Abstract
Thiazole and chalcone motifs are of research interest to medicinal chemists due to their array of synthetic and biological utility. Hence, in the present study we intended to prepare (E)-1-(2′,4′-dimethyl)-(5-acetylthiazole)-(2,4″-difluorophenyl)-prop-2-en-1-one (3c) containing both these scaffolds. The compound 3c was synthesized by the acid-catalyzed condensation of 2,4-dimethyl-5-acetylthiazole with 2,4-difluorobenzaldehyde. Purification and characterization of the compound were carried out by recrystallization and spectral techniques including UV, IR, 1H-NMR, 13C-NMR, Mass spectrometry and X-ray powdered diffractometry. The molecule 3c was successfully synthesized, purified, and characterized.
1. Introduction
Diaryl-α,β-unsaturated ketones are the biogenic originator in flavonoid biosynthesis and are recognized as chalcones. Chemically, they are open-chain flavonoids in which the two aromatic rings are joined by a three-carbon α,β-unsaturated carbonyl system [1]. Chalcones exhibit a wide range of biological activities such as antioxidant [2], antimicrobial [3], antitubercular [4], antihepatotoxic [5], neuroprotective [6], antibacterial [7], inhibitor of topoisomerase I [8], antimalarial [9], and anticancer activities [10]. Among pharmacologically important heterocyclic compounds, thiazole and its derivatives are well known in pharmaceutical chemistry because of their broad spectrum of biological activities such as antibacterial [11], antifungal [12], and anti-inflammatory activities [13] and their presence in naturally occurring compounds, e.g., antibiotics like penicillin, cephalosporin, and micrococcin, and vitamin B1 [14]. Thiazole motif is present in drug molecules such as Sulfathiazole (antimicrobial drug), Ritonavir (antiretroviral drug), and Abafungin (antifungal drug). Synthetic thiazoles offer the opportunity to increase the structural diversity of natural thiazole substrates [4]. Chalcones attract many researchers to develop efficient synthetic methods and also to produce different structural variation of chalcones that are newer and unavailable in nature. In the present study we report the successful synthesis and characterization of (E)-1-(2′,4′-dimethyl)-(5-acetylthiazole)-(2″,4″-difluorophenyl)-prop-2-en-1-one (3c). The reason for selecting the substituents i.e., methyl groups and fluorine atoms present on thiazole and phenyl rings, respectively, is to study the influence of electron-releasing (methyl) and electron-withdrawing (fluorine) substituents on the synthesis of thiazole-containing chalcone. Usually the chalcones are synthesized by base-catalyzed Claisen‒Schmidt condensation, but we were unable to derive the desired compound with this method. Hence, we utilized acid-mediated synthesis for preparing the compound 3c and were successful. The chalcone can be easily prepared in high yields by acid-catalyzed condensation reaction; it is purified by recrystallization and characterized by physicochemical and spectral analysis data.
2. Results
The title compound was synthesized by acid-catalyzed condensation of aromatic aldehyde and aromatic ketone using acetic acid in hydrochloric acid as a catalyst, as shown in Scheme 1. Firstly, the purity of the product was analyzed by performing thin-layer chromatography and then by determining its melting point. The structural elucidation of the compound was done based on spectroscopic data and the results are displayed below. The product is assumed to exist in the E configuration based on its coupling constant, J at 16 Hz.
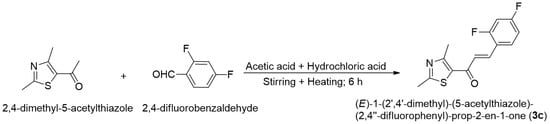
Scheme 1.
Synthesis of (E)-1-(2′,4′-dimethyl)-(5-acetylthiazole)-(2,4″-difluorophenyl)-prop-2-en-1-one (3c).
(E)-1-(2′,4′-Dimethyl)-(5-acetylthiazole)-(2,4″-difluorophenyl)-prop-2-en-1-one (3c): Shining orange crystals (0.182 g, 95%, after recrystallization) (Figure 1), m.p. 80 °C, Rf = 0.44 (30% ethyl acetate in n-hexane), MS (m/z, %) 280.1 (M + 1, 99.56); UV (λ max): 320 nm (200 µg/mL in 0.1 M HCl); IR (KBr, cm−1) 1656.38 (intense C=O conjugated band), 1596.55 (C=C of Ar), 1499.38 (str, CH=CH, conjugated), 1270.95, (C–F) and 1134.70 (C–F); 1H-NMR spectrum (400 MHz, CDCl3) δ (ppm) 2.75 (s, 3H), 2.79 (s, 3H), 6.91 (d, 1H), 7.27 (d, 1H, J = 16 Hz), 7.23 (d, 1H), 7.61 (s, 1H), 7.81 (d, 1H, J = 16 Hz). 13C-NMR spectrum (100 MHz, CDCl3) δ (ppm) 18.36 (methyl C at C-3′), 19.52 (methyl C at C-2′), 105.80 (C-5″), 112.29 (C-3″), 119.17 (C-1″), 126.80 (C-2), 126.89 (C-5′) 131.10 (C-6″), 135.97 (C-3), 160.86 (C-2″, C–F coupling constant value J = 12 Hz), 160.80 (C-4′), 162.84 (C-4″, C–F coupling constant value J = 12 Hz), 168.53 (C-2′), 182.36 (C-1). The X-ray powder diffraction pattern data of 3c are provided in Table 1.
