Abstract
A new compound belonging to a dihydrotetrazolopyrimidine derivative, that is, ethyl 5-methyl-7-(4-morpholinophenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, was successfully synthesized using a Biginelli reaction between 4-morpholinobenzaldehyde, ethyl acetoacetate, and 5-aminotetrazole with p-toluenesulfonic acid (pTSA) as a catalyst in ethanol under reflux. The molecular structure of the title compound was characterized on the basis of spectroscopic evidence, using FTIR, HRESI-MS, 1H- and 13C-NMR, and 2D NMR.
1. Introduction
A Biginelli reaction is an acid-catalyzed multicomponent condensation of three reagents that are originally from aromatic aldehyde, urea, and ethyl acetoacetate using hydrochloric acid as a catalyst [1] to furnish dihydropyrimidinone (DHPM) derivatives [2,3]. This reaction possesses a great synthetic potential to be extended via the use of new functional reagents. In its development, this reaction served successfully to be applied for the preparation of various DHPM derivatives, including a fused pyrimidine derivative and in particular azolopyrimidines [4,5,6]. Compounds displaying the tetrazolo[1,5-a]pyrimidine core moiety are well known to show a wide range of pharmacological activities, such as antituberculosis, anticonvulsant, and antidepressant properties. Furthermore, they also exhibit activities such as sodium channel blocking, the inhibition of human neutrophil elastase [7], and hypoglycemic [8], antibacterial [9], and anti-oxidant [10] properties. Therefore the synthesis of derivative compounds with a tetrazolo[1,5-a]pyrimidine core is of considerable interest for both organic synthesis and medicinal chemistry.
The introduction of a certain group into organic molecules will frequently change their physical, chemical, and physiological properties, which may result in entirely new complementary biological activity. On the basis of this consideration, we synthesized the title compound of the dihydropyrimidine derivative constructed from a tetrazolo[1,5-a]pyrimidine core and morpholino-phenyl group. The presence of a morpholinophenyl group in an organic molecule is known to be responsible for certain biological activities, such as antibacterial [11], anticancer [12], and also anti-oxidant [13] properties.
2. Results and Discussion
A Biginelli reaction is a multicomponent reaction that is known for its versatility by changing its reaction components to furnish various DHPM derivatives. In this article, we report the synthesis of tetrazolo[1,5-a]pyrimidine by replacing urea with 5-aminotetrazole and benzaldehyde with 4-morpholinobenzaldehyde using p-toluenesulfonic acid (pTSA) as a catalyst in ethanol under reflux. The target compound was obtained as a purple needle crystal in a 58% yield. The molecular structure of the product was established by spectroscopic evidence, using FTIR, HRESI-MS, and 1H- and 13C-NMR. The reaction process is described below (Figure 1).
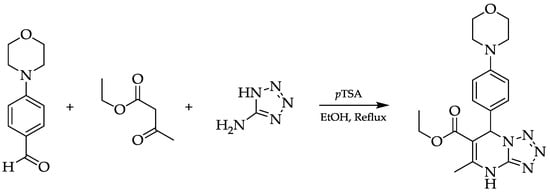
Figure 1.
Biginelli reaction of the title compound.
Ethyl 5-methyl-7-(4-morpholinophenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate: Violet needle crystal (1.0771 g, 58%). Rf = 0.47 (2:1 dichloromethane/ethylacetate). HRESI-MS: [M + H]+ calcd. for C18H22N6O3, 371.1832; found 371.1833. IR (DRS, KBr, cm−1): 3242 (N–H), 3178 (C–H aromatic), 2943 (m, C–H aliphatic), 1712 (str, C=O ester), 1577 (str, C=C aromatic), and 1099 (str, C–O ester). 1H-NMR (400 MHz, CDCl3) δH (ppm) 11.08 (s, 1H), 7.25 (d, J = 8.8 Hz, 2H), 6.83 (d, J = 8.8 Hz, 2H), 6.67 (s, 1H), 4.05 (m, 2H), 3.83 (t, J = 4.8 Hz, 4H), 3.14 (t, J = 4.8 Hz, 4H), 2.67 (s, 3H), 1.16 (t, J = 7.1 Hz, 3H). 13C-NMR (101 MHz, CDCl3) δC (ppm) 165.1, 151.5, 148.8, 145.8, 131.1, 128.4, 115.5, 99.6, 66.9, 60.6, 59.2, 48.9, 19.6, 14.2.
To describe the molecular structure of the prepared compound, first we analyzed the 1H-NMR spectrum. The spectrum displayed a singlet signal at δH 11.08 ppm (s, 1H), indicating a NH amide proton. The existence of four protons in an aromatic ring was established by two doublet signals at δH 7.25 ppm (d, J = 8.8 Hz, 2H) and 6.83 ppm (d, J = 8.8 Hz, 2H), which indicated that the protons were in a symmetrical ortho position. Eight methylene protons of a morpholino group were displayed as two triplet signals at δH 3.83 ppm (t, J = 4.8 Hz, 4H) and 3.14 ppm (t, J = 4.8 Hz, 4H). An ethyl ester group appeared as two signals at δH 4.05 ppm (m, 2H) and δH 1.16 ppm (t, J = 7.1 Hz, 3H). A proton of the methyl group was observed as a singlet signal at δH 2.67 (s, 3H) (Supplementary Materials, Figures S1 and S2). The spectrum of 13C-NMR showed 14 signals (Table 1), which represented all of the carbon atoms of the prepared compound (Supplementary Materials, Figure S3).

