Vaccine-Associated Autoimmunity: From Clinical Signals to Immune Pathways
Abstract
1. Introduction
2. Induction of Autoimmune Phenomena by COVID-19 Vaccination
2.1. Systemic Lupus Erythematosus (SLE)
2.2. Rheumatoid Arthritis (RA)
2.3. Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT)
2.4. Guillain–Barré Syndrome (GBS)
2.5. Autoimmune Hepatitis (AIH)
2.6. Type 1 Diabetes Mellitus (T1DM)
2.7. Myasthenia Gravis (MG)
2.8. Alopecia Areata (AA)
2.9. Antiphospholipid Syndrome (APS)
2.10. ANCA-Associated Vasculitis (AAV)
3. Mechanisms of Autoimmune Diseases Induced by COVID-19 Vaccines
3.1. Molecular Mimicry
3.2. Adjuvants
3.3. Bystander Activation
3.4. Epitope Spreading (ES)
3.5. Polyclonal Activation of B Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AID | Autoimmune diseases |
| SLE | Systemic lupus erythematosus |
| RA | Rheumatoid Arthritis |
| APS | Antiphospholipid Syndrome |
| AOSD | Adult-Onset Still’s Disease |
| AAV | ANCA-Associated Vasculitis |
| GCA | Giant Cell Arteritis |
| AIH | Autoimmune Hepatitis |
| T1DM | Type 1 Diabetes Mellitus |
| GBS | Guillain–Barré Syndrome |
| MG | Myasthenia Gravis |
| AA | Alopecia Areata |
| ATD | Autoimmune thyroid disease |
| VITT | Vaccine-induced Immune Thrombotic Thrombocytopenia |
| RF | Rheumatoid Factor |
| TLRs | Toll-like receptors |
| IVIG | Intravenous immune globulin |
| PEX | Plasma exchange |
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Orenstein, W.A.; Offit, P.A.; Edwards, K.M.; Plotkin, S.A. Plotkin’s Vaccines, E-Book; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef]
- Chang, R.; Yen-Ting Chen, T.; Wang, S.I.; Hung, Y.M.; Chen, H.Y.; Wei, C.J. Risk of autoimmune diseases in patients with COVID-19: A retrospective cohort study. EClinicalMedicine 2023, 56, 101783. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex. Circulation 2022, 146, 743–754. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernan, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L.; Shaw, C.A. Aluminum vaccine adjuvants: Are they safe? Curr. Med. Chem. 2011, 18, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Restrepo-Jimenez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramirez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef] [PubMed]
- Segal, Y.; Shoenfeld, Y. Vaccine-induced autoimmunity: The role of molecular mimicry and immune crossreaction. Cell Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef]
- Khavinson, V.; Terekhov, A.; Kormilets, D.; Maryanovich, A. Homology between SARS-CoV-2 and human proteins. Sci. Rep. 2021, 11, 17199. [Google Scholar] [CrossRef] [PubMed]
- Wucherpfennig, K.W. Structural basis of molecular mimicry. J. Autoimmun. 2001, 16, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Brinth, L.S.; Pors, K.; Theibel, A.C.; Mehlsen, J. Orthostatic intolerance and postural tachycardia syndrome as suspected adverse effects of vaccination against human papilloma virus. Vaccine 2015, 33, 2602–2605. [Google Scholar] [CrossRef]
- Qiu, W.; Yu, T.; Deng, G.M. The role of organ-deposited IgG in the pathogenesis of multi-organ and tissue damage in systemic lupus erythematosus. Front. Immunol. 2022, 13, 924766. [Google Scholar] [CrossRef]
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.B.; van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic lupus erythematosus. Nat. Rev. Dis. Primers 2016, 2, 16039. [Google Scholar] [CrossRef]
