Abstract
4-Amino-5-(5-methyl-1-phenyl-1H-pyrazol-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (1) upon treatment with 3,4-dimethoxybenzaldehyde in 10 mL of absolute ethanol in the presence of a catalytic amount of acetic acid produced the target compound 4-[(3,4-dimethoxybenzylidene)amino]-5-(5-methyl-1-phenyl-1H-pyrazol-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (2) in 80% yield. The obtained solid product was recrystallized from ethanol. The compound was characterized by elemental analyses, mass spectrometry, FT-IR, 1H and 13C-NMR spectroscopy. To study the binding interactions of the compound with receptor, it was docked with the human prostaglandin reductase (PTGR2). The docking pose and noncovalent interactions gave insights into its plausible inhibitory action.
1. Introduction
N-bridged heterocyclic compounds are known to possess useful properties, including antibacterial [1], anti inflammatory [2], anticancer [3], analgesic [4], antitubercular [5], antifungal [6], antiviral [7], and antitumor [8] properties. Moreover, sulfur-substituted 1,2,4-triazole ring systems represent an important group of bioactive compounds [9]. Current examples include vorozole, letrozole, and anastrozole, which are non-steroidal drugs that are also used in the treatment of cancer. Several drugs containing a pyrazole moiety, like lonazolac, pyrazofurin, fezolamie, phenylbutazone, tepoxalin, novalgin, isolan, celecoxib, ramifenazone, and apixaban, are already in the market. With this in mind, the synthesis of triazoles fused to another heterocyclic compound has attracted particular attention due to their diverse applications. Prompted by these investigations, we reported the synthesis of 4-[(3,4-dimethoxybenzylidene)amino]-5-(5-methyl-1-phenyl-1H-pyrazol-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (2).
