- Review
Recent Advances and Future Prospects Towards CO2 Methanation Reaction
- Fanying Zhang,
- Bin Lu and
- Jihao Zhang
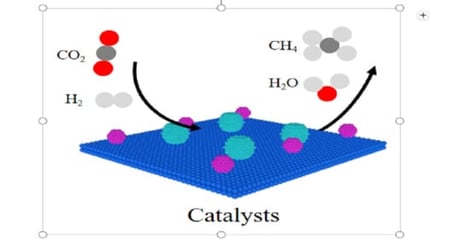
The reaction of CO2 hydrogenation into CH4 provides an industrial-scale pathway for CO2 recycling. The controllable design of catalysts with highly active and stable performance is challenging, and investigation of the reaction mechanism is of great significance. In this paper, the reasonable regulation scheme on designing excellent performance catalysts is proposed, and all the reaction paths on the surface of catalysts are also analyzed in detail. It emphasized the fundamental factors influencing the activity of catalysts, and it proposed some practical strategies to effectively improve the performance of the catalysts in combination with the structure–activity relationship. This work has great significance for the optimal performance catalysts of heterogeneous catalytic systems. Furthermore, it provided a rationalized approach to designing catalysts with specific nanostructures and surface properties, such as catalytic reforming, dehydrogenation, hydrogenation, electric catalysis, and many other reactions. In addition, a critical perspective on the future challenges and opportunities in designing high performance catalysts is provided.
1 March 2026