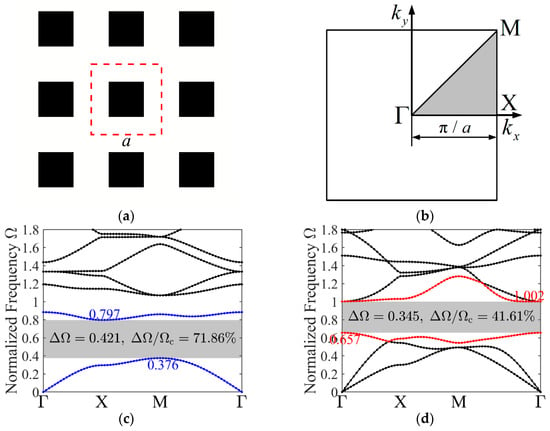
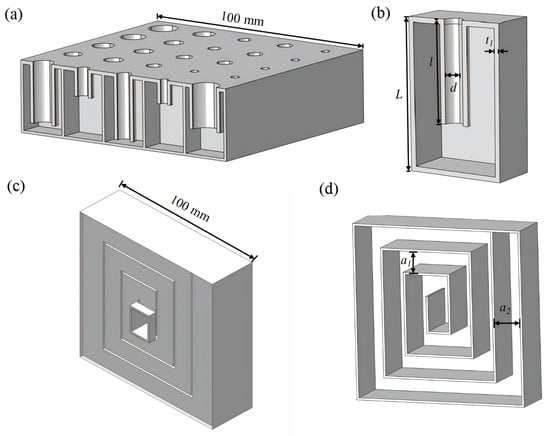
-
 Atmospheric Pressure Plasma for Carbon Material Modification and Synthesis: A Comprehensive Review
Atmospheric Pressure Plasma for Carbon Material Modification and Synthesis: A Comprehensive Review -
 A Study on Hollow Mesoporous Silica Nanoparticles with Long-Term Cycling
A Study on Hollow Mesoporous Silica Nanoparticles with Long-Term Cycling -
 Titania-Based Oxide Catalysts for Removing Nitrogen Oxides
Titania-Based Oxide Catalysts for Removing Nitrogen Oxides -
 Novel Synthesis of SnS Using SnSO4 via H2 Mediated Ultrasonic Spray Pyrolysis
Novel Synthesis of SnS Using SnSO4 via H2 Mediated Ultrasonic Spray Pyrolysis -
 Promoting CO2 Conversion: Metal Oxides, Supports in Packed DBD Plasma Reactor
Promoting CO2 Conversion: Metal Oxides, Supports in Packed DBD Plasma Reactor
Journal Description
Materials
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Ei Compendex, CaPlus / SciFinder, Inspec, Astrophysics Data System, and other databases.
- Journal Rank: JCR - Q2 (Metallurgy and Metallurgical Engineering) / CiteScore - Q1 (Condensed Matter Physics)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 15.5 days after submission; acceptance to publication is undertaken in 3.6 days (median values for papers published in this journal in the second half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
- Testimonials: See what our editors and authors say about Materials.
- Companion journals for Materials include: Electronic Materials, Construction Materials and AI Materials.
Latest Articles
E-Mail Alert
News
“Do Not Be Afraid of New Things”: Prof. Michele Parrinello on Scientific Curiosity and the Importance of Fundamental Research
1st International Online Conference on Optics (IOCO 2026)–Registration Deadline—23 March 2026

Topics
Deadline: 25 February 2026
Deadline: 20 March 2026
Deadline: 31 March 2026
Deadline: 13 April 2026
Conferences
Special Issues
Deadline: 10 February 2026
Deadline: 10 February 2026
Deadline: 10 February 2026
Deadline: 10 February 2026