Prostaglandins are important lipid mediators which are derived from arachidonic acid and play variety and important roles throughout the body, including within the central nervous system (CNS), cardiovascular, endocrine, immune, and genitourinary systems. The specific role of each prostaglandin was examined according to their receptor expression profile and the cellular context. Prostaglandin D2 synthase (PGDS) catalyzes the isomerization of PGH2 to PGD2. The release of PGD2 results in a various set of responses, ranging from sleep promotion and inhibition of platelet aggregation to the attraction of bronchoconstriction and inflammatory cells [10]. In the present study, we report the synthesis and in silico evaluation of the title compound 2 as prostaglandin D synthase inhibitor.
2. Results
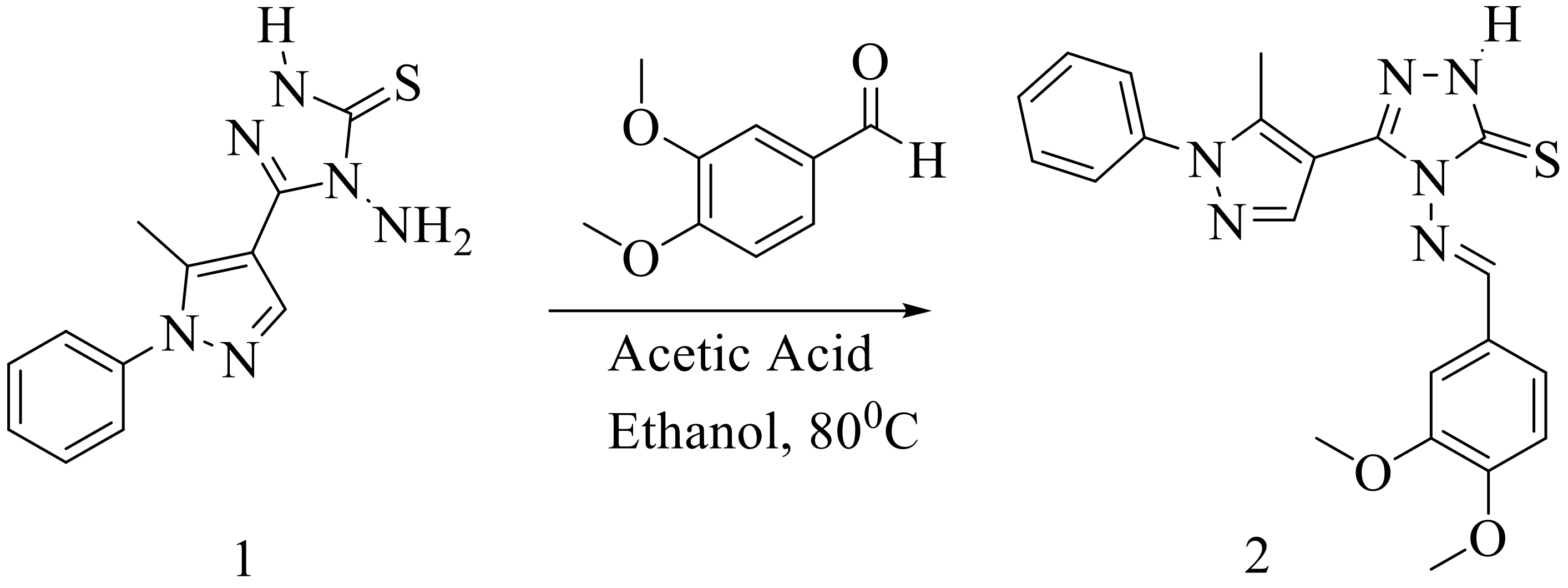
We synthesized 4-amino-5-(5-methyl-1-phenyl-1H-pyrazol-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (1) according to a previously published procedure [11,12], where 5-methyl-1-phenyl-1H-pyrazole-4-carbohydrazide was treated with carbon disulfide (CS2) in the presence of ethanolic potassium hydroxide and refluxed. The obtained product was acidified with dil. HCl and was then treated with hydrazine hydrate to obtain the intermediate 4-amino-5-(5-methyl-1-phenyl-1H-pyrazol-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (1). The resulting intermediate was then refluxed with 3,4-dimethoxybenzaldehyde in 10 mL of absolute ethanol in the presence of a catalytic amount of acetic acid. After completion of the reaction, the reaction mixture was cooled to allow precipitation of the product, which was filtered, washed with water, and dried. The crude product obtained was recrystallized from ethanol to afford compound (2) with 80% yield (Scheme 1). The structure of the compound was confirmed by FT-IR, 1H and 13C-NMR, and mass spectroscopic techniques (Supplementary Materials).
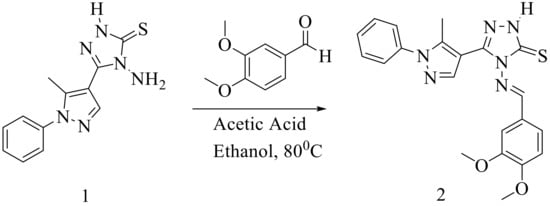
Scheme 1.
Synthetic protocol for 4-[(3,4-dimethoxybenzylidene)amino]-5-(5-methyl-1-phenyl-1H-pyrazol-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (2).
