- Article
A Theoretical Proposal to Localize and Determine the Amount of Methane, Ammonia and Carbon Dioxide in Nano-Cages of Water Clathrate Through the Space Infrared Spectroscopic Observations
- Azzedine Lakhlifi,
- Pierre R. Dahoo and
- Mustapha Meftah
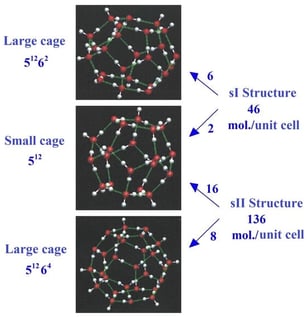
This paper investigates the different relaxation channels of a single symmetric top NH3 and a spherical top CH4 molecule trapped at low temperature in a clathrate hydrate nano-cage in the infrared absorption domain of their vibrational degrees of freedom. The approach utilizes the Born–Oppenheimer approximation and the extended site inclusion model applied to CO2 in a previous work, which was based on pairwise atom–atom effective interaction potentials. The calculations show that trapping the methane or ammonia molecule is energetically more favorable in a type sI clathrate structure than in an sII one, and entropic considerations show that methane can be released much more easily than ammonia from clathrate hydrate nano-cages. In the small (s) and large (l) nano-cages with the sI structure, the CH4 molecule exhibits a more or less perturbed rotational motion, while the NH3 molecule shows a strongly hindered orientational motion that tends to a three-dimension librational motion (oscillation motion) around its orientational equilibrium configuration. The calculated orientational energy level schemes are quite different from those of the molecular free rotation. In the static field inside the cage, degenerate and vibrational modes of methane and ammonia molecules are shifted and split. Moreover, for ammonia molecules, the and modes are shifted, and the inversion motion is no longer allowed. The non-radiative and radiative relaxation channels of CH4, NH3 and CO2 in clathrate nano-cages are discussed with reference to the matrix isolation spectroscopic results. Upon laser excitation, then, from the energy levels calculated for the different degrees of freedom, NH3 and CO2 are expected to fluoresce, while for CH4, non-radiative relaxation should lead to evaporation at the surface of clathrates. Experimental setups are suggested to localize and study these species underneath ice surfaces on distant planets or planetesimals from mobile detectors such as drones or CubeSats equipped with appropriate laser sources and telescopes with 2D imaging detectors.
5 February 2026



![Methane (CH4) fluxes in agricultural (a) and natural (b) soils from four Cerrado sites (Araras, Brasília, Itirapina, Sorocaba) in response to ammonium sulfate [(NH4)2SO4] addition. Boxplots represent mean values (box) and confidence intervals (lines), with points indicating outliers. “No” indicates the absence of nitrogen, and “yes” indicates nitrogen addition. Values are expressed in µg CH4 m−2 h−1, with scales adjusted for agricultural (a) and natural (b) soils. Significant differences (p < 0.05) between treatments are indicated by asterisks (*), while “ns” denotes no significant difference.](https://mdpi-res.com/cdn-cgi/image/w=281,h=192/https://mdpi-res.com/methane/methane-05-00006/article_deploy/html/images/methane-05-00006-ag-550.jpg)