Materials
The following are available online: Figure S1: 1H-NMR spectrum of 3; Figure S2: 13C-NMR spectrum of 3; Figure S3: IR spectrum of 3; Figure S4: High-resolution mass spectrum of 3.
Acknowledgments
This work was supported by project CORFO 13CTI-21526-P1.
Author Contributions
B.D.: experimental synthetic work, literature research, IR, NMR, mass spectrum interpretation, and writing of manuscript; G.R.-G.: synthesis planning, IR, NMR interpretation, and proofreading of manuscript; C.F.L.: literature research, IR, and NMR interpretation.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 11β-HSD1 | 11β-hydroxysteroid dehydrogenase type 1 |
| IR | infrared spectroscopy |
| NMR | nuclear magnetic resonance spectroscopy |
| HRMS | high-resolution mass spectroscopy |
| TMS | tetramethylsilane |
References
- Seckl, J.R.; Walker, B.R. Minireview: 11beta-hydroxysteroid dehydrogenase type 1—A tissue-specific amplifier of glucocorticoid action. Endocrinology 2001, 142, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Staab, C.A.; Maser, E. 11beta-hydroxysteroid dehydrogenase type 1 is an important regulator at the interface of obesity and inflammation. J. Steroid Biochem. Mol. Biol. 2010, 119, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Wake, D.J.; Walker, B.R. 11 beta-hydroxysteroid dehydrogenase type 1 in obesity and the metabolic syndrome. Mol. Cell. Endocrinol. 2004, 215, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Baudrand, R.; Dominguez, J.M.; Carvajal, C.A.; Riquelme, A.; Campino, C.; Macchiavello, S.; Bozinovic, M.; Morales, M.; Pizarro, M.; Solis, N.; et al. Overexpression of hepatic 5α-reductase and 11β-hydroxysteroid dehydrogenase type 1 in visceral adipose tissue is associated with hyperinsulinemia in morbidly obese patients. Metabolism 2011, 60, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Morton, N.M.; Paterson, J.M.; Masuzaki, H.; Holmes, M.C.; Staels, B.; Fievet, C.; Walker, B.R.; Flier, J.S.; Mullins, J.J.; Seckl, J.R. Novel adipose tissue—Mediated resistance to diet-induced visceral obesity in 11β-hydroxysteroid dehydrogenase type 1—Deficient mice. Diabetes 2004, 53, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Lagos, C.F.; Vecchiola, A.; Allende, F.; Fuentes, C.A.; Tichauer, J.E.; Valdivia, C.; Solari, S.; Campino, C.; Tapia-Castillo, A.; Baudrand, R.; et al. Identification of novel 11β-hsd1 inhibitors by combined ligand- and structure-based virtual screening. Mol. Cell. Endocrinol. 2014, 384, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Porcheddu, A.; Cadoni, R.; De Luca, L. A fast and efficient one-pot microwave assisted synthesis of variously di-substituted 1,2,4-oxadiazoles. Org. Biomol. Chem. 2011, 9, 7539–7546. [Google Scholar] [CrossRef] [PubMed]
- Eloy, F.; Lenaers, R. The chemistry of amidoximes and related compounds. Chem. Rev. 1962, 62, 155–183. [Google Scholar] [CrossRef]
- Kandre, S.; Bhagat, P.R.; Sharma, R.; Gupte, A. Microwave assisted synthesis of 3,5-disubstituted 1,2,4-oxadiazoles from substituted amidoximes and benzoyl cyanides. Tetrahedron Lett. 2013, 54, 3526–3529. [Google Scholar] [CrossRef]
- Xia, G.; You, X.; Liu, L.; Liu, H.; Wang, J.; Shi, Y.; Li, P.; Xiong, B.; Liu, X.; Shen, J. Design, synthesis and sar of piperidyl-oxadiazoles as 11β-hydroxysteroid dehydrogenase 1 inhibitors. Eur. J. Med. Chem. 2013, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vorona, S.; Artamonova, T.; Zevatskii, Y.; Myznikov, L. An improved protocol for the preparation of 5-substituted tetrazoles from organic thiocyanates and nitriles. Synthesis 2014, 46, 781–786. [Google Scholar]
- Cantillo, D.; Gutmann, B.; Kappe, C.O. An experimental and computational assessment of acid-catalyzed azide-nitrile cycloadditions. J. Org. Chem. 2012, 77, 10882–10890. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).