Figure 1.
Titled compound 3c after recrystallization with methanol.

Table 1.
X-ray powder pattern of compound 3c.
3. Materials and Methods
3.1. General
The organic solvents such as methanol, hexane, and ethyl acetate were of spectral grade and used as such without further purification. Anhydrous methanol was obtained by fractional distillation and storing over type 4A molecular sieves. Some of the solvents were purchased from local manufacturers and some from S.D. Fine Chem. Ltd, Mumbai, India. All the chemicals used in the synthesis were obtained from standard commercial sources. TLC analysis was carried out on Merck grade precoated TLC silica gel 60 F254 plates (Merck KGaA, Darmstadt, Germany) and the spots were visualized under a UV lamp. 2,4-dimethyl-5-acetylthiazole was purchased from Tokyo Chemical Industry (Tokyo Chemical Industries Co., Ltd. Toshima, Kita-Ku, Tokyo, Japan). 2,4-difluorobenzaldehyde was purchased from Sigma Aldrich Chemical Co. (Milwaukee, WI, USA). Melting point was determined in open capillaries, using a Boetius melting point apparatus (expressed in °C) (Rapido, Dresden, Germany), and are uncorrected. UV spectra were recorded on an Elico SL-210 UV-Visible spectrophotometer (ELICO Ltd. B-90, A.P.I.E., Sanathnagar, Hyderabad 500 018, A.P., India). FT-IR spectra were recorded using Bruker alpha-T (BRUKER biospin International AG., Zug, Switzerland) and the 1H- and 13C-NMR spectra of the compound were recorded on a Bruker 400 Avance NMR spectrophotometer using TMS as an internal standard (values are expressed in δ ppm). MS spectra were recorded on an Agilent LC-MS spectrometer (Agilent technologies, 5301 Stevens Creek Blvd, Santa Clara, CA, USA.).
3.2. Preparation of the Title Compound
First, 135 µL (1 mmol) of 2,4-dimethyl-5-acetylthiazole were dissolved in a mixture of 4 mL of glacial acetic acid and 2 mL of hydrochloric acid. To the above solution, 109 µL (1 mmol) of 2,4-difluorobenzaldehyde were added and stirred on a magnetic stirrer at a temperature of 70 °C for 6 h. After completion of the reaction (observed through spots on the TLC plate using 30% ethyl acetate in n-hexane), the mixture was transferred onto crushed ice. This led to the precipitation of the compound, which was then filtered, washed thoroughly with cold water, and dried. The precipitate was further recrystallized with methanol to get the orange crystals of the title compound 3c (Scheme 1).
4. Conclusions
We have demonstrated the synthesis of a (E)-1-(2′,4′-dimethyl)-(5-acetylthiazole)-(2,4″-difluorophenyl)-prop-2-en-1-one through an acid-catalyzed condensation reaction and characterization by physicochemical and spectral methods.
Supplementary Materials
UV, FTIR, 1H-NMR, 13C-NMR, MS, and X-ray spectra of the synthesized compounds are available online.
Author Contributions
Conceptualization, A.S.; Data curation, S.S.; Formal analysis, S.S.; Funding acquisition, S.B.P.; Investigation, A.S. and S.S.; Methodology, A.S.; Project administration, A.S.; Resources, S.B.P.; Supervision, A.S.; Validation, A.S. and S.B.P.; Visualization, A.S. and S.S.; Writing—original draft, S.S.; Writing—review & editing, A.S.
Funding
This research received no external funding.