3. Molecular Docking
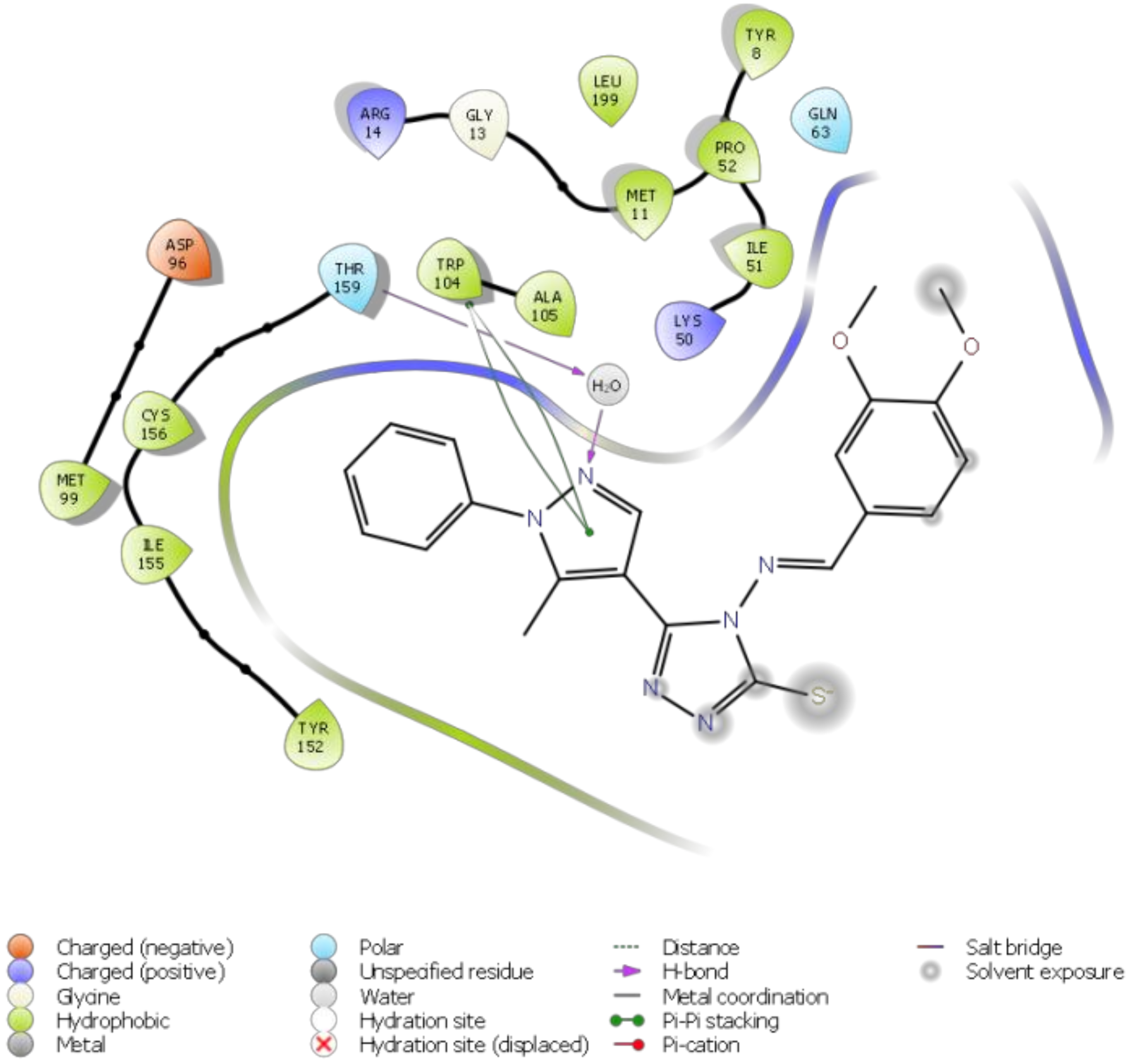
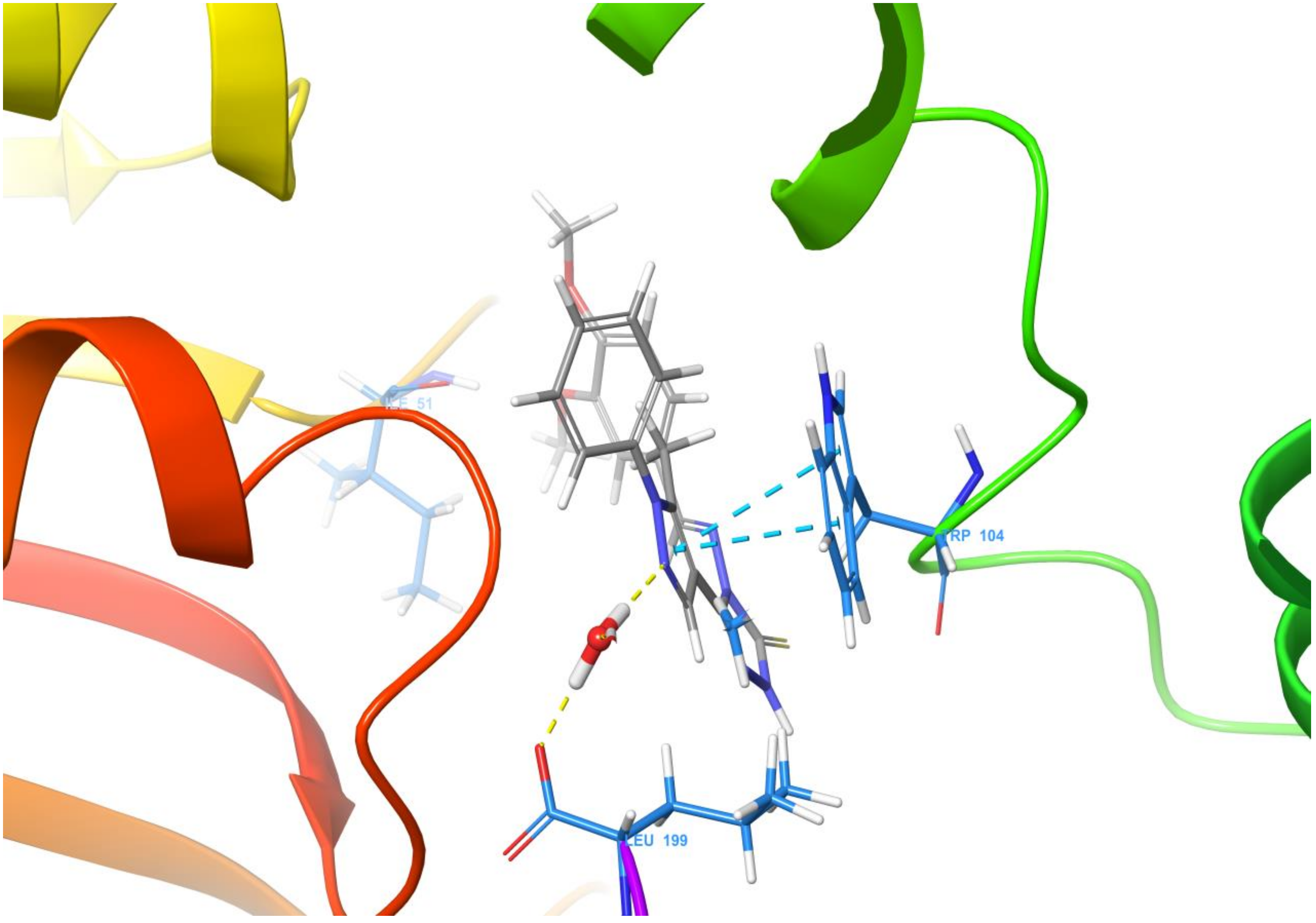
Molecular docking of the target compound (2) and the target protein 2VCW showed a strong interaction (Figure 1 and Figure 2). The N-2 of the pyrazole acts as a donor and makes hydrogen bond with a water molecule, and that water molecule made hydrogen bond interaction with Thr159. The pyrazole ring exhibited π–π stacking interactions with Trp104. Besides these