- Article
Immunogenicity of HIV-1 Env-Gag VLP mRNA and Adenovirus Vector Vaccines in Mice
- Jing Yang,
- Qi Ma and
- Xiaozhou He
- + 4 authors
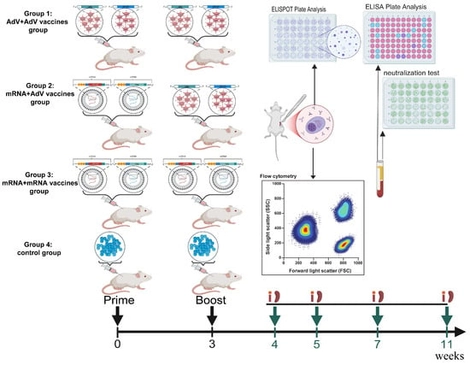
Background/Objectives: In previous studies, we demonstrated that the HIV-1 Env-Gag VLP mRNA vaccine elicited superior cellular immune responses. In this study, we further evaluated the immunogenicity of the Env-Gag VLP mRNA and adenovirus vector vaccines when administered individually or in combination in mice. Methods: BALB/c mice were divided into four groups and immunized twice at a 3-week interval. The three groups received either the Env-Gag VLP mRNA vaccine, the adenovirus vector vaccines expressing env and gag genes, or PBS as a control. The fourth group received a prime-boost regimen, primed with the Env-Gag mRNA vaccine and boosted with the adenovirus vector vaccines. The HIV-1 specific cellular and humoral immune responses were measured 1, 2, 4 and 8 weeks after the last immunization. Results/Conclusions: The results showed that the mRNA vaccines prime-adenovirus vector vaccines boost elicited higher cellular immune responses than those induced by homologous regimens at multiple time points, especially 8 weeks after the last immunization. Although the level of gp120 binding antibody in the combined immunization group is significantly lower than that of in the VLP mRNA vaccine group, a more balanced Th1/Th2 responses were induced in the combined immunization group, and significantly higher and longer-lasting neutralizing antibody levels were detected in this group making it a very promising HIV vaccine strategy.
14 December 2025