Phase Behaviour of Multicomponent Mixtures of Hydrocarbons: MD Simulation
Abstract
1. Introduction
2. Model and Method
2.1. System Description
2.2. Model and Simulation Method
2.3. Molecular Model of Hydrocarbons
2.3.1. Linear Molecules
2.3.2. One Branched Molecule for a Component
2.3.3. Many Branched Molecules for a Component
2.3.4. Molecules Generation Algorithm
3. Simulation Results and Discussion
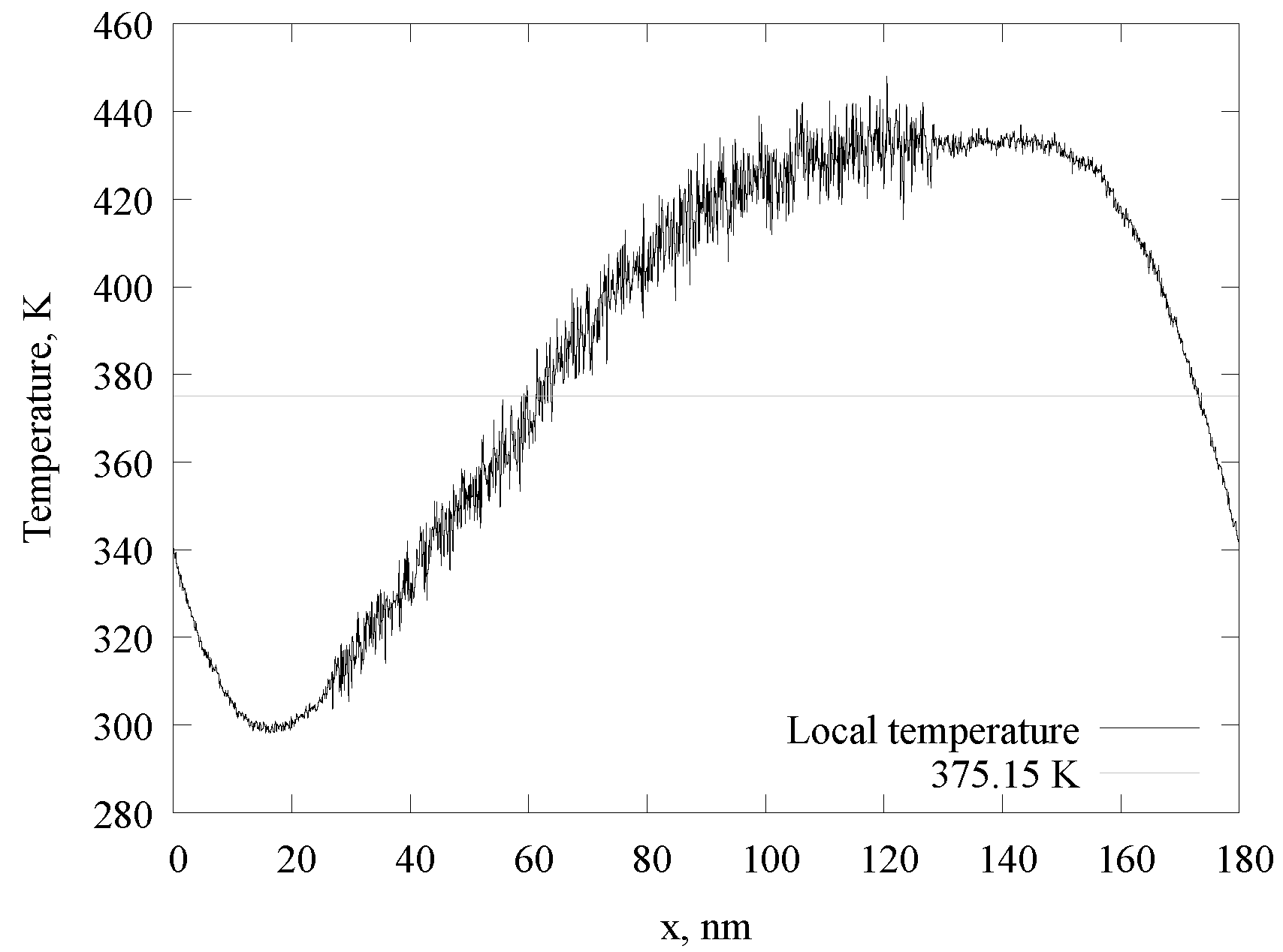
3.1. Phase Diagram for a Mixture with a Spherical Gas–Liquid Interface
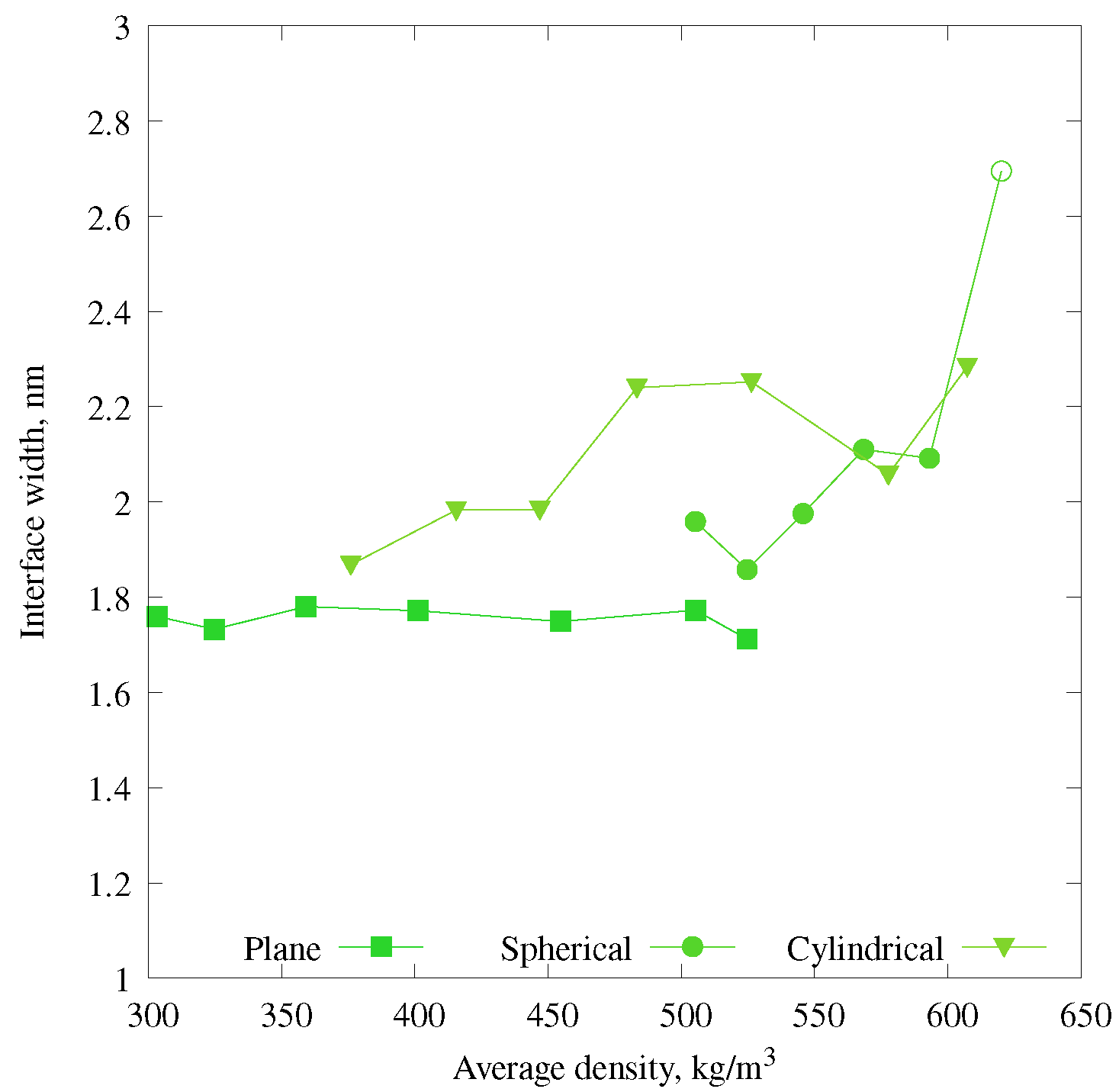
3.2. Phase Diagram for Mixtures with Gas–Liquid Interfaces of Different Shapes
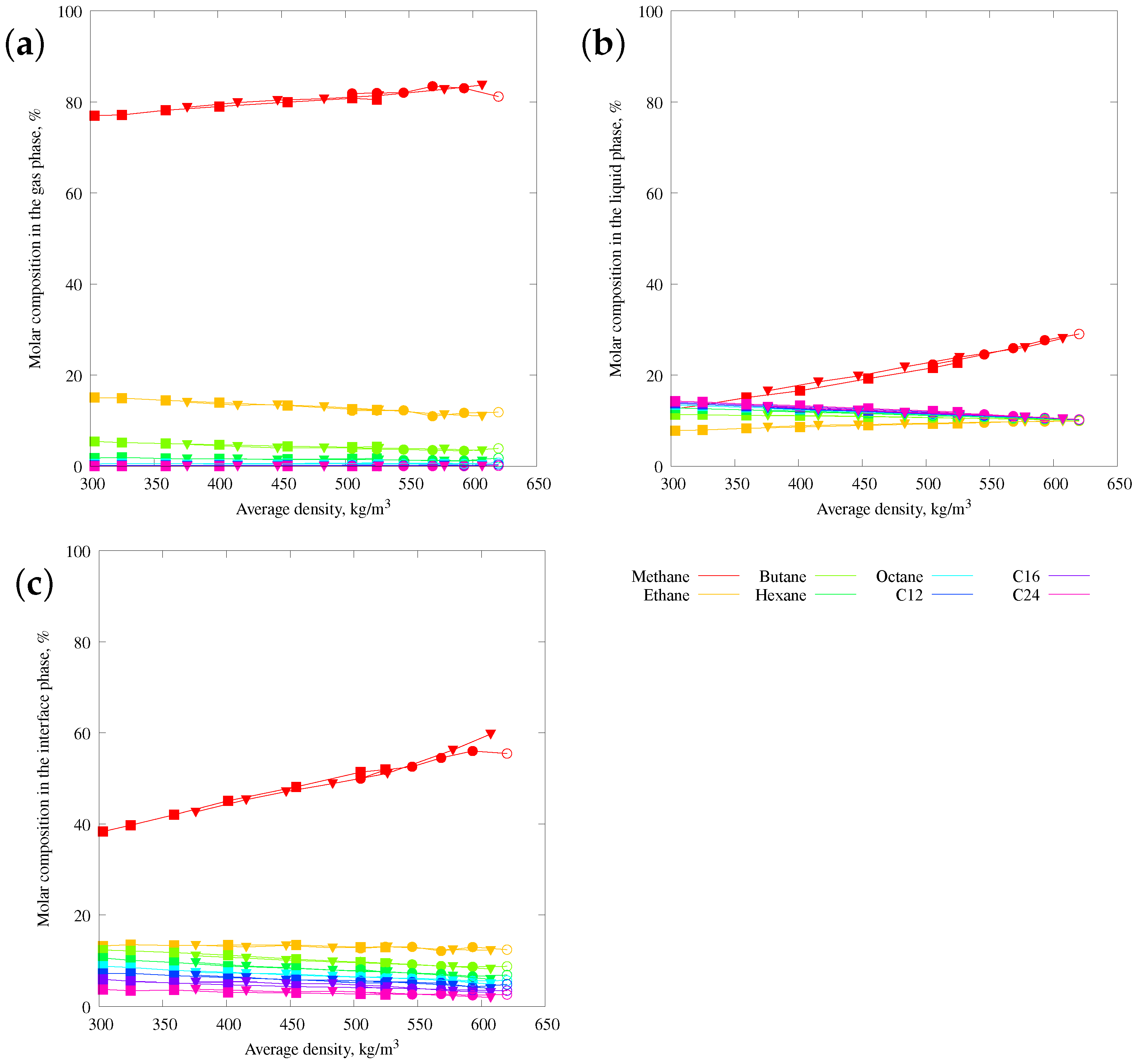