interactions, the targeted molecule exhibited hydrophobic interactions with the residues around the binding pocket, such as Tyr152, Ile155, Cys156, Thr159, Ala105, Lys50, Ile51, and Gln63. All of the observed interactions revealed that the molecule has a great affinity towards the enzyme. The inhibitor has a better docking score of −7.648 kcal/mol; the reference inhibitor gives a docking score of −5.247 kcal/mol.
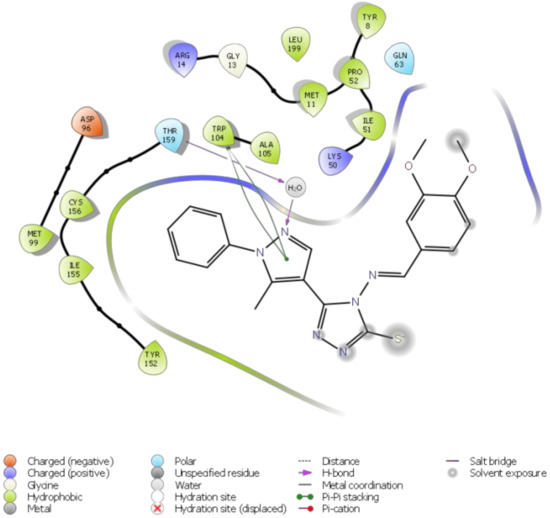
Figure 1.