- Crow, M.K. Pathogenesis of systemic lupus erythematosus: Risks, mechanisms and therapeutic targets. Ann. Rheum. Dis. 2023, 82, 999–1014. [Google Scholar] [CrossRef]
- Patil, S.; Patil, A. Systemic lupus erythematosus after COVID-19 vaccination: A case report. J. Cosmet. Dermatol. 2021, 20, 3103–3104. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, C.; Padilla, C.; Krampe, N.; Doerfler, S.; Morgenlander, A.; Thiel, B.; Aggarwal, R. Systemic lupus erythematous after Pfizer COVID-19 vaccine: A case report. Clin. Rheumatol. 2022, 41, 1597–1601. [Google Scholar] [CrossRef]
- Moriyama, M.; Noda, K.; Ito, H.; Matsushita, T.; Kurosaka, D. Clinical features of newly diagnosed systemic lupus erythematosus after SARS-CoV-2 vaccination. Mod. Rheumatol. Case Rep. 2023, 8, 63–68. [Google Scholar] [CrossRef]
- Raviv, Y.; Betesh-Abay, B.; Valdman-Grinshpoun, Y.; Boehm-Cohen, L.; Kassirer, M.; Sagy, I. First Presentation of Systemic Lupus Erythematosus in a 24-Year-Old Male following mRNA COVID-19 Vaccine. Case Rep. Rheumatol. 2022, 2022, 9698138. [Google Scholar] [CrossRef]
- Kaur, I.; Zafar, S.; Capitle, E.; Khianey, R. COVID-19 Vaccination as a Potential Trigger for New-Onset Systemic Lupus Erythematosus. Cureus 2022, 14, e21917. [Google Scholar] [CrossRef]
- Baez-Negron, L.; Vila, L.M. New-Onset Systemic Lupus Erythematosus after mRNA SARS-CoV-2 Vaccination. Case Rep. Rheumatol. 2022, 2022, 6436839. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, A.; Licciardi-Fernandez, M.J.; Burmann, S.N.; Burkert, B.; Oellig, F.; Michalowitz, A.L. Induction and exacerbation of subacute cutaneous lupus erythematosus following mRNA-based or adenoviral vector-based SARS-CoV-2 vaccination. Clin. Exp. Dermatol. 2022, 47, 161–163. [Google Scholar] [CrossRef]
- Matsuda, M.; Funakubo Asanuma, Y.; Emoto, K.; Sakai, S.; Okumura, N.; Yazawa, H.; Maruyama, T.; Tsuzuki Wada, T.; Yokota, K.; Araki, Y.; et al. New-onset of rheumatic diseases following COVID-19 vaccination: The report of three cases and a literature review. Immunol. Med. 2024, 47, 205–216. [Google Scholar] [CrossRef]
- Saleh, A.M.; Khalid, A.; Alshaya, A.K.; Alanazi, S.M.M. Systemic lupus erythematosus with acute pancreatitis and vasculitic rash following COVID-19 vaccine: A case report and literature review. Clin. Rheumatol. 2022, 41, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Nune, A.; Iyengar, K.P.; Ish, P.; Varupula, B.; Musat, C.A.; Sapkota, H.R. The Emergence of new-onset SLE following SARS-CoV-2 vaccination. QJM Int. J. Med. 2021, 114, 739–740. [Google Scholar] [CrossRef]
- Sagy, I.; Zeller, L.; Raviv, Y.; Porges, T.; Bieber, A.; Abu-Shakra, M. New-onset systemic lupus erythematosus following BNT162b2 mRNA COVID-19 vaccine: A case series and literature review. Rheumatol. Int. 2022, 42, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Molina-Rios, S.; Rojas-Martinez, R.; Estevez-Ramirez, G.M.; Medina, Y.F. Systemic lupus erythematosus and antiphospholipid syndrome after COVID-19 vaccination. A case report. Mod. Rheumatol. Case Rep. 2023, 7, 43–46. [Google Scholar] [CrossRef]
- Hidaka, D.; Ogasawara, R.; Sugimura, S.; Fujii, F.; Kojima, K.; Nagai, J.; Ebata, K.; Okada, K.; Kobayashi, N.; Ogasawara, M.; et al. New-onset Evans syndrome associated with systemic lupus erythematosus after BNT162b2 mRNA COVID-19 vaccination. Int. J. Hematol. 2022, 115, 424–427. [Google Scholar] [CrossRef]