3.3. Composition Analysis of the Gas and Liquid Phases and Interface Layer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. MD Simulation Details
Appendix A.1. Force Field
| Type | Mass (a.m.u.) | (kJ/mol) | (nm) |
|---|---|---|---|
| CH4 | 16.043 | 1.2305400 | 0.3730 |
| CH3e | 15.035 | 0.8364349 | 0.3825 |
| CH3 | 15.035 | 0.8647041 | 0.3910 |
| CH3s | 15.035 | 0.6901004 | 0.3820 |
| CH2 | 14.027 | 0.3808024 | 0.3930 |
| CH1 | 13.019 | 0.3300842 | 0.3850 |
| CH0 | 12.011 | 0.1413459 | 0.3910 |
| Bond | (nm) | (kJ/mol/nm2) | ||
| X-X | 0.154 | 80234.99 | ||
| Angle | (deg.) | (kJ/mol/rad2) | ||
| X-CH2-X | 114.00 | 519.657 | ||
| X-CH1-X | 109.47 | 519.657 | ||
| X-CH0-X | 109.47 | 519.657 | ||
| Dihedral | (kJ/mol) | (kJ/mol) | (kJ/mol) | (kJ/mol) |
| X-CH2-CH2-X | 5.903802 | −1.133933 | 13.158878 | 0.000000 |
| X-CH2-CH1-X | 6.623336 | 2.313428 | −14.986067 | 0.000000 |
| X-CH2-CH0-X | 0.000000 | 0.000000 | 27.200077 | 0.000000 |
| X-CH1-CH1-X | 0.000000 | 0.000000 | 27.200077 | 0.000000 |
| X-CH1-CH0-X | 0.000000 | 0.000000 | 27.200077 | 0.000000 |
| X-CH0-CH0-X | 0.000000 | 0.000000 | 27.200077 | 0.000000 |
Appendix A.2. Single Precision vs. Double Precision in GROMACS
Appendix B. Molecules Generation Algorithm
Appendix C. Analysis Methods
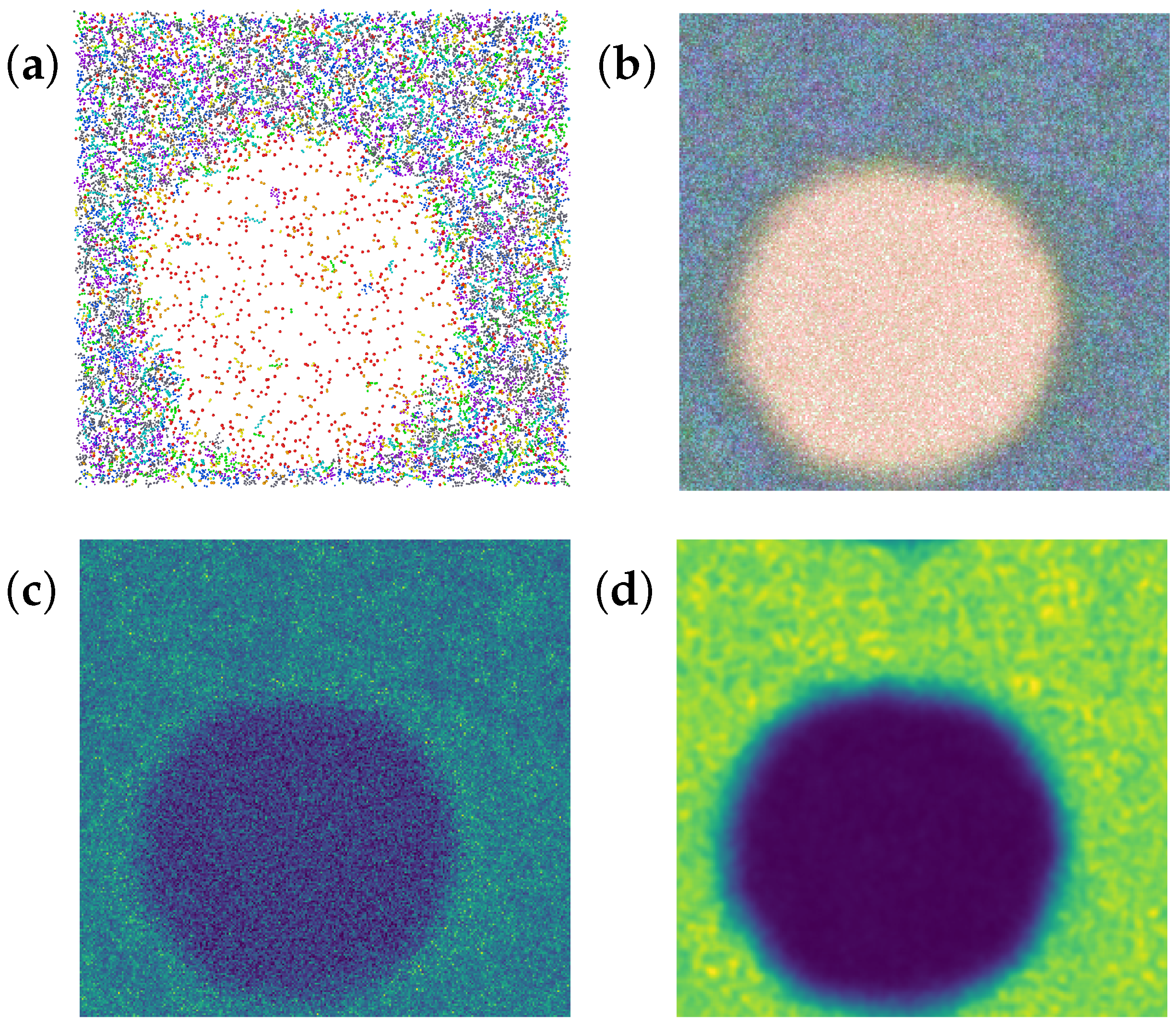
- 1.
