- Case Report
Primary Renal Neuroendocrine Tumor: Diagnostic Challenges in a Rare Entity—A Case Report
- Raphaela D. Lewetag,
- Katharina Kluthe and
- Martina Hinterleitner
- + 7 authors
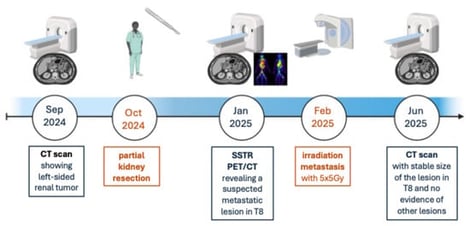
Neuroendocrine neoplasms (NENs) represent a rare, heterogeneous group of malignancies. Within this tumor entity, primary renal neuroendocrine tumors (PRNETs) are exceedingly rare, with only 160 cases reported worldwide. Due to the absence of native neuroendocrine cells in the renal parenchyma, their cellular origin remains unclear. Clinical and diagnostic challenges are reflected by the low incidence, non-specific clinical presentation, resemblance to common renal neoplasms, and the need for specialized histopathological diagnostic examination. Here, we present the case of a 61-year-old female with an incidentally diagnosed left-sided PRNET in September 2024. This case highlights the diagnostic complexity of PRNET and, importantly, underscores its genomic heterogeneity. It demonstrates a lack of typical renal cell carcinoma (RCC) or common NEN-associated gene alterations, emphasizing their unique molecular landscape.
6 February 2026