- Zavala-Miranda, M.F.; Gonzalez-Ibarra, S.G.; Perez-Arias, A.A.; Uribe-Uribe, N.O.; Mejia-Vilet, J.M. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int. 2021, 100, 1340–1341. [Google Scholar] [CrossRef]
- Kim, H.J.; Jung, M.; Lim, B.J.; Han, S.H. New-onset class III lupus nephritis with multi-organ involvement after COVID-19 vaccination. Kidney Int. 2022, 101, 826–828. [Google Scholar] [CrossRef]
- Nelson, M.C.; Rytting, H.; Greenbaum, L.A.; Goldberg, B. Presentation of SLE after COVID vaccination in a pediatric patient. BMC Rheumatol. 2022, 6, 81. [Google Scholar] [CrossRef]
- Salas, A.; Fatola, A.; Krimins, R.; Kamel, I.R.; Geetha, D.; Fine, D.; Monroy-Trujillo, M.; Rosenberg, A.; Arend, L.; Timlin, H. COVID vaccine-induced lupus nephritis: Case report and review of the literature. Lupus 2024, 33, 176–182. [Google Scholar] [CrossRef]
- Pedersen, H.L.; Horvei, K.D.; Thiyagarajan, D.; Seredkina, N.; Rekvig, O.P. Murine and Human Lupus Nephritis: Pathogenic Mechanisms and Theoretical Strategies for Therapy. Semin. Nephrol. 2015, 35, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Suwannalai, P.; Trouw, L.A.; Toes, R.E.; Huizinga, T.W. Anti-citrullinated protein antibodies (ACPA) in early rheumatoid arthritis. Mod. Rheumatol. 2012, 22, 15–20. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Yonezawa, H.; Ohmura, S.I.; Ohkubo, Y.; Miyamoto, T. New-onset Seropositive Rheumatoid Arthritis Following COVID-19 Vaccination in a Patient with Seronegative Status. Intern. Med. 2022, 61, 3449–3452. [Google Scholar] [CrossRef]
- Nahra, V.; Makandura, M.; Anthony, D.D.; Mattar, M. A Case Series on the COVID-19 Vaccines and Possible Immune-Related Adverse Events: A New Challenge for the Rheumatologists. Cureus 2022, 14, e29660. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.M.; Harada, M.; Kishimoto, E.; Suzuki, K.; Nakagawa, E.; Hiramatsu, T.; Nakai, S.; Murakami, Y.; Nishimoto, K.; Matsushima, S.; et al. BNT162b2 coronavirus disease-2019 vaccination accelerated rheumatoid arthritis disease activity in chronic eosinophilic pneumonia: A case report. Medicine 2022, 101, e30806. [Google Scholar] [CrossRef] [PubMed]
- Safary, A.; Esalatmanesh, K.; Eftekharsadat, A.T.; Jafari Nakjavani, M.R.; Khabbazi, A. Autoimmune inflammatory rheumatic diseases post-COVID-19 vaccination. Int. Immunopharmacol. 2022, 110, 109061. [Google Scholar] [CrossRef]
- Baimukhamedov, C.; Makhmudov, S.; Botabekova, A. Seropositive rheumatoid arthritis after vaccination against SARS-CoV-2 infection. Int. J. Rheum. Dis. 2021, 24, 1440–1441. [Google Scholar] [CrossRef]
- Watanabe, T.; Minaga, K.; Hara, A.; Yoshikawa, T.; Kamata, K.; Kudo, M. Case Report: New-Onset Rheumatoid Arthritis Following COVID-19 Vaccination. Front. Immunol. 2022, 13, 859926. [Google Scholar] [CrossRef] [PubMed]
- Almouslem, A.; Al Lawati, T.; Al Shirawi, A.; Al Amri, U. New-onset Seropositive Rheumatoid Arthritis Post-mRNA COVID-19 Vaccine: A Case Report. Oman Med. J. 2024, 39, e597. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Pai, M.; Huisman, M.V.; Makris, M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022, 9, e73–e80. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sorvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattor, T.H.; Tjonnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef]
- Clerici, B.; Pontisso, E.; Aloise, C.; Peroni, B.; Perricone, R.; Pisetta, C.; Scavone, M.; Birocchi, S.; Podda, G.M. Thrombosis and Bleeding in Patients with Vaccine-Induced Immune Thrombotic Thrombocytopenia: A Systematic Review of Published Cases. Thromb. Haemost. 2024, 124, 423–431. [Google Scholar] [CrossRef]