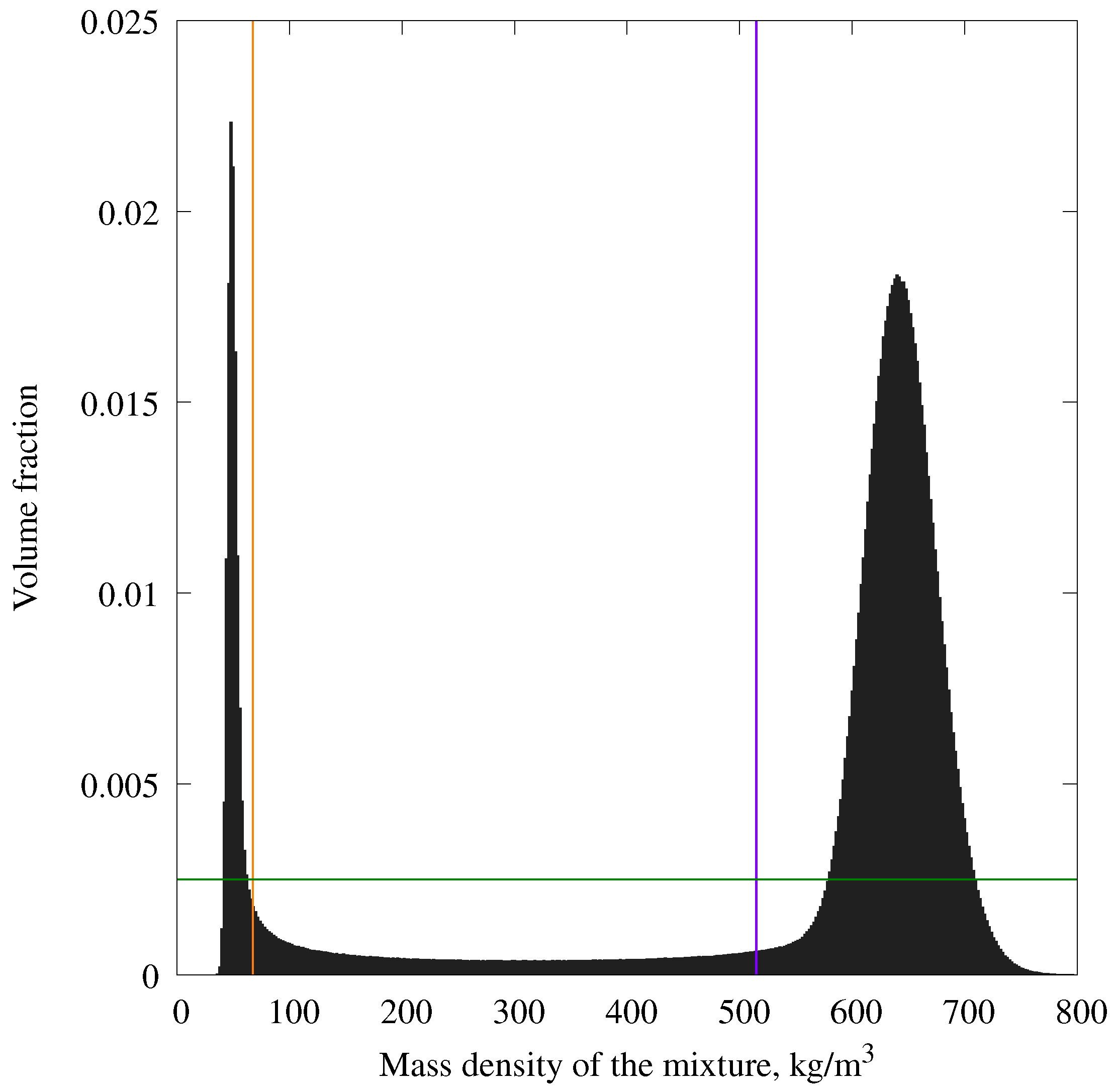
- A plotted histogram of the volume fraction (number of bins) on density (Figure A3). The bin size of this histogram was 2 kg/m3. There are two spikes on this plot—one refers to the gas phase, another refers to the liquid phase. Volume with intermediate density refers to the gas–liquid interface.
- 2.
- Evaluate approximate average densities of gas and liquid (, ) and their dispersions (, ). For the calculation of these values, only values exceeding 0.25% of volume were used: small values on the histogram (below the green line in Figure A3), which refer to the phase interface, were omitted. Remaining values were divided into two groups (gas and liquid). For these groups, average values and dispersions were calculated.
- 3.
- Evaluate two density values: a higher limit of gas density and a lower limit of liquid density ( and , correspondingly). These values were calculated using the following formulas and (orange and purple lines in Figure A3).
- 4.
- Get a rough estimate of the density of the intermediate layer as a mean between the density of the gas and liquid phases (), to set all voxels with a local density less than as “gas”, voxels with a local density more than as “liquid”, and voxels with a density equal to as the “intermediate layer”. This is a temporary labeling of voxels. Some “intermediate” voxels are labeled as “gas” or “liquid” after this step.
- 5.
- Iteratively find all voxels with intermediate densities laying between gas and liquid phases. At each step, one needs to label as “intermediate” all voxels which are neighboring current “intermediate” ones and have local densities between and values. The process repeats until the number of “intermediate” voxels does not rise.
- 6.
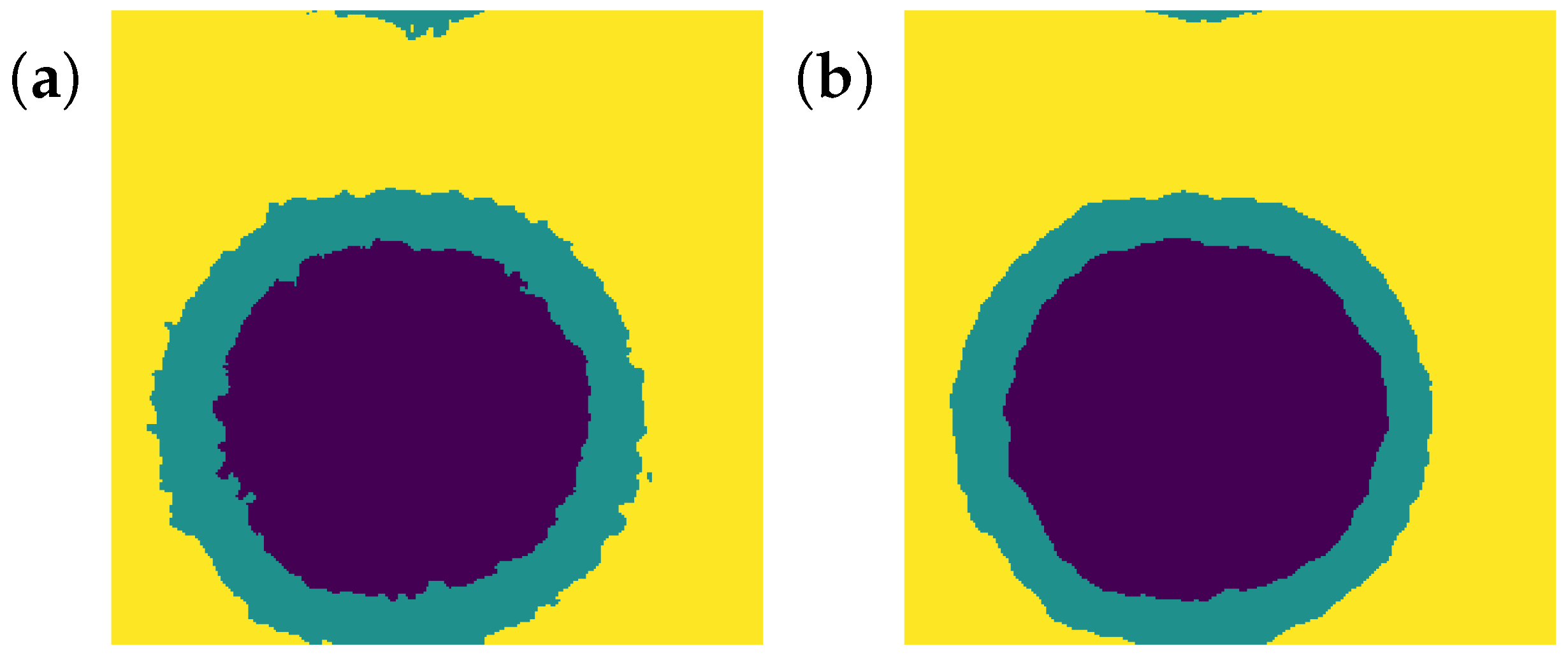
- After the previous step, “intermediate” volume can have parts going deeply into a different phase (Figure A4a), so one should smooth surfaces which distinguish phases. This can be done with the following process: every voxel changes its label to the dominant label among its 26 neighbours. After some iterations, the label map stops changing, and the boundaries are smooth (Figure A4b).