Table 1.
NMR data of the prepared compound in CDCl3.
The FTIR spectrum consecutively exhibited the signals of secondary N–H bonding, C–H aliphatic bonding, the carbonyl of an ester group, the aromatic C=C functional group, and the C–O functional group of an ester at wavenumbers (cm−1) of 3242, 3178, 2943, 1712, 1577, and 1099 (see Supplementary Materials, Figure S4), whereas the HRESI-MS spectrum displayed a positive molecular ion [M + H]+ at m/z = 371.1833, indicating a molecular formula of C18H22N6O3 and 11 degrees of unsaturation (see Supplementary Materials, Figure S5).
The existence of the dihydrotetrazolopyrimidine was established by a HMBC experiment, which showed a correlation of a proton at C-7 with an olefinic carbon atom (δC 145.8 (C-5); 99.6 (C-6)) and a carbonyl ester group (δC 165.1 (C-9)). In addition, four correlations of a proton attached at a nitrogen atom with an olefinic carbon (δC 145.8 (C-5) and 99.6 (C-6)), a carbonyl ester (δC 165.1, C-9), and an aminotetrazolo-type carbon atom (δC 148.8 (C-5a)) were observed. The presence of an aryl substituent attached at C-7 was proved by a long-range correlation of the C-7 proton with three aromatic carbons (δC 131.1 (C-1′) and 128.4 (C2′; C-6′)). Moreover, the position of the methyl group at C-8 was proved by a proton long-range correlation with the olefinic carbon atom (δC 145.8 (C-5) and 99.6 (C-6)) and carbonyl ester group (δC 165.1 (C-9)). The proton–carbon correlations from the HMBC experiment (Supplementary Materials, Figure S6) corresponded with the prepared compound and are presented in Figure 2. On the basis of the spectroscopic evidence, the prepared compound is a new compound that has never been published previously.
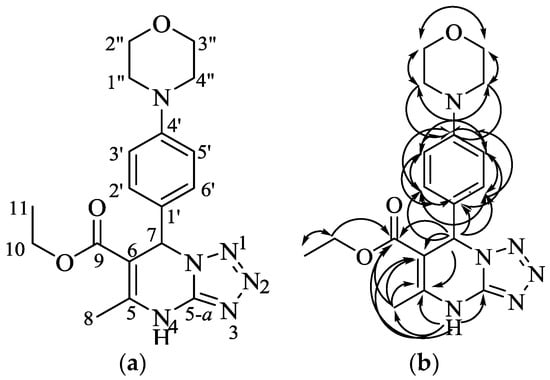
Figure 2.
Structure, numbering (a), and HMBC correlations of the prepared compound (b).
3. Materials and Methods
3.1. General
All reagents and solvents (E. Merck (Darmstadt, Germany) or Sigma-Aldrich (St. Louis, MO, USA)) were used without further purification. The reaction progress was monitored by TLC on silica gel GF254 aluminum sheets (0.25 mm) using various developing system. Spots were detected under UV light (λ = 254 nm). IR spectra were recorded in KBr powder with a diffuse reflectance method on an IRTracer-100 spectrophotometer (Shimadzu, Kyoto, Japan). Mass spectra were recorded on a HR Waters LCT Premier XE mass spectrometer (Santa Clara, CA, USA). NMR spectra (1H-, 13C-NMR, and HMBC) were recorded using a JEOL JNM-ECS400 spectrometer (at 400 and 100 MHz) (Tokyo, Japan) with CDCl3 as the solvent and internal standard.
3.2. Synthesis Procedure of the Prepared Compound
The synthesis of the title compound followed the procedure reported by Suwito et al. (2017) [14]. A mixture of 5 mmol of 4-morpholinobenzaldehyde, 5 mmol of ethyl acetoacetate, 6 mmol of 5-aminotetrazole monohydrate, and 1 mmol of pTSA was dissolved in 3 mL of ethanol and refluxed in a 25 mL round bottom flask. The progress of the reaction was followed by the TLC experiment. The reaction mixture was stopped after 7 h of reflux, cooled to room temperature, and then precipitated by the addition of cool water. The violet precipitate was filtered off and recrystallized with aqueous ethanol to give purple needle crystals.
4. Conclusions
In conclusion, we successfully synthesized as a new compound ethyl 5-methyl-7-(4-morpholinophenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate using a three-component Biginelli reaction starting from 4-morpholinobenzaldehyde, ethyl acetoacetate, and 5-amino-1-tetrazole using pTSA as the catalyst under reflux.