2D ligand interaction diagram of the title compound 2 with human prostaglandin reductase (PTGR2).
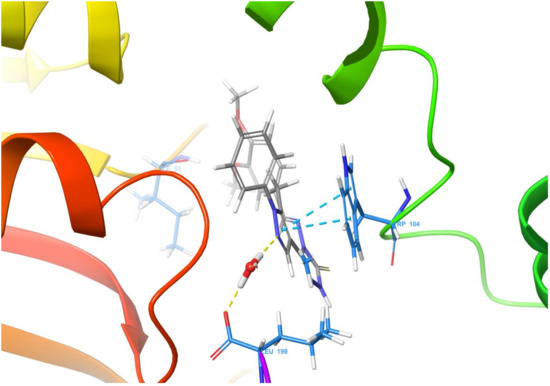
Figure 2.
Ligand interaction diagram of the title compound 2 with human prostaglandin reductase (PTGR2).
4. Discussion
The IR spectrum of compound (2) exhibited an absorption band at 1597 cm−1, corresponding to the C=N group of the pyrazoline and triazole ring. The absorption band that appeared at 1504 cm−1 is due to the presence of the C=C group. Absorption bands observed at 3409 and 1249 cm−1 supported the presence of aromatic N–H and C=S groups. The observed IR bands support the formation of the target compound. The 1H-NMR spectrum of compound 2 showed a singlet at 2.38 ppm for CH3 protons. The two methoxy protons appeared at 3.95 ppm and 3.97 ppm as two distinct singlets. The proton in–NH resonated at 4.83 ppm and appeared as a singlet. Aromatic protons appeared as a multiplet in the range 7.26–7.52 ppm. A doublet in the range 7.90–7.92 ppm was assigned to an aromatic proton with a coupling constant of 8.4 Hz. A singlet that appeared at 8.11 ppm corresponds to the proton in the second position of the 3,4-dimethoxyphenyl substituent. One more doublet that appeared in the range 8.15–8.17 ppm was assigned to an aromatic proton with a coupling constant of 8.4 Hz. The pyrazole proton resonated as a singlet at 8.99 ppm, and another singlet signal that appeared at 9.19 ppm was assigned to the benzylidene proton. The 13C-NMR spectrum of the targeted molecule showed signals at 171.4 and 172.8 ppm for its C=N and C=S carbons, respectively. The signal that appeared at 12.8 ppm indicated the presence of methyl in the compound. The appearance of two signals at 55.9 and 56.0 ppm exhibited the presence of methoxy carbon atoms. Twelve signals of aromatic carbons appeared in the range of 108.8–168.3 ppm. The formation of the title compound was further confirmed by the molecular ion peak at m/z 421.05 [M + H]+ which is in agreement with the molecular formula C21H20N6O2S. The elemental analysis gave satisfactory values for the percentage of C, H, and N present in the synthesized molecule, and is presented in the experimental section.
5. Materials and Methods
All chemicals and solvents of appropriate grade were obtained from Spectrochem Pvt. Ltd. (Bangalore, India) and Sigma-Aldrich (Bangalore, India), and were used without further purification. The purity of the compounds was examined by thin layer chromatography (TLC) on silica-coated aluminum sheet (silica gel 60F254) using hexane and ethyl acetate mixtures and visualized under UV at 254 nm. The melting points of the new synthesized compounds were determined using open glass capillary tubes and left uncorrected. FT-IR spectra were recorded on a Shimadzu FT-IR157 Spectrometer (Shimadzu Corporation, Kyoto, Japan). NMR spectra were measured using a Bruker AvanceII 400 spectrometer (Bruker BioSpin AG, Fällanden, Switzerland) operating at 400 MHz for 1H and at 100 MHz for 13C nuclei respectively, using tetramethylsilane (TMS) as an internal standard. The chemical shifts (δ) and the coupling constants (J) are reported in parts per million (ppm) and in hertz, respectively. Mass spectra were recorded in an Agilent Technology LC mass spectrometer (Agilent Technologies, Santa Clara, CA, United States) with ESI ionization in positive mode. Elemental analysis was performed using CHNS Elementar Vario EL III (Elementar, Langenselbold, Germany).