References
- Salahshoor, S.; Fahes, M.; Teodoriu, C. A review on the effect of confinement on phase behavior in tight formations. J. Nat. Gas Sci. Eng. 2018, 51, 89–103. [Google Scholar] [CrossRef]
- Bao, B.; Zandavi, S.H.; Li, H.; Zhong, J.; Jatukaran, A.; Mostowfi, F.; Sinton, D. Bubble nucleation and growth in nanochannels. Phys. Chem. Chem. Phys. 2017, 19, 8223–8229. [Google Scholar] [CrossRef]
- Chen, F.; Bi, R.; Nasrabadi, H. Molecular Simulation of Multi-scale Multi-Component Hydrocarbon Phase Behavior in Liquid-Rich Shale Reservoirs. In Proceedings of the Unconventional Resources Technology Conference, Houston, TX, USA, 26–28 July 2021; pp. 2464–2476. [Google Scholar] [CrossRef]
- Yen, T.H.; Chen, Y.L. Analysis of Gas Nanoclusters in Water Using All-Atom Molecular Dynamics. Langmuir 2022, 38, 13195–13205. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.M.; Jamieson, G.W. Oil and Organic Matter in Source Rocks of Petroleum1. AAPG Bull. 1956, 40, 477–488. [Google Scholar] [CrossRef]
- Sidorenkov, A.; Aslyamov, T.; Ilinov, D.; Stukan, M. Methane storage in nano-pores: Molecular dynamics simulation and density functional theory. Geoenergy Sci. Eng. 2023, 222, 211419. [Google Scholar] [CrossRef]
- Sidorenkov, A.; Stukan, M.; Ivanov, V. Methane flow in nanopores: Analytical approximation based on MD simulations. Fuel 2023, 332, 126070. [Google Scholar] [CrossRef]
- Herdes, C.; Petit, C.; Mejía, A.; Müller, E.A. Combined Experimental, Theoretical, and Molecular Simulation Approach for the Description of the Fluid-Phase Behavior of Hydrocarbon Mixtures within Shale Rocks. Energy Fuels 2018, 32, 5750–5762. [Google Scholar] [CrossRef]
- Mao, S.; Kuldinow, D.; Haataja, M.P.; Košmrlj, A. Phase behavior and morphology of multicomponent liquid mixtures. Soft Matter 2019, 15, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Sadus, R.J. Chapter 1—Survey of the High Pressure Phase Behaviour of Fluids. In High Pressure Phase Behaviour of Multicomponent Fluid Mixtures; Sadus, R.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 1–12. [Google Scholar] [CrossRef]
- McCain, W. The Properties of Petroleum Fluids; PennWell Books: Tulsa, OK, USA, 1990. [Google Scholar]
- McCain, W. Heavy Components Control Reservoir Fluid Behavior. J. Pet. Technol. 1994, 46, 746–750. [Google Scholar] [CrossRef]
- MacDowell, L.G.; Virnau, P.; Müller, M.; Binder, K. The evaporation/condensation transition of liquid droplets. J. Chem. Phys. 2004, 120, 5293–5308. [Google Scholar] [CrossRef] [PubMed]
- MacDowell, L.G.; Shen, V.K.; Errington, J.R. Nucleation and cavitation of spherical, cylindrical, and slablike droplets and bubbles in small systems. J. Chem. Phys. 2006, 125, 034705. [Google Scholar] [CrossRef]
- Díaz-Herrera, E.; Cerón-García, E.; Gutiérrez, A.B.; Chapela, G.A. Finite size effect on the existence of the liquid–vapour spinodal curve. Mol. Phys. 2022, 120, e1989071. [Google Scholar] [CrossRef]
- MacDowell, L.G. Comment on ‘Finite size effect on the existence of the liquid–vapour spinodal curve’. Mol. Phys. 2022, 120, e2084466. [Google Scholar] [CrossRef]
- Yushchenko, T.S.; Brusilovsky, A.I. Step-by-step algorithm for creating and tuning a PVT model for a reservoir hydrocarbon system. Arab. J. Geosci. 2025, 18, 1866–7511. [Google Scholar] [CrossRef]
- Nath, S.K.; Khare, R. New forcefield parameters for branched hydrocarbons. J. Chem. Phys. 2001, 115, 10837–10844. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Available online: http://www.gromacs.org/ (accessed on 1 August 2025).
- Available online: https://manual.gromacs.org/current/reference-manual/algorithms/molecular-dynamics.html (accessed on 1 August 2025).
- Sheremata, J.M.; Gray, M.R.; Dettman, H.D.; McCaffrey, W.C. Quantitative molecular representation and sequential optimization of Athabasca asphaltenes. Energy Fuels 2004, 18, 1377–1384. [Google Scholar] [CrossRef]
- Lagache, M.H.; Ungerer, P.; Boutin, A. Prediction of thermodynamic derivative properties of natural condensate gases at high pressure by Monte Carlo simulation. Fluid Phase Equilibria 2004, 220, 211–223. [Google Scholar] [CrossRef]
- Boek, E.S.; Yakovlev, D.S.; Headen, T.F. Quantitative molecular representation of asphaltenes and molecular dynamics simulation of their aggregation. Energy Fuels 2009, 23, 1209–1219. [Google Scholar] [CrossRef]
- Uddin, M.; Coombe, D.; Ivory, J. Quantifying physical properties of Weyburn oil via molecular dynamics simulation. Chem. Eng. J. 2016, 302, 249–259. [Google Scholar] [CrossRef]
- Iwase, M.; Sugiyama, S.; Liang, Y.; Masuda, Y.; Morimoto, M.; Matsuoka, T.; Boek, E.S.; Ueda, R.; Nakagawa, K. Development of Digital Oil for Heavy Crude Oil: Molecular Model and Molecular Dynamics Simulations. Energy Fuels 2018, 32, 2781–2792. [Google Scholar] [CrossRef]
- Iwase, M.; Liang, Y.; Masuda, Y.; Morimoto, M.; Matsuoka, T.; Boek, E.S.; Kaito, Y.; Nakagawa, K. Application of a Digital Oil Model to Solvent-Based Enhanced Oil Recovery of Heavy Crude Oil. Energy Fuels 2019, 33, 10868–10877. [Google Scholar] [CrossRef]
- Mullins, O.C.; Forsythe, J.C.; Pomerantz, A.E.; Wilkinson, T.; Winkelman, B.; Mishra, V.K.; Canas, J.A.; Chen, L.; Jackson, R.; Betancourt, S.S.; et al. Downhole Fluid Analysis and Gas Chromatography; a Powerful Combination for Reservoir Evaluation. Petrophysics 2018, 59, 649–671. [Google Scholar] [CrossRef]
- Chen, X.; Hou, L.; Wei, X.; Bedrov, D. Transport Properties of Waxy Crude Oil: A Molecular Dynamics Simulation Study. ACS Omega 2020, 5, 18557–18564. [Google Scholar] [CrossRef]
- Fall, W.S.; Baschnagel, J.; Lhost, O.; Meyer, H. Role of Short Chain Branching in Crystalline Model Polyethylenes. Macromolecules 2022, 55, 8438–8450. [Google Scholar] [CrossRef]
- Ambrose, D.; Tsonopoulos, C. Vapor-Liquid Critical Properties of Elements and Compounds. 2. Normal Alkanes. J. Chem. Eng. Data 1995, 40, 531–546. [Google Scholar] [CrossRef]
- Available online: https://webbook.nist.gov/chemistry/fluid/ (accessed on 1 August 2025).