- Sistanizad, M.; Sabaghian, T.; Amini, H.; Hadavand, F.; Nabavi, M.; Kouchek, M.; Miri, M.M.; Salarian, S.; Shojaei, S.; Moradi, O. Sinopharm (HB02)-associated vaccine-induced immune thrombotic thrombocytopenia: A case report. J. Med. Case Rep. 2023, 17, 383. [Google Scholar] [CrossRef]
- Gabarin, N.; Arnold, D.M.; Nazy, I.; Warkentin, T.E. Treatment of vaccine-induced immune thrombotic thrombocytopenia (VITT). Semin. Hematol. 2022, 59, 89–96. [Google Scholar] [CrossRef]
- Roberge, G.; Cote, B.; Calabrino, A.; Gilbert, N.; Gagnon, N. Acute lower limb ischemia caused by vaccine-induced immune thrombotic thrombocytopenia: Focus on perioperative considerations for 2 cases. Thromb. J. 2022, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Roberge, G.; Carrier, M. Long VITT: A case report. Thromb. Res. 2023, 223, 78–79. [Google Scholar] [CrossRef]
- Gunther, A.; Bramer, D.; Pletz, M.W.; Kamradt, T.; Baumgart, S.; Mayer, T.E.; Baier, M.; Autsch, A.; Mawrin, C.; Schonborn, L.; et al. Complicated Long Term Vaccine Induced Thrombotic Immune Thrombocytopenia-A Case Report. Vaccines 2021, 9, 1344. [Google Scholar] [CrossRef]
- Mouta Nunes de Oliveira, P.; Mendes-de-Almeida, D.P.; Bertollo Gomes Porto, V.; Crespo Cordeiro, C.; Vitiello Teixeira, G.; Saraiva Pedro, R.; Roberto Gomes Takey, P.; Kegele Lignani, L.; Reis Xavier, J.; Cardoso Doria da Gama, V.; et al. Vaccine-induced immune thrombotic thrombocytopenia after COVID-19 vaccination: Description of a series of 39 cases in Brazil. Vaccine 2022, 40, 4788–4795. [Google Scholar] [CrossRef]
- Lin, T.C.; Fu, P.A.; Hsu, Y.T.; Chen, T.Y. Vaccine-Induced Immune Thrombotic Thrombocytopenia following BNT162b2 mRNA COVID-19 Booster: A Case Report. Vaccines 2023, 11, 1115. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.; Reza, R.R.; Agboola, A.A.; Mohamed, K.H.; Mohamed, A.S.; Zahid, N.; Haseeb, M.; Nasir, H. Immune Thrombocytopenia and Cerebral Venous Sinus Thrombosis Following COVID-19 Vaccination: A Case Report. Cureus 2023, 15, e34272. [Google Scholar] [CrossRef]
- Chen, Q.T.; Liu, Y.; Chen, Y.C.; Chou, C.H.; Lin, Y.P.; Lin, Y.Q.; Tsai, M.C.; Chang, B.K.; Ho, T.H.; Lu, C.C.; et al. Case report: Vaccine-induced immune thrombotic thrombocytopenia complicated by acute cerebral venous thrombosis and hemorrhage after AstraZeneca vaccines followed by Moderna COVID-19 vaccine booster and surgery. Front. Neurol. 2022, 13, 989730. [Google Scholar] [CrossRef]
- Bellamy, S.E.; Loor, B.A. A Case of Vaccine-Induced Thrombocytopenic Thrombosis Manifesting as Cerebral Venous Thrombosis and Intracerebral Bleed. Cureus 2023, 15, e33318. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Dilibe, A.; Rai, S.; Awosika, A.; Omole, A.E.; Ahmed, M.; Nwosu, S. Cerebral Sinus Thrombosis and Immune Thrombocytopenia Post COVID-19 Vaccination: A Case Report and Narrative Review. Cureus 2023, 15, e34550. [Google Scholar] [CrossRef]
- Castan, M.; Damin-Pernik, M.; Thiery, G.; Page, D.; Smadja, D.M.; Bertoletti, L. A case report of vaccine-induced immune thrombocytopenia and thrombosis syndrome after Ad26.COV2.S vaccine (Janssen/Johnson & Johnson). Therapie 2022, 77, 734–737. [Google Scholar] [CrossRef]
