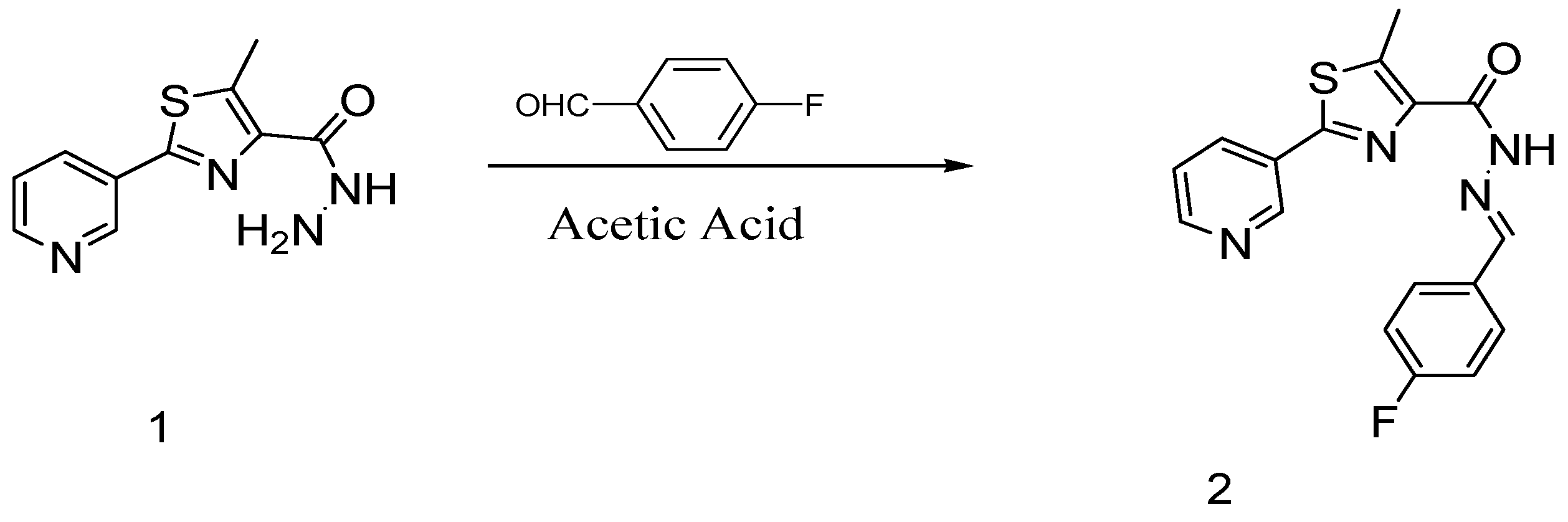
(E)-N′-(4-Fluorobenzylidene)-5-methyl-2-(pyridin-3-yl)thiazole-4-carbohydrazide
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zadafiya, S.K.; Tailor, J.H.; Malik, G.M. Disperse Dyes Based on Thiazole, Their Dyeing Application on Polyester Fiber and Their Antimicrobial Activity. J. Chem. 2012, 2013, 1–5. [Google Scholar] [CrossRef]
- Krishna, C.J.; Pathak, V.N.; Arya, P. Synthesis of Some New Fluorine Containing Condensed Thiazoles and Their Fungicidal Activity. Agric. Biol. Chem. 1977, 41, 543–546. [Google Scholar]
- Phillips, W.G.; Rejda-Heath, J.M. Thiazole Carboxanilide Fungicides: A New Structure-Activity Relationship for Succinate Dehydrogenase Inhibitors. Pestic. Sci. 1993, 38, 1–7. [Google Scholar] [CrossRef]
- Andreani, A.; Rambaldi, M.; Carloni, P.; Greci, L.; Stipa, P. Imidazo[2,1-b]thiazole carbamates and acylureas as potential insect control agents. J. Heterocycl. Chem. 1989, 26, 525–529. [Google Scholar] [CrossRef]
- Dondoni, A. Heterocycles in organic synthesis: Thiazoles and triazoles as exemplar cases of synthetic auxiliaries. Org. Biomol. Chem. 2010, 8, 3366–3385. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.R.; Singh, E.K.; Wahyudi, H.; Alexander, L.D.; Kunicki, J.B.; Nazarova, L.A.; Fairweather, K.A.; Giltrap, A.M.; Jolliffe, K.A.; McAlpine, S.R. Synthesis of sansalvamide A peptidomimetics: Triazole, oxazole, thiazole, and pseudoproline containing compounds. Tetrahedron 2012, 68, 1029–1051. [Google Scholar] [CrossRef] [PubMed]
- Santosh, R.; Prabhu, A.; Selvam, K.M.; Panchangam, M.K.; Nagaraja, G.K.; Rekha, P.D. Design, synthesis, and pharmacology of some oxadiazole and hydroxypyrazoline hybrids bearing thiazoyl scaffold: Antiproliferative activity, molecular docking and DNA binding studies. Heliyon 2019, 5, e01255. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Okolo, C.T.; Alsharif, Z.A.; Whitt, J.; Chambers, S.A.; Varma, R.S.; Alam, M.A. Benign synthesis of thiazolo-androstenone derivatives as potent anticancer agents. Org. Lett. 2018, 20, 5927–5932. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Malini, K.V.; Rao, B.S.; Sarojini, B.K.; Kumari, N.S. Synthesis of some new 2,4-disubstituted thiazoles as possible antibacterial and anti-inflammatory agents. Eur. J. Med. Chem. 2003, 38, 313–318. [Google Scholar] [CrossRef]
- Vijesh, A.M.; Isloor, A.M.; Prabhu, V.; Ahmad, S.; Malladi, S. Synthesis, characterization and anti-microbial studies of some novel 2,4-disubstituted thiazoles. Eur. J. Med. Chem. 2010, 45, 5460–5464. [Google Scholar] [CrossRef] [PubMed]
- Brider, J.; Rowe, T.; Gibler, D.J.; Gottsponer, A.; Delancey, E.; Branscum, M.D.; Ontko, A.; Gilmore, D.; Alam, M.A. Synthesis and antimicrobial studies of azomethine and N-arylamine derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-methicillin-resistant Staphylococcus aureus agents. Med. Chem. Res. 2016, 25, 2691–2697. [Google Scholar] [CrossRef]