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Wilhelmsen, O.; Bedeaux, D.; Kjelstrup, S.; Reguera, D. Thermodynamic stability of nanosized multicomponent bubbles/droplets: The square gradient theory and the capillary approach. J. Chem. Phys. 2014, 140, 024704. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, W.; Sun, W.; Wang, B. Understanding the Stabilization of a Bulk Nanobubble: A Molecular Dynamics Analysis. Langmuir 2021, 37, 11281–11291. [Google Scholar] [CrossRef]
- Voevodin, V.V.; Antonov, A.S.; Nikitenko, D.A.; Shvets, P.A.; Sobolev, S.I.; Sidorov, I.Y.; Stefanov, K.S.; Voevodin, V.V.; Zhumatiy, S.A. Supercomputer Lomonosov-2: Large Scale, Deep Monitoring and Fine Analytics for the User Community. Supercomput. Front. Innov. 2019, 6, 4–11. [Google Scholar] [CrossRef]
- Available online: https://manual.gromacs.org/current/reference-manual/functions/nonbonded-interactions.html (accessed on 1 August 2025).
- Available online: https://github.com/AlexanderSidorenkov/HydrocarbonGenerator (accessed on 1 August 2025).
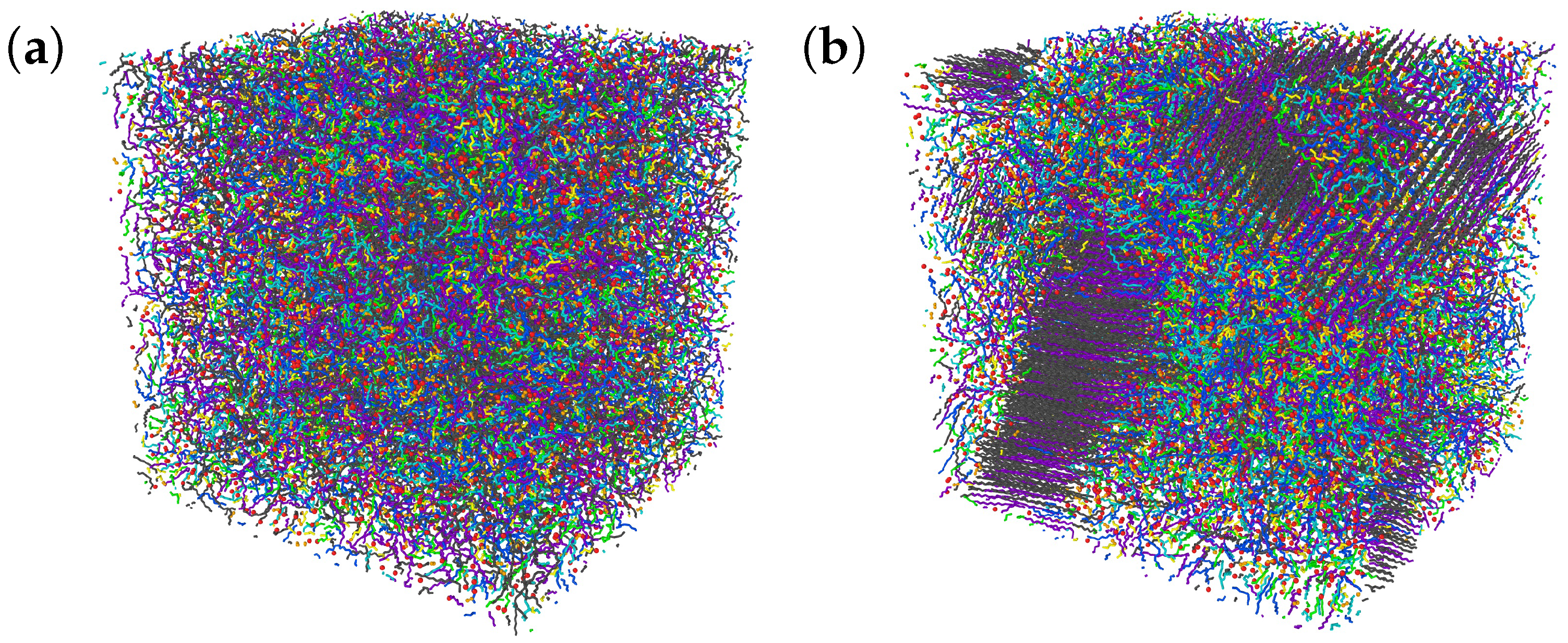
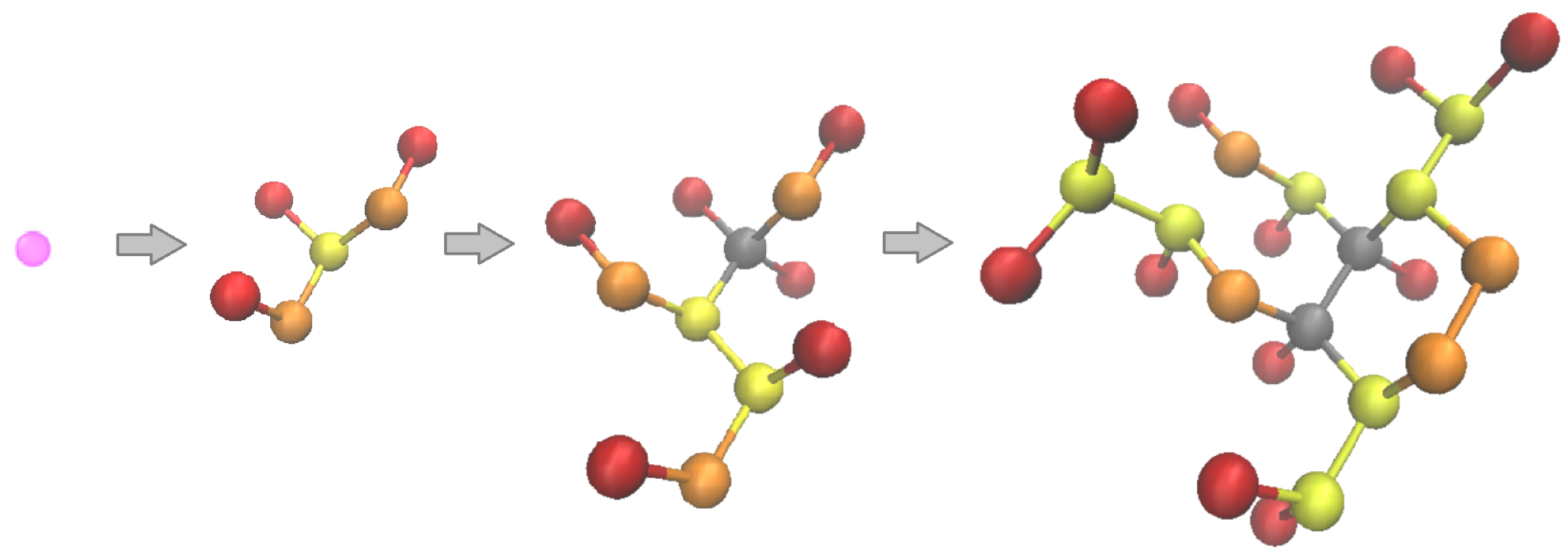
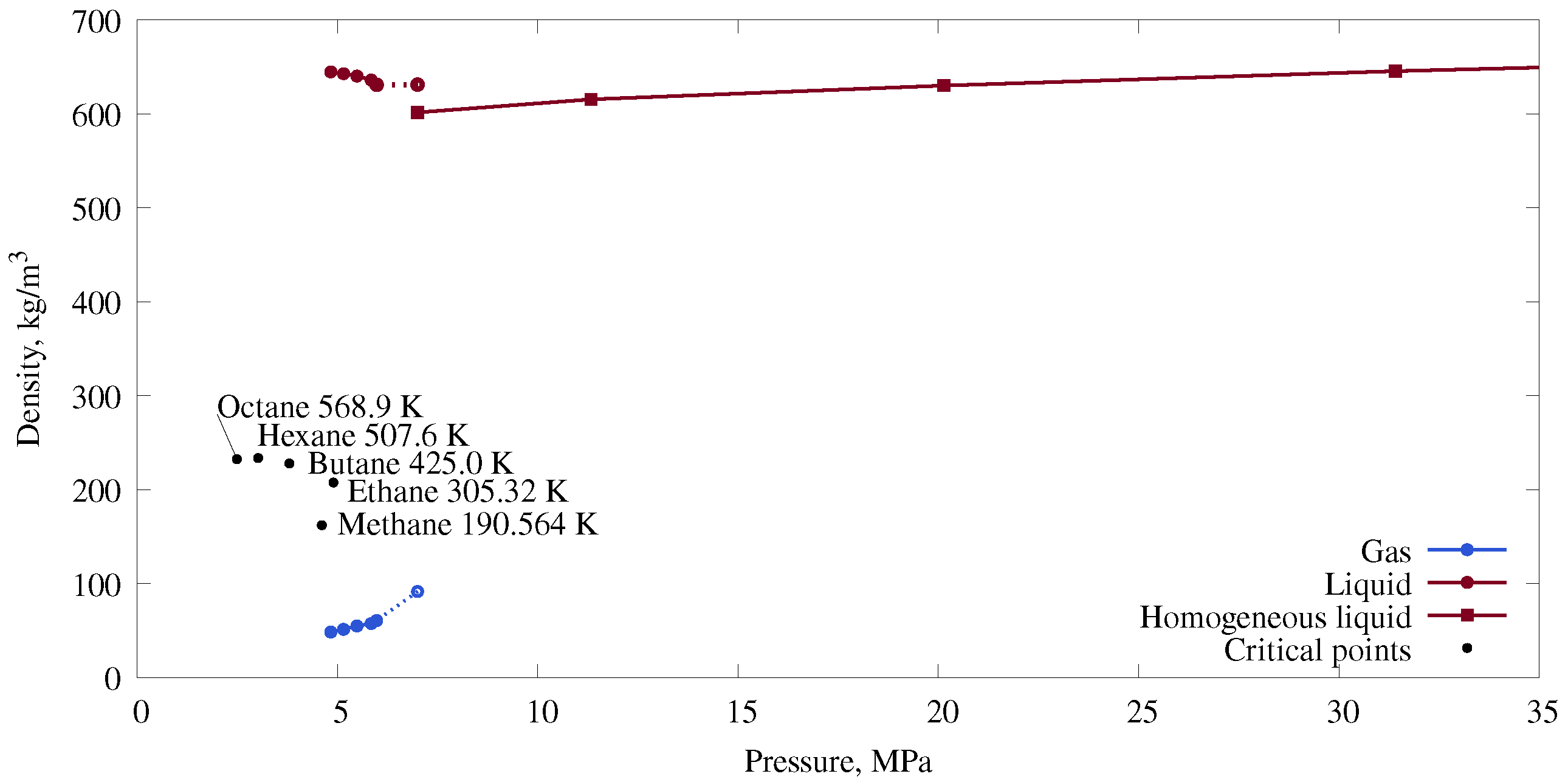

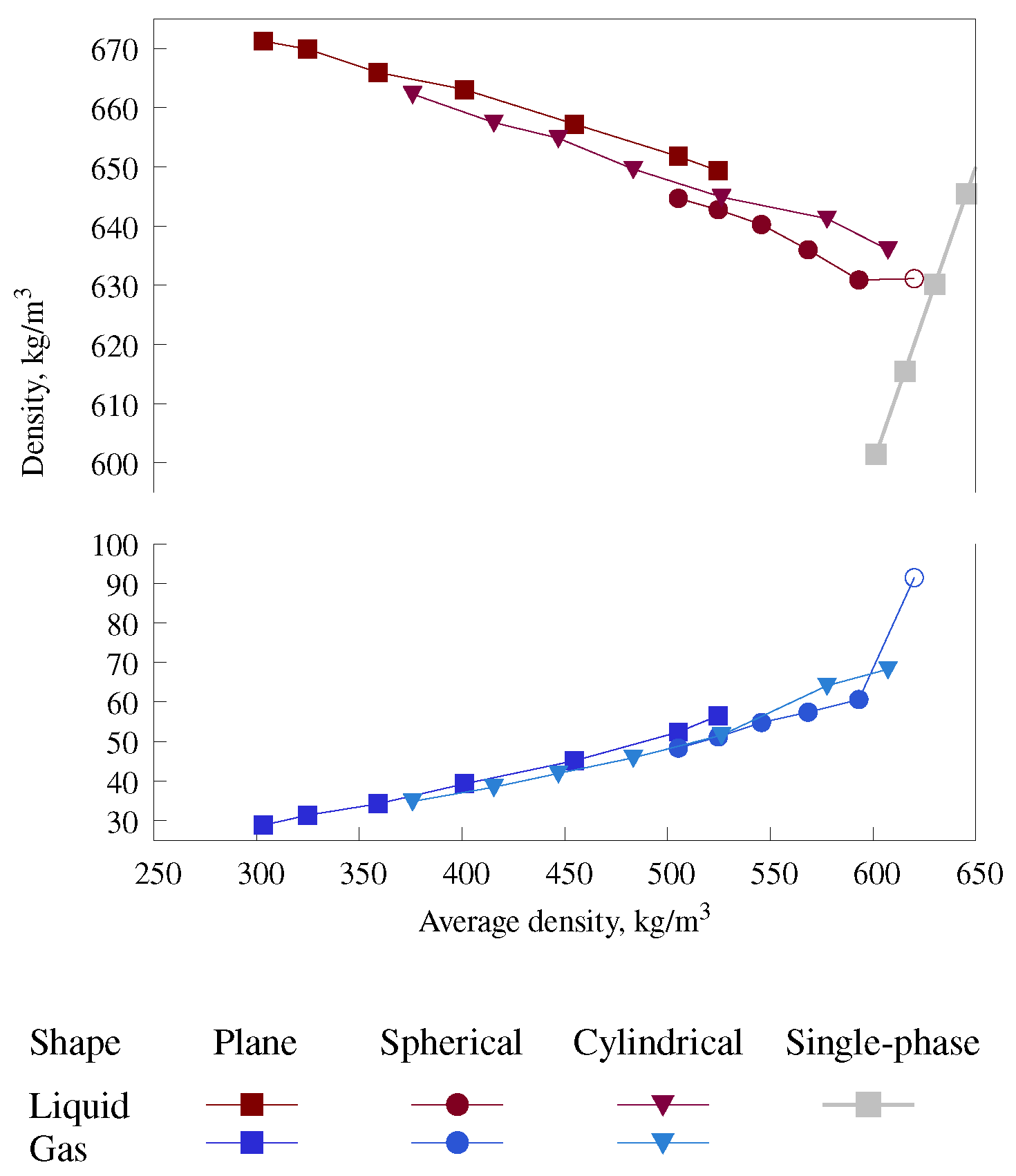



