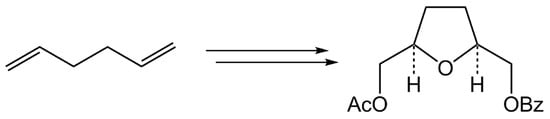
(±)-((2S,5R)-5-(Acetoxymethyl)tetrahydrofuran-2-yl)methyl Benzoate
Abstract
:1. Introduction
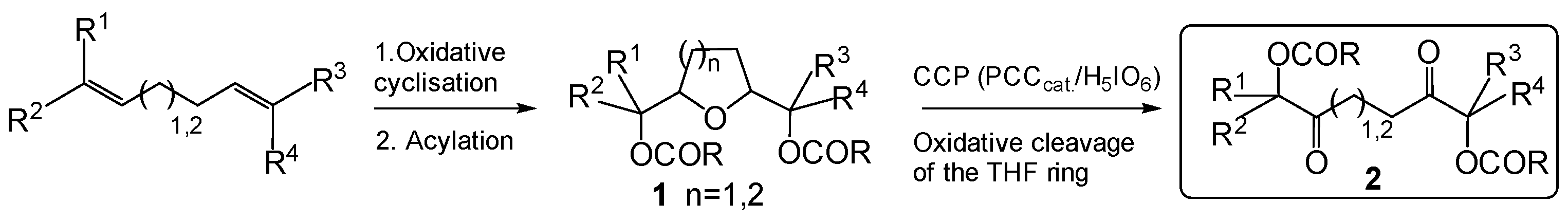
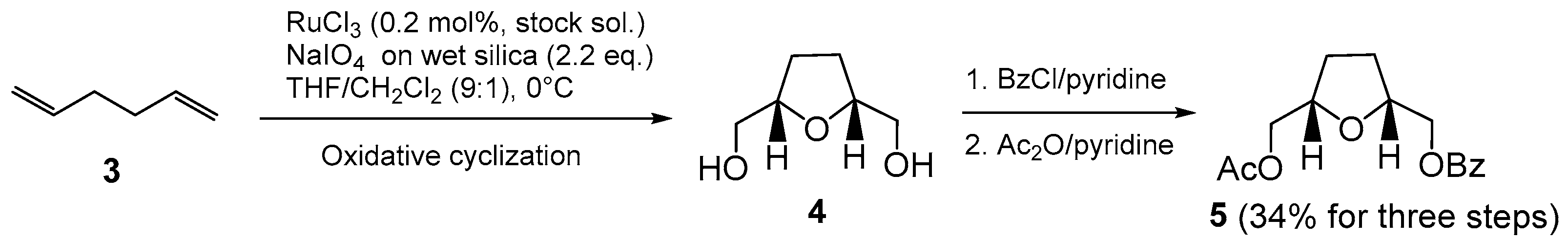
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of (±)-((2S,5R)-5-(Acetoxymethyl)tetrahydrofuran-2-yl)methyl Benzoate (5)
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mijs, W.J.; De Jonge, C.R.H.I. (Eds.) Organic Syntheses by Oxidation with Metal Compounds; Plenum Press: New York, NY, USA, 1986. [Google Scholar]
- Baeckvall, J.-E. (Ed.) Modern Oxidation Methods, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Piccialli, V. Oxidative cyclization of dienes and polyenes mediated by transition metal oxo-species. Synthesis 2007, 2585–2607. [Google Scholar] [CrossRef]
- Piccialli, V. Ruthenium tetroxide and perruthenate chemistry. Recent advances and related transformations mediated by other transition metal oxo-species. Molecules 2014, 19, 6534–6582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scire, S.; Fiorenza, R.; Bellardita, M.; Palmisano, L. Catalytic Applications of TiO2. In Titanium Dioxide (TiO2) and Its Applications; Parrino, F., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 637–679. [Google Scholar]
- Puttaswamy, K.N.V.; Gowda, K.N.N. Os(VIII) as an Efficient Homogeneous Catalyst for the Oxidative Decolorization of Methylene Blue Dye with Alkaline Chloramine-T: Kinetic, Mechanistic, and Platinum Metal Ions Reactivity Studies. Ind. Eng. Chem. Res. 2010, 49, 3137–3145. [Google Scholar]
- Piccialli, V.; Zaccaria, S.; Borbone, N.; Oliviero, G.; D’Errico, S.; Hemminki, A.; Cerullo, V.; Romano, V.; Tuzi, A.; Centore, R. Discovery of a novel one-step RuO4-catalyzed tandem oxidative polycyclization/double spiroketalization process. Access to a new type of polyether bis-spiroketal compound displaying antitumor activity. Org. Biomol. Chem. 2009, 7, 3036–3039. [Google Scholar] [CrossRef]
- Piccialli, V.; Zaccaria, S.; Oliviero, G.; D’Errico, S.; D’Atri, V.; Borbone, N. Insight into pyridinium chlorochromate chemistry: Catalytic oxidation of tetrahydrofuran compounds and synthesis of umbelactone. Eur. J. Org. Chem. 2012, 4293–4305. [Google Scholar] [CrossRef]
- Zaccaria, S.; Borbone, N.; Oliviero, G.; D’Errico, S.; Piccialli, V. Pyridinium chlorochromate chemistry. New insight into oxidation of tetrahydrofurans. Arkivoc 2017, 273–290. [Google Scholar] [CrossRef] [Green Version]
- Piccialli, V.; Borbone, N.; Oliviero, G. Ruthenium-catalyzed oxidative cyclization of 1,7-dienes. A novel diasteroselective synthesis of 2,7-disubstitueted trans-oxepane diols. Tetrahedron Lett. 2007, 48, 5131–5135. [Google Scholar] [CrossRef]
- Piccialli, V.; D’Errico, S.; Borbone, N.; Oliviero, G.; Centore, R.; Zaccaria, S. A general synthesis of bis-α-acyloxy-1,4- and -1,5-diketones through catalytic oxidative opening of acylated THF and THP diols. Eur. J. Org. Chem. 2013, 1781–1789. [Google Scholar] [CrossRef]
- Okamura, A.; Kitani, M.; Murata, M. Kinetic studies of the catalytic oxygen exchange of chromate ions with water by periodate ions. Bull. Chem. Soc. Jpn. 1994, 67, 1522–1530. [Google Scholar] [CrossRef]
- Piccialli, V.; Cavallo, N. Improved RuO4-catalysed oxidative cyclisation of geraniol-type 1,5-dienes to cis-2,5-bis(hydroxymethyl)tetrahydrofuranyldiols. Tetrahedron Lett. 2001, 42, 4695–4699. [Google Scholar] [CrossRef]
