1-[(1S)-(4-Fluorophenyl)-((1′S)-1′-naphthalen-1-yl-ethylamino)-methyl]-naphthalen-2-trifluoromethanesulfonate
Abstract
:1. Introduction
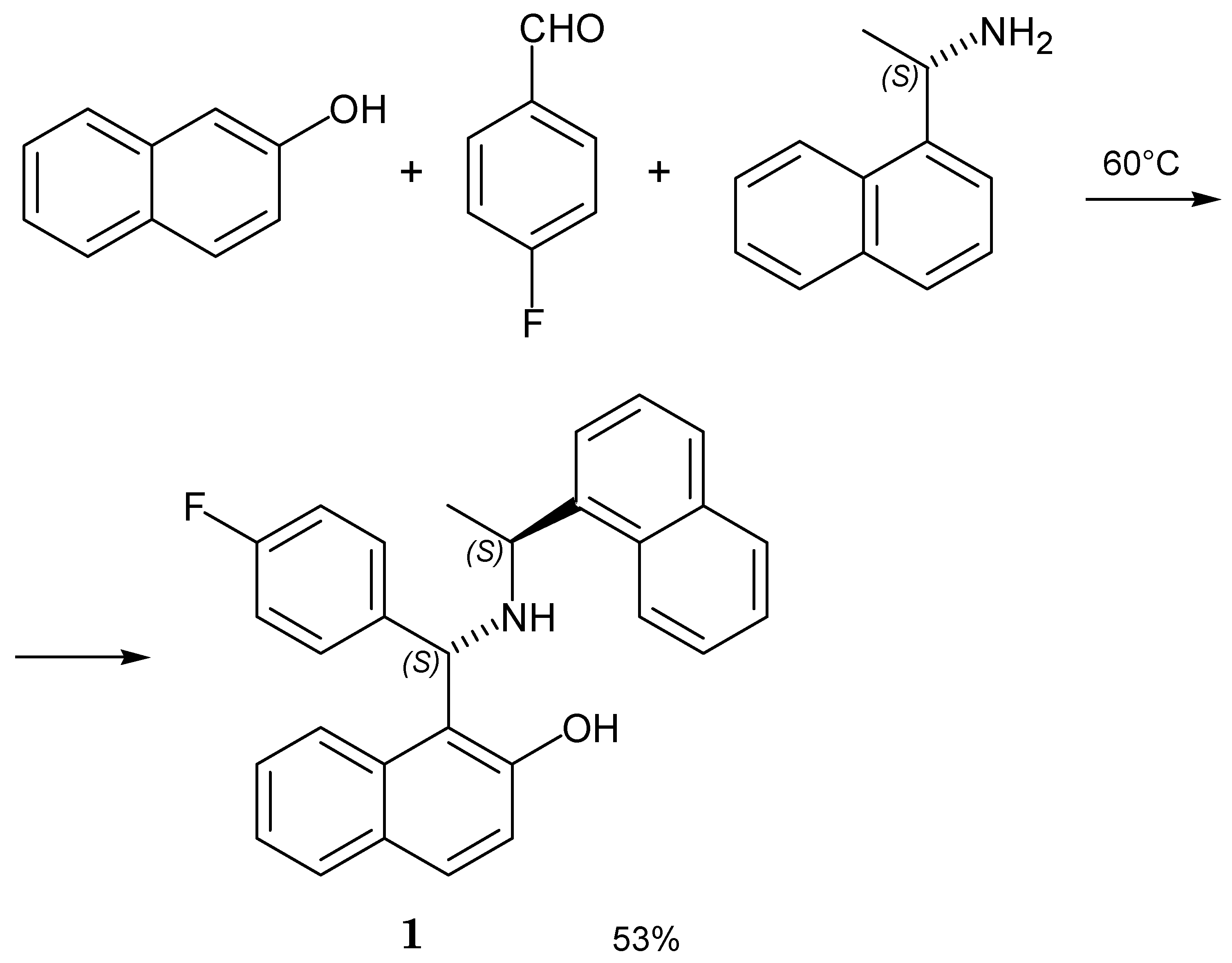
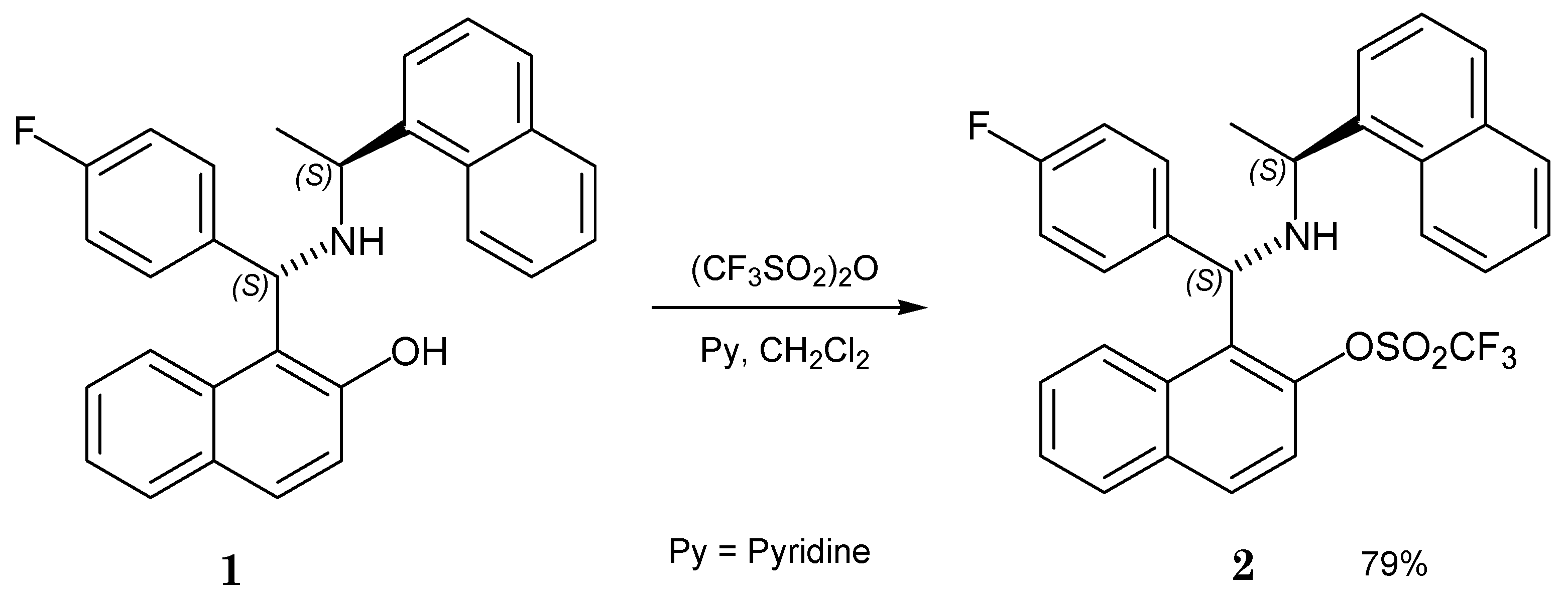
2. Results
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardellicchio, C.; Capozzi, M.A.M.; Naso, F. The Betti base: The awakening of a sleeping beauty. Tetrahedron Asymmetry 2010, 21, 507–517. [Google Scholar] [CrossRef]
- Naso, F. Mario Betti: A Giant in the Chemistry Scenario of the Twentieth Century. Substantia 2017, 1, 111–121. [Google Scholar]
- Iftikhar, R.; Kamran, M.; Iftikhar, A.; Parveen, S.; Naeem, N.; Jamil, N. Recent Advances in the green synthesis of Betti bases and their application: A review. Mol. Divers. 2023, 27, 543–569. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Zahoor, A.F.; Ahmad, S.; Parveen, B.; Ali, K.G. Novel synthetic methods toward the synthesis of Betti bases: An update. Chem. Pap. 2023. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Ding, K. Synthesis of a new type of chiral amino phosphine ligands for asymmetric catalysis. Tetrahedron Asymmetry 2002, 13, 1291–1297. [Google Scholar] [CrossRef]
- Chakraborty, S.; Konieczny, K.; Müller, B.H.; Spannenberg, A.; Kamer, P.C.J.; de Vries, J.G. Betti base derived P-stereogenic phosphine-diamidophosphite ligands with a single atom spacer and their application in asymmetric catalysis. Catal. Sci. Technol. 2022, 12, 1392–1399. [Google Scholar] [CrossRef]