Computer-aided drug design plays an important role in the medicinal field. Structure-based drug design mainly aims at the identification of candidate drugs, which includes the design and optimization of a chemical structure with the goal of identifying a compound which is suitable for clinical testing. With the Glide module in Schrodinger, the docking of the ligands to the structural protein model was carried out. Then, the receptor grid was generated at the site having lowest energy using Glide software and using the ligand-docking panel in Glide, molecular docking was carried out. Using 2D sketcher, the structures of the ligands were drawn and then optimized with LigPrep to generate energy-optimized 3D structures. The ionization states were generated at pH range 7.0 ± 2 using the Epik module in LigPrep with default options. The high-resolution 3D structure of UPPS (PDB ID: 2VCW) protein was downloaded from Protein Data Bank and was prepared with the Protein Preparation Wizard in Maestro using default options, and the missing hydrogen’s amino acid side chains were added, and any erroneous bond orders were corrected. The structure was finally minimized to release any possible strain. The prepared molecules were successively and flexibly docked at the protein grid using extra precession (XP) mode. The protein-ligand complex was further analyzed and visualized for ligand fitting and interactions using Maestro.
4-Amino-5-(5-methyl-1-phenyl-1H-pyrazol-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (1) was prepared according to a previously published procedure [11,12].
4-[(3,4-Dimethoxybenzylidene)amino]-5-(5-methyl-1-phenyl-1H-pyrazol-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (2)
A mixture of compound (1, 0.01 mol) and 3,4-dimethoxybenzaldehyde (0.01 mol) in 10 mL of absolute ethanol in the presence of catalytic amount of acetic acid was refluxed for 4 h. The reaction was cooled upon completion (TLC), and the precipitated product was filtered, washed with water, and dried. The obtained solid product was recrystallized from ethanol to get the target compound (2, 80%) a pale yellow colored solid, m.p. 160–162 °C (EtOH); FT-IR (ATR, υmax, cm−1): 3409 (N–H), 1597 (C=N), 1504 (C=C), 1249 (C=S); δH (400 MHz, CDCl3): 2.38 (3H, s, –CH3 ), 3.95 (3H, s, –OCH3 ), 3.97 (3H, s, –OCH3 ),4.83 (1H, s, –NH), 7.26–7.52 (5H, m, Ar–H), 7.90–7.92 (1H, d, Ar–H, J = 8.4 Hz), 8.11 (1H, s, Ar–H), 8.15–8.17 (1H, d, Ar–H, J = 8.4 Hz), 8.99 (1H, s, pyrazole–CH), 9.19 (1H, s, =CH); 13C-NMR (400 MHz, CDCl3, δ ppm): 12.8, 55.9, 56.0, 108.8, 110.7, 116.5, 123.9, 126.1, 127.3, 129.0, 130.7, 149.4, 151.8, 161.2, 168.3, 171.4, 172.8;EI–MS: m/z (M + 1, 421.05); Anal. calcd for C21H20N6O2S C, 59.98; H, 4.79; N, 19.99. Found: C, 59.99; H, 4.83; N, 19.96.
6. Conclusions
In the present study, 4-[(3,4-dimethoxybenzylidene)amino]-5-(5-methyl-1-phenyl-1H-pyrazol-4-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (2) was successfully obtained using a simple and straightforward method. The structure of the new synthesized compound was confirmed using spectroscopic techniques. The potency of the compound to act as a ligand to the target protein 2VCW was also evaluated by a docking method. The results revealed the presence of hydrogen bond interactions and a π–π stacking interaction along with a good docking score. The docking studies indicate that the title compound might have some pharmacological activity.