- Edmonds, R.; Schonborn, L.; Habben, S.; Paparoupa, M.; Greinacher, A.; Schuppert, F. Vaccine-induced immune thrombotic thrombocytopenia (VITT) after SARS-CoV-2 vaccination: Two cases from Germany with unusual presentation. Clin. Case Rep. 2023, 11, e6883. [Google Scholar] [CrossRef]
- Yelne, P.; Kabra, R.; Mathurkar, S.; Gaidhane, S.A.; Acharya, S.; Kumar, S. Relationship Between Vaccination and Immune Thrombotic Thrombocytopenia: Coincidental or Causal? Cureus 2022, 14, e28560. [Google Scholar] [CrossRef]
- Hung, C.T.; Hsu, H. Microsurgical complication associated with vaccine-induced immune thrombotic thrombocytopenia (VITT): A case report. Medicine 2023, 102, e33013. [Google Scholar] [CrossRef]
- Jain, N.; Chaudhary, P.; Shrivastava, A.; Kaur, T.; Kaur, S.; Brar, H.S.; Jindal, R. Thrombosis with Thrombocytopenia Syndrome (TTS) After ChAdOx1 nCoV-19 Immunization: An Investigative Case Report. Am. J. Case Rep. 2023, 24, e938878. [Google Scholar] [CrossRef]
- Ge, M.; Ladha, D.; Lymer, J.; Pancic, S.; Carrier, M.; Le Gal, G.; Castellucci, L.A. Thrombocytopenia with and without thrombosis following COVID-19 vaccination: Long-term management. Res. Pract. Thromb. Haemost. 2024, 8, 102357. [Google Scholar] [CrossRef]
- Comolli, S.; Del Vecchio, L.; De Micheli, V.; Tucci, B.; D’Amico, M.; Fumagalli, G.; Gallelli, B.; Gervasi, F.; Mezzina, N.; Tettamanti, M.; et al. Possible vaccine-induced immune thrombotic thrombocytopenia in a patient with diabetes and chronic kidney disease or random association? G. Ital. Nefrol. 2022, 39, 2022-vol6. [Google Scholar] [PubMed]
- Ling, V.W.T.; Fan, B.E.; Lau, S.L.; Lee, X.H.; Tan, C.W.; Lee, S.Y. Severe Thrombocytopenia, Thrombosis and Anti-PF4 Antibody after Pfizer-BioNTech COVID-19 mRNA Vaccine Booster-Is It Vaccine-Induced Immune Thrombotic Thrombocytopenia? Vaccines 2022, 10, 2023. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.; Davis, L.; Sivaloganathan, H.; Chevassut, T. A Single-Centre Experience of Post-COVID-19 Vaccine-Related Immune-Mediated Complications. Case Rep. Hematol. 2022, 2022, 4742639. [Google Scholar] [CrossRef] [PubMed]
- Doubrovinskaia, S.; Mooshage, C.M.; Seliger, C.; Lorenz, H.M.; Nagel, S.; Lehnert, P.; Purrucker, J.; Wildemann, B.; Bendszus, M.; Wick, W.; et al. Neurological autoimmune diseases following vaccinations against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): A follow-up study. Eur. J. Neurol. 2023, 30, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Savulescu, F.; Cirlan, C.; Iordache-Petrescu, M.I.; Iordache, M.; Petrescu, A.B.; Blajut, C. Portal Vein and Mesenteric Artery Thrombosis Following the Administration of an Ad26.COV2-S Vaccine-First Case from Romania: A Case Report. Vaccines 2022, 10, 1950. [Google Scholar] [CrossRef]
- Zaheri, H.; Kiani, A.; Afaghi, S.; Rahimi, F.; Banitorfi, M.; Norozi, A.K.; Hashemi, S.; Abedini, A. Lower limb arterial thrombosis followed by sub-massive pulmonary thromboembolism after Sinopharm BBIBP-CorV COVID-19 vaccination. Arch. Clin. Cases 2022, 9, 150–153. [Google Scholar] [CrossRef]
- Schonborn, L.; Thiele, T.; Kaderali, L.; Greinacher, A. Decline in Pathogenic Antibodies over Time in VITT. N. Engl. J. Med. 2021, 385, 1815–1816. [Google Scholar] [CrossRef]
- Huynh, A.; Kelton, J.G.; Arnold, D.M.; Daka, M.; Nazy, I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature 2021, 596, 565–569. [Google Scholar] [CrossRef]
- Wang, J.J.; Schonborn, L.; Warkentin, T.E.; Chataway, T.; Grosse, L.; Simioni, P.; Moll, S.; Greinacher, A.; Gordon, T.P. Antibody Fingerprints Linking Adenoviral Anti-PF4 Disorders. N. Engl. J. Med. 2024, 390, 1827–1829. [Google Scholar] [CrossRef]