- Metlushka, K.E.; Sadkova, D.N.; Shaimardanova, L.N.; Nikitina, K.A.; Lodochnikova, O.A.; Kataeva, O.N.; Al’fonsov, V.A. Phosphorylation of Betti bases. Russ. J. Org. Chem. 2014, 50, 1573–1578. [Google Scholar] [CrossRef]
- Cardellicchio, C.; Capozzi, M.A.M.; Alvarez-Larena, A.; Piniella, J.F.; Capitelli, F. Investigation on the weak interactions assembling the crystal structures of Betti bases. CrystEngComm 2012, 14, 3972–3981. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Cardellicchio, C. (S,S)-1-(phenyl((1′-4-nitrophenyl-ethyl)amino)methyl)-2-naphthol. Molbank 2022, 2022, 1522. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Terraneo, G.; Cardellicchio, C. Structural insights into methyl- or methoxy-substituted 1-(α−aminobenzyl)-2-naphthol structures: The role of C-H···π interactions. Acta Cryst. 2019, C75, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M. CH/π hydrogen bonds in crystals. CrystEngComm 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Cardellicchio, C. Stereoselection in the Betti reaction of valine methyl esters. Tetrahedron Asymmetry 2017, 28, 1792–1796. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Alvarez-Larena, A.; Piniella Febrer, J.F.; Cardellicchio, C. A combined structural and computational investigation of aminobenzylnaphthol compounds derived from the Betti reaction using valine methyl ester. New J. Chem. 2021, 45, 20735–20742. