9-Aminoquino[2',3':3,4]pyrrolo[2,1-b]quinazolin-11(13H)-one
Abstract
:1. Introduction
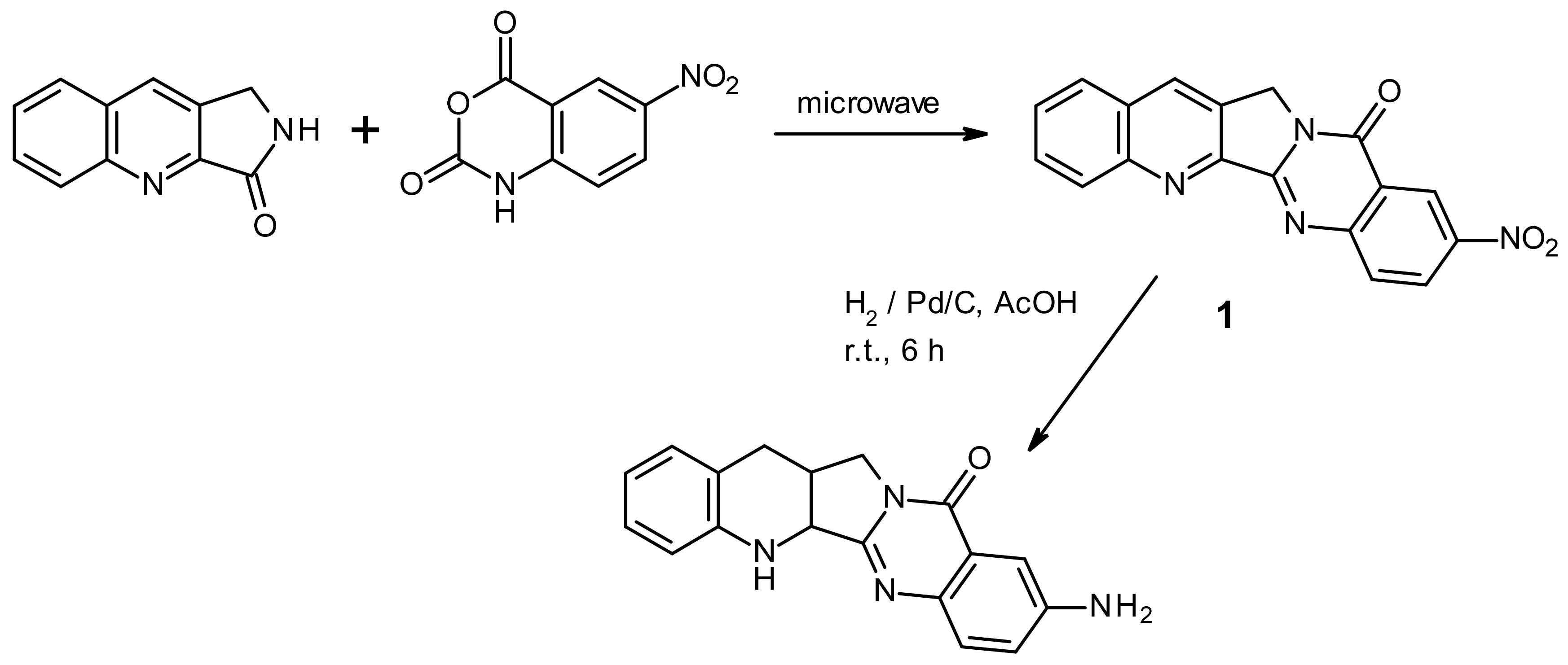
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 9-Aminoquino[2′,3′:3,4]pyrrolo[2,1-b]quinazolin-11(13H)-one (2)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Z.-Z.; Hano, Y.; Nomura, T.; Chen, Y.-J. Two new pyrroloquinazolinoquinoline alkaloids from Peganum Nigellastrum. Heterocycles 1997, 46, 541–546. [Google Scholar] [CrossRef]
- Cagir, A.; Jones, S.H.; Gao, R.; Eisenhauer, B.M.; Hecht, S.M.; Luotonin, A. A naturally occurring human DNA topoisomerase I poison. J. Am. Chem. Soc. 2003, 125, 13628–13629. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.L.; Cha, H.C.; Jahng, Y. Recent advances in the studies on Luotonins. Molecules 2011, 16, 4861–4883. [Google Scholar] [CrossRef] [PubMed]
- Ibric, A.; Eckerstorfer, S.; Eder, M.; Louko, I.; Tunjic, L.; Heffeter, P.; Schueffl, H.H.; Marian, B.; Haider, N. Position-Selective Synthesis and Biological Evaluation of Four Isomeric A-Ring Amino Derivatives of the Alkaloid Luotonin A. Molecules 2019, 24, 716. [Google Scholar] [CrossRef] [PubMed]
- Nacro, K.; Zha, C.; Guzzo, P.R.; Herr, R.J.; Peace, D.; Friedrich, T.D. Synthesis and topoisomerase poisoning activity of A-ring and E-ring substituted Luotonin A derivatives. Bioorg. Med. Chem. 2007, 15, 4237–4246. [Google Scholar] [CrossRef] [PubMed]
- Cagir, A.; Eisenhauer, B.M.; Gao, R.; Thomas, S.J.; Hecht, S.M. Synthesis and topoisomerase I properties of Luotonin A analogues. Bioorg. Med. Chem. 2004, 12, 6287–6299. [Google Scholar] [CrossRef] [PubMed]
- Dallavalle, S.; Merlini, L.; Beretta, G.L.; Tinelli, S.; Zunino, F. Synthesis and cytotoxic activity of substituted Luotonin A derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 5757–5761. [Google Scholar] [CrossRef] [PubMed]
- Sugasawa, T.; Toyota, T.; Sasakura, K.; Hidaka, T. Synthesis of 1-and 3-Oxo-dihydro-2H-pyrrolo [3,4-b] quinoline and Their Reaction with Phosphorus Pentasulfide. Chem. Pharm. Bull. 1971, 19, 1971–1974. [Google Scholar] [CrossRef]