- Wijdicks, E.F.; Klein, C.J. Guillain-Barre Syndrome. Mayo Clin. Proc. 2017, 92, 467–479. [Google Scholar] [CrossRef]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Taylor, R.S. Guillain-Barre Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- McKean, N.; Chircop, C. Guillain-Barre syndrome after COVID-19 vaccination. BMJ Case Rep. 2021, 14, e244125. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.Y.; Park, S.; Jung, J.; Kim, S.H.; Kim, T.S.; Choi, Y.J.; Kim, H.J.; Eom, H.S.; Hyun, J.W. Guillain-Barre syndrome after vaccination against COVID-19. Lancet Neurol. 2022, 21, 117–119. [Google Scholar] [CrossRef]
- Hasan, T.; Khan, M.; Khan, F.; Hamza, G. Case of Guillain-Barre syndrome following COVID-19 vaccine. BMJ Case Rep. 2021, 14, e243629. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Grimshaw, M.; Michel-Chavez, A.; Vera-Zertuche, J.M.; Galnares-Olalde, J.A.; Hernandez-Vanegas, L.E.; Figueroa-Cucurachi, M.; Paredes-Ceballos, O.; Reyes-Teran, G.; Carbajal-Sandoval, G.; Ceballos-Liceaga, S.E.; et al. Guillain-Barre syndrome is infrequent among recipients of the BNT162b2 mRNA COVID-19 vaccine. Clin. Immunol. 2021, 230, 108818. [Google Scholar] [CrossRef]
- Castiglione, J.I.; Crespo, J.M.; Lecchini, L.; Silveira, F.O.; Luis, M.B.; Cotti, N.; Simison, C.J.; Aguirre, F.; Piedrabuena, M.A.; Alonso, R.N.; et al. Bilateral facial palsy with paresthesias, variant of Guillain-Barre syndrome following COVID-19 vaccine: A case series of 9 patients. Neuromuscul. Disord. 2022, 32, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Germano, F.; Bellucci, M.; Grisanti, S.; Beronio, A.; Grazzini, M.; Coco, E.; Tassinari, T.; Della Cava, F.; De Michelis, C.; Baldi, O.; et al. COVID-19 vaccine-related Guillain-Barre syndrome in the Liguria region of Italy: A multicenter case series. J. Neurol. Sci. 2022, 440, 120330. [Google Scholar] [CrossRef]
- Waheed, S.; Bayas, A.; Hindi, F.; Rizvi, Z.; Espinosa, P.S. Neurological Complications of COVID-19: Guillain-Barre Syndrome Following Pfizer COVID-19 Vaccine. Cureus 2021, 13, e13426. [Google Scholar] [CrossRef]
- Razok, A.; Shams, A.; Almeer, A.; Zahid, M. Post-COVID-19 vaccine Guillain-Barre syndrome; first reported case from Qatar. Ann. Med. Surg. 2021, 67, 102540. [Google Scholar] [CrossRef]
- Patel, S.U.; Khurram, R.; Lakhani, A.; Quirk, B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. 2021, 14, e242956. [Google Scholar] [CrossRef]
- Allen, C.M.; Ramsamy, S.; Tarr, A.W.; Tighe, P.J.; Irving, W.L.; Tanasescu, R.; Evans, J.R. Guillain-Barre Syndrome Variant Occurring after SARS-CoV-2 Vaccination. Ann. Neurol. 2021, 90, 315–318. [Google Scholar] [CrossRef]
- Maramattom, B.V.; Krishnan, P.; Paul, R.; Padmanabhan, S.; Cherukudal Vishnu Nampoothiri, S.; Syed, A.A.; Mangat, H.S. Guillain-Barre Syndrome following ChAdOx1-S/nCoV-19 Vaccine. Ann. Neurol. 2021, 90, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Hernandez, O.; Sanchez-Cardoza, S. Case report of Guillain-Barre Syndrome after COVID BNT162b2 mRNA vaccine. Vacunas 2022, 23, 68–70. [Google Scholar] [CrossRef]
- Fakhari, M.S.; Poorsaadat, L.; Mahmoodiyeh, B. Guillain-Barre syndrome following COVID-19 vaccine: A case report. Clin. Case Rep. 2022, 10, e6451. [Google Scholar] [CrossRef]