Supplementary Materials
The following are available online, Figure S1: FT-IR spectrum, Figure S2: Mass spectrum, Figures S3–S5: 1H-NMR spectrum, Figure S6: 13C-NMR Spectrum.
Author Contributions
Experiments, Writing—Original Draft Preparation, and Formal Analysis, S.G; Supervision, B.P.
Funding
This research received no external funding.
Acknowledgments
The authors are thankful to the Department of Biochemistry, Mangalore University for providing the docking facility. The authors thank IISC-Bangalore for the spectral data.
Conflicts of Interest
The authors have no conflicts of interest.
References
- Sharma, P.K. A review: Antimicrobial agents based on nitrogen and sulfur-containing heterocycles. Asian. J. Pharm. Clin. Res. 2017, 10, 47–49. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Patil, S.A.; Gacche, R.N.; Korbad, B.L.; Hote, B.S.; Kinkar, S.N.; Jalde, S.S. Synthesis and biological evaluation of nitrogen-containing chalcones as possible anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. Lett. 2010, 20, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Ruddarraju, R.R.; Murugulla, A.C.; Kotla, R.; Tirumalasetty, M.C.B.; Wudayagiri, R.; Donthabakthuni, S.; Maroju, R. Design, synthesis, anticancer activity and docking studies of theophylline containing 1,2,3-triazoles with variant amide derivatives. Med. Chem.Commun. 2016, 8, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Khanage, S.G.; Raju, A.; Mohite, P.B.; Pandhare, R.B. Analgesic Activity of Some 1,2,4-triazole Heterocycles Clubbed with Pyrazole, Tetrazole, Isoxazole and Pyrimidine. Adv. Pharm. Bull. 2013, 3, 13–18. [Google Scholar]
- Fernandes, G.F.S.; Chin, C.M.; Santos, J.L. Advances in Drug Discovery of New Antitubercular Multidrug-Resistant Compounds. Pharmaceuticals 2017, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Shalini, K.; Kumar, N.; Drabu, S.; Sharma, P.K. Advances in synthetic approach to and antifungal activity of triazoles. Beilstein J. Org. Chem. 2011, 7, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, Q.; Yang, G. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.S.; Poojary, B.; Prasad, D.J.; Naik, P.; Holla, B.S. Synthesis and antitumor activity studies of some new fused 1,2,4-triazole derivatives carrying 2,4-dichloro-5-fluorophenyl moiety. Eur. J. Med. Chem. 2009, 44, 5066–5070. [Google Scholar] [CrossRef] [PubMed]
- Karegoudar, P.; Prasad, D.J.; Ashok, M.; Mahalinga, M.; Poojary, P.; Holla, B.S. Synthesis, antimicrobial and anti-inflammatory activities of some 1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles and 1,2,4-triazolo[3,4-b][1,3,4]thiadiazines bearing trichlorophenyl moiety. Eur. J. Med. Chem. 2008, 43, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Hohwy, M.; Spadola, L.; Lundquist, B.; Hawtin, P.; Dahmen, J.; Groth-Clausen, I.; Nilsson, E.; Persdotter, S.; Wachenfeldt, K.V.; Folmer, R.H.A.; et al. Novel Prostaglandin D Synthase Inhibitors Generated by Fragment-Based Drug Design. J. Med. Chem. 2008, 51, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Viveka, S.; Viveka, S.; Dinesha; Shama, P.; Nagaraja, G.K.; Deepa, N.; Sreenivasa, M.Y. Design, synthesis, and pharmacological studies of some new Mannich bases and S-alkylated analogs of pyrazole integrated 1,3,4-oxadiazole. Res. Chem. Intermed. 2016, 42, 2597–2617. [Google Scholar] [CrossRef]
- Farghaly, A.; Clercq, E.D.; El-Kashef, H. Synthesis and antiviral activity of novel[1,2,4]triazolo[3,4-b][1,3,4]thiadiazoles, [1,2,4]triazolo[3,4-b] [1,3,4]thiadiazines and [1,2,4]triazolo[3,4-b][1,3,4] thiadiazepines. Arkivoc 2006, 137–151. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).