| Number of Molecules | Cell Size in x, y, and z Directions (nm) | Pressure (MPa) | Phase Interface Shape |
|---|---|---|---|
| 48,000 | 170. 12.5 12.5 | plane | |
| 48,000 | 150. 12.5 12.5 | plane | |
| 48,000 | 130. 12.5 12.5 | plane | |
| 48,000 | 110. 12.5 12.5 | plane | |
| 48,000 | 90. 12.5 12.5 | plane | |
| 48,000 | 45.0 25.0 25.0 | plane | |
| 48,000 | 42.0 25.0 25.0 | plane | |
| 48,000 | 38.0 25.0 25.0 | plane | |
| 48,000 | 34.0 25.0 25.0 | plane | |
| 48,000 | 30.0 25.0 25.0 | plane | |
| 48,000 | 27.0 25.0 25.0 | plane | |
| 48,000 | 26.0 25.0 25.0 | plane | |
| 48,000 | 27.0 25.0 25.0 | sphere | |
| 48,000 | 26.0 25.0 25.0 | sphere | |
| 48,000 | 25.0 25.0 25.0 | sphere | |
| 48,000 | 24.0 25.0 25.0 | sphere | |
| 48,000 | 23.0 25.0 25.0 | sphere | |
| 48,000 | 22.0 25.0 25.0 | interface only | |
| 48,000 | 21.5 25.0 25.0 | — | |
| 48,000 | 21.0 25.0 25.0 | — | |
| 48,000 | 20.5 25.0 25.0 | — | |
| 48,000 | 20.0 25.0 25.0 | — | |
| 48,000 | 63.0 8.0 45.0 | cylinder | |
| 48,000 | 57.0 8.0 45.0 | cylinder | |
| 48,000 | 53.0 8.0 45.0 | cylinder | |
| 48,000 | 49.0 8.0 45.0 | cylinder | |
| 48,000 | 45.0 8.0 45.0 | cylinder | |
| 48,000 | 41.0 8.0 45.0 | cylinder | |