- Rao, S.J.; Khurana, S.; Murthy, G.; Dawson, E.T.; Jazebi, N.; Haas, C.J. A case of Guillain-Barre syndrome following Pfizer COVID-19 vaccine. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 597–600. [Google Scholar] [CrossRef]
- Bellucci, M.; Germano, F.; Grisanti, S.; Castellano, C.; Tazza, F.; Mobilia, E.M.; Visigalli, D.; Novi, G.; Massa, F.; Rossi, S.; et al. Case Report: Post-COVID-19 Vaccine Recurrence of Guillain-Barre Syndrome Following an Antecedent Parainfectious COVID-19-Related GBS. Front. Immunol. 2022, 13, 894872. [Google Scholar] [CrossRef] [PubMed]
- Masuccio, F.G.; Comi, C.; Solaro, C. Guillain-Barre syndrome following COVID-19 vaccine mRNA-1273: A case report. Acta Neurol. Belg. 2022, 122, 1369–1371. [Google Scholar] [CrossRef] [PubMed]
- Kripalani, Y.; Lakkappan, V.; Parulekar, L.; Shaikh, A.; Singh, R.; Vyas, P. A Rare Case of Guillain-Barre Syndrome following COVID-19 Vaccination. Eur. J. Case Rep. Intern. Med. 2021, 8, 002707. [Google Scholar] [CrossRef]
- Thant, H.L.; Morgan, R.; Paese, M.M.; Persaud, T.; Diaz, J.; Hurtado, L. Guillain-Barre Syndrome After Ad26.COV2.S Vaccination. Am. J. Case Rep. 2022, 23, e935275. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.F.; da Silva, C.F.; Oliveira, R.; Romancini, F.; Mendes, R.M.; Locks, A.; Longo, M.F.M.; Moro, C.H.C.; Longo, A.L.; Braatz, V.L. Guillain-Barre syndrome after coronavirus disease 2019 vaccine: A temporal association. Clin. Exp. Neuroimmunol. 2022, 13, 92–94. [Google Scholar] [CrossRef]
- Wan, M.M.; Lee, A.; Kapadia, R.; Hahn, C. Case Series of Guillain-Barre Syndrome After the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) Vaccine. Neurol. Clin. Pract. 2022, 12, 149–153. [Google Scholar] [CrossRef]
- Censcak, D.; Ungermann, L.; Stetkarova, I.; Ehler, E. Guillan-Barre Syndrome after First Vaccination Dose against COVID-19: Case Report. Acta Medica 2021, 64, 183–186. [Google Scholar] [CrossRef]
- Oo, W.M.; Giri, P.; de Souza, A. AstraZeneca COVID-19 vaccine and Guillain- Barre Syndrome in Tasmania: A causal link? J. Neuroimmunol. 2021, 360, 577719. [Google Scholar] [CrossRef] [PubMed]
- Scendoni, R.; Petrelli, C.; Scaloni, G.; Logullo, F.O. Electromyoneurography and laboratory findings in a case of Guillain-Barre syndrome after second dose of Pfizer COVID-19 vaccine. Hum. Vaccin. Immunother. 2021, 17, 4093–4096. [Google Scholar] [CrossRef] [PubMed]
- Sriwastava, S.; Shrestha, A.K.; Khalid, S.H.; Colantonio, M.A.; Nwafor, D.; Srivastava, S. Spectrum of Neuroimaging Findings in Post-COVID-19 Vaccination: A Case Series and Review of Literature. Neurol. Int. 2021, 13, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Suri, V.; Pandey, S.; Singh, J.; Jena, A. Acute-onset chronic inflammatory demyelinating polyneuropathy after COVID-19 infection and subsequent ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2021, 14, e245816. [Google Scholar] [CrossRef] [PubMed]
- Introna, A.; Caputo, F.; Santoro, C.; Guerra, T.; Ucci, M.; Mezzapesa, D.M.; Trojano, M. Guillain-Barre syndrome after AstraZeneca COVID-19-vaccination: A causal or casual association? Clin. Neurol. Neurosurg. 2021, 208, 106887. [Google Scholar] [CrossRef]
- Bouattour, N.; Hdiji, O.; Sakka, S.; Fakhfakh, E.; Moalla, K.; Daoud, S.; Farhat, N.; Damak, M.; Mhiri, C. Guillain-Barre syndrome following the first dose of Pfizer-BioNTech COVID-19 vaccine: Case report and review of reported cases. Neurol. Sci. 2022, 43, 755–761. [Google Scholar] [CrossRef]