Acknowledgments
The authors are thankful to the management of Vignan Pharmacy College, Vadlamudi, Guntur District, Andhra Pradesh, India for providing the chemicals and laboratory facilities to perform the research work. The authors are also grateful to Andhra University College of Pharmaceutical Sciences, Visakhapatnam, Andhra Pradesh, India for providing the IR, NMR and mass spectral data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Suwito, H.; Haq, K.U.; Rahmah, N.N.D.; Kristanti, A.N.; Puspaningsih, N.N.T. 4-({4-[(2E)-3-(2,5-Dimethoxyphenyl)prop-2-enoyl]phenyl}amino)-4-oxobutanoic Acid. Molbank 2017, 2017, M938. [Google Scholar] [CrossRef]
- Ayati, A.; Bakhshaiesh, T.O.; Moghimi, S.; Esmaeili, R.; Majidzadeh-A, K.; Safavi, M.; Firoozpour, L.; Emami, S.; Foroumadi, A. Synthesis and biological evaluation of new coumarins bearing 2,4-diaminothiazole-5-carbonyl moiety. Eur. J. Med. Chem. 2018, 155, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Liaras, K.; Geronikaki, A.; Glamoclija, J.; Ciric, A.; Sokovic, M. Thiazole-based chalcones as potent antimicrobial agents. Synth. Biol. Eval. 2011, 19, 3135–3140. [Google Scholar] [CrossRef]
- Karuvalam Ranjith, P.; Haridas Karickal, R.; Nayak Susanta, K.; Guru Row Tayur, N.; Rajeesh, P.; Rishikesan, R.; Suchetha Kumari, N. Design, synthesis of some new (2-aminothiazol-4-yl)methylester derivatives as possible antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2012, 49, 172–118. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Ahmed, B.; Alam, T. Synthesis and hepatotoxic activity of some new chalcones containing 1,4-dioxane ring system. Pak. J. Pharm. Sci. 2006, 19, 290–294. [Google Scholar] [PubMed]
- Jung, J.-C.; Jang, S.; Lee, Y.; Min, D.; Lim, E.; Jung, H.; Oh, M.; Oh, S.; Jumg, M. Efficient synthesis and neuroprotective effect of substituted 1,3-diphenyl-2-propen-1-ones. J. Med. Chem. 2008, 51, 4054–4058. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chaturvedi, V.; Manju, Y.K.; Bhatnagar, S.; Srivastava, K.; Puri, S.K.; Chauhan, P.M.S. Substituted quinolinyl chalcones and quinolinyl pyrimidines as a new class of anti-infective agents. Eur. J. Med. Chem. 2009, 44, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.; Kang, B.Y.; Cheon, S.H. Topoisomerase I inhibition and cytotoxicity of lichochalcones A and E from Glycyrrhiza inflate. Arch. Pharm. Res. 2007, 30, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Suwito, H.; Pudjiastuti, P.; Fanani, M.Z.; Kimata-Ariga, Y.; Katahira, R.; Kawakami, T.; Fujiwara, T.; Hase, T.; Sirat, H.M.; Puspaningsih, N.N.T. Design and synthesis of chalcone derivatives as inhibitors of the ferredoxin—Ferredoxin-NADP+ reductase interaction of plasmodium falciparum: Pursuing new antimalarial agents. Molecules 2014, 19, 21473–21488. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Sever, B.; Çiftçi, G.A.; Özdemir, A. Design, Synthesis, and Evaluation of a New Series of Thiazole-Based Anticancer Agents as Potent Akt Inhibitors. Molecules 2018, 23, 1318. [Google Scholar] [CrossRef] [PubMed]
- Sadek, B.; Al-Tabakha, M.M.; Fahelelbom, K.M.S. Antimicrobial Prospect of Newly Synthesized 1,3-Thiazole Derivatives. Molecules 2011, 16, 9386–9396. [Google Scholar] [CrossRef] [PubMed]
- Kaplancıklı, Z.A.; Levent, S.; Osmaniye, D.; Sağlık, B.N.; Çevik, U.A.; Çavuşoğlu, B.K.; Özkay, Y.; Ilgın, S. Synthesis and Anticandidal Activity Evaluation of New Benzimidazole-Thiazole Derivatives. Molecules 2017, 22, 2051. [Google Scholar] [CrossRef] [PubMed]
- Januario, J.P.; de Souza, T.B.; Lavorato, S.N.; Maiolini, T.C.S.; Domingos, O.S.; Baldim, J.L.; Folquitto, L.R.S.; Soares, M.G.; Chagas-Paula, D.A.; Dias, D.F.; et al. Design and Synthesis of New Benzophenone Derivatives with In Vivo Anti-Inflammatory Activity through Dual Inhibition of Edema and Neutrophil Recruitment. Molecules 2018, 23, 1859. [Google Scholar] [CrossRef] [PubMed]
- Shivananda, W.; Vasudeva Adhikari, A.; Suchetha Kumari, N. Synthesis of Some Novel 2,4-Disubstituted Thiazoles as Possible Antimicrobial Agents. Phosporus Sulfur Silicon Relat. Elem. 2008, 183, 1285–1300. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).