- Karegoudar, P.; Karthikeyan, M.S.; Prasad, D.J.; Mahalinga, M.; Holla, B.S.; Kumari, N.S. Synthesis of some novel 2,4-disubstituted thiazoles as possible antimicrobial agents. Eur. J. Med. Chem. 2008, 43, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Bing, G.; Zhang, X.; Qin, Z.; Yu, H.; Qin, X.; Dai, H.; Miao, W.; Wu, S.; Fang, J. Synthesis and herbicidal activities of 2-cyano-3-benzylaminoacrylates containing thiazole moiety. Bioorg. Med. Chem. Lett. 2010, 20, 3348–3351. [Google Scholar] [CrossRef] [PubMed]
- Steinbaugh, B.A.; Hamilton, H.W.; Patt, W.C.; Rapundalo, S.T.; Batley, B.L.; Lunney, E.A.; Ryan, M.J.; Hicks, G.W. Tetrahydroisoquinoline as a phenylalanine replacement in renin inhibitors. Bioorg. Med. Chem. Lett. 1994, 4, 2029–2034. [Google Scholar] [CrossRef]
- Bugg, T.D.H.; Braddick, D.; Dowson, C.G.; Roper, D.I. Bacterial cell wall assembly: Still an attractive antibacterial target. Trends Biotechnol. 2011, 29, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Almendral, M.J.; Curto, Y.; Criado, J.J.; Rodriguez, E.; Manzano, J.L. Determination of the DNA-binding characteristics of ethidiumbromide, proflavine, and cisplatin by flow injection analysis: Usefulness in studies on antitumor drugs. Anal. Biochem. 2006, 355, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Santosh, R.; Selvam, K.M.; Kanekar, S.U.; Nagaraja, G.K.; Kumar, M. Design, Synthesis, DNA Binding, and Docking Studies of Thiazoles and Thiazole-Containing Triazoles as Antibacterials. Chem. Select. 2018, 3, 3892–3898. [Google Scholar] [CrossRef]
- Alsharif, Z.A.; Alam, M.A. Modular synthesis of thiazoline and thiazole derivatives by using a cascade protocol. RSC Adv. 2017, 7, 32647–32651. [Google Scholar] [CrossRef] [PubMed]
| Tested Organism | S. aureus | B. subtilis | E. coli | P. aeruginosa |
|---|---|---|---|---|
| Tetracycline | 1 | 0.5 | 1 | 1 |
| Streptomycin | 1 | 0.5 | 1 | 1 |
| Compound 2 | 3 | 6 | 3 | 3 |
| Compound | Free | Bound | ∆λmax (nm) | H% | Kb × 106 M−1 |
|---|---|---|---|---|---|
| 2 | 316 | 320 | 4 | 70.58 | 2.26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamat, V.; Santosh, R.; Nayak, S.P. (E)-N′-(4-Fluorobenzylidene)-5-methyl-2-(pyridin-3-yl)thiazole-4-carbohydrazide. Molbank 2019, 2019, M1058. https://doi.org/10.3390/M1058
Kamat V, Santosh R, Nayak SP. (E)-N′-(4-Fluorobenzylidene)-5-methyl-2-(pyridin-3-yl)thiazole-4-carbohydrazide. Molbank. 2019; 2019(2):M1058. https://doi.org/10.3390/M1058
Chicago/Turabian StyleKamat, Vinuta, Rangappa Santosh, and Suresh P. Nayak. 2019. "(E)-N′-(4-Fluorobenzylidene)-5-methyl-2-(pyridin-3-yl)thiazole-4-carbohydrazide" Molbank 2019, no. 2: M1058. https://doi.org/10.3390/M1058
APA StyleKamat, V., Santosh, R., & Nayak, S. P. (2019). (E)-N′-(4-Fluorobenzylidene)-5-methyl-2-(pyridin-3-yl)thiazole-4-carbohydrazide. Molbank, 2019(2), M1058. https://doi.org/10.3390/M1058