| 48,000 | 39.0 8.0 45.0 | cylinder |
| C24 Molecules | Number Density (nm−3) |
|---|---|
| n-tetracosane | ≈1.6 (non-uniform crystal structure) |
| c-tetracosane | 1.29 |
| methyltridecane | 1.03 |
| 2-7-diheptylbicyclodecane | 1.36 |
| randomly generated molecules | 1.45 |
| C | CH | CH2 | CH3 | |
|---|---|---|---|---|
| C12 | 334 | 1343 | 2408 | 3115 |
| C16 | 540 | 1961 | 3182 | 3917 |
| C24 | 1115 | 3171 | 4577 | 5537 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorenkov, A.; Ivanov, V. Phase Behaviour of Multicomponent Mixtures of Hydrocarbons: MD Simulation. Methane 2025, 4, 24. https://doi.org/10.3390/methane4040024
Sidorenkov A, Ivanov V. Phase Behaviour of Multicomponent Mixtures of Hydrocarbons: MD Simulation. Methane. 2025; 4(4):24. https://doi.org/10.3390/methane4040024
Chicago/Turabian StyleSidorenkov, Alexander, and Viktor Ivanov. 2025. "Phase Behaviour of Multicomponent Mixtures of Hydrocarbons: MD Simulation" Methane 4, no. 4: 24. https://doi.org/10.3390/methane4040024
APA StyleSidorenkov, A., & Ivanov, V. (2025). Phase Behaviour of Multicomponent Mixtures of Hydrocarbons: MD Simulation. Methane, 4(4), 24. https://doi.org/10.3390/methane4040024