- Haider, N.; Nuß, S. Weinreb amidation as the cornerstone of an improved synthetic route to A-ring-modified derivatives of Luotonin A. Molecules 2012, 17, 11363–11378. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Meng, G.; Roger, S.; Wank, S. An efficient and selective access to 1-substituted and 3-substituted derivatives of Luotonin A. Tetrahedron 2013, 69, 7066–7072. [Google Scholar] [CrossRef]
- Atia, M.; Bogdán, D.; Brügger, M.; Haider, N.; Mátyus, P. Remarkable regioselectivities in the course of the synthesis of two new Luotonin A derivatives. Tetrahedron 2017, 73, 3231–3239. [Google Scholar] [CrossRef]
- Ibric, A.; Dutter, K.; Marian, B.; Haider, N. A facile oxidative opening of the C-ring in Luotonin A and derivatives. Molecules 2017, 22, 1540. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Nishiyama, H. Recent topics of transfer hydrogenation. Tetrahedron Lett. 2014, 55, 3133–3146. [Google Scholar] [CrossRef]
- Haider, N.; Tropper, K. Dimethyl 6-amino-1-methyl-9H-carbazole-2,3-dicarboxylate. Molbank 2015, M849. [Google Scholar] [CrossRef]
- Deady, L.W.; Sette, R.M. Lithiation of Pivaloylamino Derivatives of Dibenzofuran and 9-Methylcarbazole. Aust. J. Chem. 2001, 54, 177–180. [Google Scholar] [CrossRef]
- Althuis, T.H.; Moore, P.F.; Hess, H.-J. Development of ethyl 3,4-dihydro-4-oxopyrimido[4,5-b]quinoline-2-carboxylate, a new prototype with oral antiallergy activity. J. Med. Chem. 1979, 22, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Gege, C.; Schneider, M.; Chevrier, C.; Deng, H.; Sucholeiki, I.; Gallagher, B.M.; Bosies, M.; Steeneck, C.; Wu, X.; Hochguertel, M.; et al. Preparation of heterobicyclic metalloprotease inhibitors. PCT Int. Appl. WO 2008063668.


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, E.; Haider, N. 9-Aminoquino[2',3':3,4]pyrrolo[2,1-b]quinazolin-11(13H)-one. Molbank 2019, 2019, M1050. https://doi.org/10.3390/M1050
Schneider E, Haider N. 9-Aminoquino[2',3':3,4]pyrrolo[2,1-b]quinazolin-11(13H)-one. Molbank. 2019; 2019(1):M1050. https://doi.org/10.3390/M1050
Chicago/Turabian StyleSchneider, Eugen, and Norbert Haider. 2019. "9-Aminoquino[2',3':3,4]pyrrolo[2,1-b]quinazolin-11(13H)-one" Molbank 2019, no. 1: M1050. https://doi.org/10.3390/M1050
APA StyleSchneider, E., & Haider, N. (2019). 9-Aminoquino[2',3':3,4]pyrrolo[2,1-b]quinazolin-11(13H)-one. Molbank, 2019(1), M1050. https://doi.org/10.3390/M1050