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-8599/2018/2/0/s1: HRESI-MS, FTIR, 1H-NMR, 13C-NMR, and HMBC spectra reported in the supplementary materials as Figures S1–S6 and structure refinement parameters in Table S1.
Author Contributions
H.S. proposed the idea, managed the research, and wrote the paper. N.K. performed the synthesis. K.U.H. and I.I. analyzed the whole spectra. A.A. corrected the draft. All the authors read the draft.
Funding
This research was funded by Universitas Airlangga through Hibah Riset Mandat 2018 research scheme, grant number 1919/UN3.1.8/LT/2018.
Acknowledgments
The authors acknowledge Lembaga Penelitian dan Inovasi of Airlangga University for the funding through the Riset Mandat 2018 Research Grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clarck, J.H.; Macquarrie, D.J.; Sherwood, J. The combined role of catalysis and solvent effects on the Biginelli reaction: Improving efficiency and sustainability. Chem. Eur. J. 2013, 19, 5174–5182. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O.; Stadler, A. The Biginelli dihydropyrimidine synthesis. Org. React. 2004, 63, 1–116. [Google Scholar]
- Kappe, C.O. Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc. Chem. Res. 2000, 33, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Gein, V.L.; Gein, L.F.; Tsyplyakova, E.P.; Panova, O.S. Synthesis of 6-Acyl-7-aryl-4,7-dihydrotetrazolo[1,5-a]pyrimidine-5-carboxylic acids and their methyl esters. Russ. J. Org. Chem. 2007, 43, 1382–1386. [Google Scholar] [CrossRef]
- Gein, V.L.; Zamaraeva, T.M.; Dmitriev, M.V. Synthesis of diethyl 6-aryl-3,6-dihydrotetrazolo-[1,5-a]pyrimidine-4,5-dicarboxylates. Russ. J. Org. Chem. 2016, 52, 558–561. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Toghraei-Semiromi, Z.; Amiri, M. Karimi-Nami, R., One-pot synthesis of tetrazolo[1,5-a]pyrimidines under solvent-free conditions. Mol. Divers. 2013, 17, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-J.; Yao, C.-S.; Yu, C.-X.; Wang, X.-S.; Tu, S.-J. Ionic liquid-mediated one pot synthesis of 5-(trifluoromethyl)-4,7-dihydrotetrazolo-[1,5-a]pyrimidine derivatives. Synth. Commun. 2012, 42, 2728–2738. [Google Scholar] [CrossRef]
- Gein, V.L.; Zamaraeva, T.M.; Mishunin, V.V.; Kotegov, V.P. Synthesis and Hypoglycemic Activity of Methyl 6-aryl(hetaryl)-5-(2-furanoyl)-3,6-dihydrotetrazolo[1,5-a]pyrimidine-4-carboxylates. Russ. J. Org. Chem. 2016, 86, 286–290. [Google Scholar] [CrossRef]
- Gein, V.L.; Zamaraeva, T.M.; Kurbatova, A.A.; Voronina, E.V.; Vakhrin, M.I. Synthesis and Antimicrobial Activity of N,7-diaryl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxamides. Pharm. Chem. J. 2010, 44, 27–29. [Google Scholar]
- Haleel, A.; Mahendiran, D.; Veena, V.; Sakthivel, N.; Rahiman, A.K. Antioxidant, DNA interaction, VEGFR2 kinase, Topoisomerase I and in vitro cytotoxic activities of heteroleptic copper (II) complexes of tetrazolo[1,5-a]pyrimidines and diimines. Mater. Sci. Eng. C Mater. 2016, 68, 366–382. [Google Scholar] [CrossRef] [PubMed]
- Piccionello, A.P.; Musumeci, R.; Cocuzza, C.; Fortuna, C.G.; Guarcello, A.; Pierro, P.; Pace, A. Synthesis and preliminary antibacterial evaluation of Linezolid-like 1,2,4-oxadiazole derivatives. Eur. J. Med. Chem. 2012, 50, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Dastagiri, D.; Ramaiah, M.J.; Reddy, S.; Bharathi, E.V.; Reddy, M.K.; Sagar, M.V.P.; Reddy, T.L.; Pushpavalli, S.N.C.V.L.; Pal-Bhadra, M. Synthesis and apoptosis inducing ability of new anilino substituted pyrimidine sulfonamides as potential anticancer agent. Eur. J. Med. Chem. 2011, 46, 5817–5824. [Google Scholar] [CrossRef] [PubMed]
- Maddilaa, S.; Jonnalagadda, S.B. New class of pyrimidinesulfamoyl containing pyrazole and pyrrole derivatives and their antioxidant activity. Lett. Org. Chem. 2011, 6, 374–379. [Google Scholar] [CrossRef]
- Suwito, H.; Zulqaida, S.; Ul Haq, K.; Kristanti, A.N.; Indriani, I. Ethyl 4-[5-methoxymethyl)furan-2-yl]-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank 2017, 2007, M954. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).