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Capitelli, F.; Bottoni, A.; Calvaresi, M.; Cardellicchio, C. Stacked Naphthyl and Weak Hydrogen-bond Interactions Govern the Conformational Behavior of P-Resolved Cyclic Phosphonamides: A Combined Experimental and Computational Study. J. Org. Chem. 2014, 79, 11101–11109. [Google Scholar] [CrossRef] [PubMed]
- Metlushka, K.E.; Sadkova, D.N.; Nikitina, K.A.; Shaimardanova, L.N.; Kataeva, O.N.; Alfonsov, V.A. Phosphorylation of Betti base with diethyl chlorophosphate. Russ. J. Gen. Chem. 2016, 86, 515–521. [Google Scholar] [CrossRef]
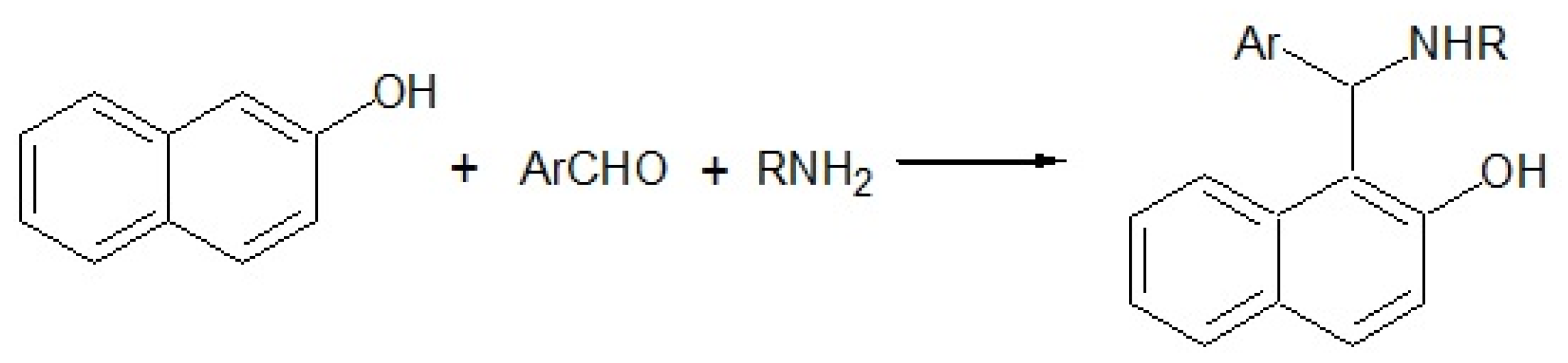

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardellicchio, C.; Capozzi, M.A.M. 1-[(1S)-(4-Fluorophenyl)-((1′S)-1′-naphthalen-1-yl-ethylamino)-methyl]-naphthalen-2-trifluoromethanesulfonate. Molbank 2023, 2023, M1695. https://doi.org/10.3390/M1695
Cardellicchio C, Capozzi MAM. 1-[(1S)-(4-Fluorophenyl)-((1′S)-1′-naphthalen-1-yl-ethylamino)-methyl]-naphthalen-2-trifluoromethanesulfonate. Molbank. 2023; 2023(3):M1695. https://doi.org/10.3390/M1695
Chicago/Turabian StyleCardellicchio, Cosimo, and Maria Annunziata M. Capozzi. 2023. "1-[(1S)-(4-Fluorophenyl)-((1′S)-1′-naphthalen-1-yl-ethylamino)-methyl]-naphthalen-2-trifluoromethanesulfonate" Molbank 2023, no. 3: M1695. https://doi.org/10.3390/M1695
APA StyleCardellicchio, C., & Capozzi, M. A. M. (2023). 1-[(1S)-(4-Fluorophenyl)-((1′S)-1′-naphthalen-1-yl-ethylamino)-methyl]-naphthalen-2-trifluoromethanesulfonate. Molbank, 2023(3), M1695. https://doi.org/10.3390/M1695