- Albarella, L.; Musumeci, D.; Sica, D. Reaction of RuO4 with carbon-carbon double bonds, 9. Reactions of 1,5-dienes with ruthenium tetraoxide: Stereoselective synthesis of tetrahydrofurandiols. Eur. J. Org. Chem. 2001, 5, 997–1003. [Google Scholar] [CrossRef]
- Roth, S.; Göhler, S.; Cheng, H.; Stark, C.B.W. A highly efficient procedure for ruthenium tetroxide catalyzed oxidative cyclizations of 1,5-dienes. Eur. J. Org. Chem. 2005, 4109–4118. [Google Scholar] [CrossRef]
- Piccialli, V. RuO4-catalyzed oxidative cyclization of 1,6-dienes to trans-2,6-bis(hydroxymethyl)tetrahydropyranyldiols. A novel stereoselective process. Tetrahedron Lett. 2000, 41, 3731–3733. [Google Scholar] [CrossRef]
- Roth, S.; Stark, C.B.W. Efficient oxidative cyclization of 1,6-dienes: A highly diastereoselective entry to substituted tetrahydropyrans. Angew. Chem. Int. Ed. 2006, 45, 6218–6221. [Google Scholar] [CrossRef] [PubMed]
- de Champdorè, M.; Lasalvia, M.; Piccialli, V. OsO4-catalyzed oxidative cyclization of geranyl and neryl acetate to cis-2,5-bis(hydroxymethyl)tetrahydrofurans. Tetrahedron Lett. 1998, 39, 9781–9784. [Google Scholar] [CrossRef]
- Donohoe, T.J.; Butterworth, S. A general oxidative cyclization of 1,5-dienes using catalytic osmium tetroxide. Angew. Chem. Int. Ed. 2003, 42, 948–951. [Google Scholar] [CrossRef]
- Lee, J.C.; Jin, Y.S.; Choi, J.-H. Synthesis of α-acetoxy and formyloxy ketones by thallium(III) promoted α-oxidation. Chem. Commun. 2001, 956–957. [Google Scholar] [CrossRef]
- Beshara, C.S.; Hall, H.; Jenkins, R.L.; Jones, K.L.; Jones, T.C.; Killeen, N.M.; Taylor, P.H.; Thomas, S.P.; Tomkinson, N.C.O. A general method for the α-acyloxylation of carbonyl compounds. Org. Lett. 2005, 7, 5729–5732. [Google Scholar] [CrossRef]
- Uyanik, M.; Suzuki, D.; Yasui, T.; Ishihara, K. In situ generated (hypo)iodite catalysts for the direct α-oxyacylation of carbonyl compounds with carboxylic acids. Angew. Chem. Int. Ed. 2011, 50, 5331–5334. [Google Scholar] [CrossRef]
- Prasad, P.K.; Reddi, R.N.; Arumugam, S. Recent methods for the synthesis of α-acyloxy ketones. Org. Biomol. Chem. 2018, 16, 9334–9348. [Google Scholar] [CrossRef]
- Chen, X.; Xin, Y.; Zhao, Z.-W.; Hou, Y.-J.; Wang, X.-X.; Xia, W.-J.; Li, Y.-M. Decarboxylative oxyacyloxylation of propiolic acids: Construction of alkynyl-containing α-acyloxy ketones. J. Org. Chem. 2021, 86, 8216–8225, and references therein. [Google Scholar] [CrossRef] [PubMed]
- Connolly, T.J.; Considine, J.L.; Ding, Z.; Forsatz, B.; Jennings, M.N.; MacEwan, M.F.; McCoy, K.M.; Place, D.W.; Sharma, A.; Sutherland, K. Efficient Synthesis of 8-Oxa-3-aza-bicyclo[3.2.1]octane Hydrochloride. Org. Process Res. Dev. 2010, 14, 459–465. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccialli, V. (±)-((2S,5R)-5-(Acetoxymethyl)tetrahydrofuran-2-yl)methyl Benzoate. Molbank 2022, 2022, M1349. https://doi.org/10.3390/M1349
Piccialli V. (±)-((2S,5R)-5-(Acetoxymethyl)tetrahydrofuran-2-yl)methyl Benzoate. Molbank. 2022; 2022(1):M1349. https://doi.org/10.3390/M1349
Chicago/Turabian StylePiccialli, Vincenzo. 2022. "(±)-((2S,5R)-5-(Acetoxymethyl)tetrahydrofuran-2-yl)methyl Benzoate" Molbank 2022, no. 1: M1349. https://doi.org/10.3390/M1349
APA StylePiccialli, V. (2022). (±)-((2S,5R)-5-(Acetoxymethyl)tetrahydrofuran-2-yl)methyl Benzoate. Molbank, 2022(1), M1349. https://doi.org/10.3390/M1349