- Min, Y.G.; Ju, W.; Ha, Y.E.; Ban, J.J.; Lee, S.A.; Sung, J.J.; Shin, J.Y. Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: Report of two cases and review of literature. J. Neuroimmunol. 2021, 359, 577691. [Google Scholar] [CrossRef]
- Nasuelli, N.A.; De Marchi, F.; Cecchin, M.; De Paoli, I.; Onorato, S.; Pettinaroli, R.; Savoini, G.; Godi, L. A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy after ChAdOx1 nCoV-19 vaccine. Neurol. Sci. 2021, 42, 4747–4749. [Google Scholar] [CrossRef]
- Morehouse, Z.P.; Paulus, A.; Jasti, S.A.; Bing, X. A Rare Variant of Guillain-Barre Syndrome Following Ad26.COV2.S Vaccination. Cureus 2021, 13, e18153. [Google Scholar] [CrossRef]
- Andreozzi, V.; D’Arco, B.; Pagliano, P.; Toriello, A.; Barone, P. Bilateral facial palsy after COVID-19 vaccination. Neurol. Sci. 2022, 43, 4069–4079. [Google Scholar] [CrossRef]
- Tabatabaee, S.; Rezania, F.; Alwedaie, S.M.J.; Malekdar, E.; Badi, Z.; Tabatabaei, S.M.; Mirzaasgari, Z. Post COVID-19 vaccination Guillain-Barre syndrome: Three cases. Hum. Vaccin. Immunother. 2022, 18, 2045153. [Google Scholar] [CrossRef]
- Hughes, D.L.; Brunn, J.A.; Jacobs, J.; Todd, P.K.; Askari, F.K.; Fontana, R.J. Guillain-Barre Syndrome After COVID-19 mRNA Vaccination in a Liver Transplantation Recipient With Favorable Treatment Response. Liver Transpl. 2022, 28, 134–137. [Google Scholar] [CrossRef]
- Rohilla, R.; Kakkar, A.K.; Divyashree, K.; Mohindra, R.; Suri, V. Recombinant protein subunit COVID-19 vaccine-induced Guillain-Barre Syndrome in an adolescent: A case report. Br. J. Clin. Pharmacol. 2023, 89, 556–560. [Google Scholar] [CrossRef]
- Biswas, A.; Pandey, S.K.; Kumar, D.; Vardhan, H. Post coronavirus disease-2019 vaccination Guillain-Barre syndrome. Indian J. Public Health 2021, 65, 422–424. [Google Scholar] [CrossRef]
- Dalwadi, V.; Hancock, D.; Ballout, A.A.; Geraci, A. Axonal-Variant Guillian-Barre Syndrome Temporally Associated With mRNA-Based Moderna SARS-CoV-2 Vaccine. Cureus 2021, 13, e18291. [Google Scholar] [CrossRef]
- Rossetti, A.; Gheihman, G.; O’Hare, M.; Kosowsky, J.M. Guillain-Barre Syndrome Presenting as Facial Diplegia after COVID-19 Vaccination: A Case Report. J. Emerg. Med. 2021, 61, e141–e145. [Google Scholar] [CrossRef]
- Lanman, T.A.; Wu, C.; Cheung, H.; Goyal, N.; Greene, M. Guillain-Barre Syndrome with Rapid Onset and Autonomic Dysfunction Following First Dose of Pfizer-BioNTech COVID-19 Vaccine: A Case Report. Neurohospitalist 2022, 12, 388–390. [Google Scholar] [CrossRef]
- Dang, Y.L.; Bryson, A. Miller-Fisher Syndrome and Guillain-Barre Syndrome overlap syndrome in a patient post Oxford-AstraZeneca SARS-CoV-2 vaccination. BMJ Case Rep. 2021, 14, e246701. [Google Scholar] [CrossRef]
- Kim, Y.; Zhu, Z.; Kochar, P.; Gavigan, P.; Kaur, D.; Kumar, A. A Pediatric Case of Sensory Predominant Guillain-Barre Syndrome Following COVID-19 Vaccination. Child. Neurol. Open 2022, 9, 2329048X221074549. [Google Scholar] [CrossRef]
- Ogata, S.; Ishii, Y.; Asano, K.; Kobayashi, E.; Kubota, S.; Takahashi, K.; Miyaji, Y.; Higashiyama, Y.; Joki, H.; Doi, H.; et al. Sensory Ataxic Guillain-Barre Syndrome with Dysgeusia after mRNA COVID-19 Vaccination. Intern. Med. 2022, 61, 1757–1760. [Google Scholar] [CrossRef]
- Nagalli, S.; Shankar Kikkeri, N. Sub-acute Onset of Guillain-Barre Syndrome Post-mRNA-1273 Vaccination: A Case Report. SN Compr. Clin. Med. 2022, 4, 41. [Google Scholar] [CrossRef]
- Aldeeb, M.; Okar, L.; Mahmud, S.S.; Adeli, G.A. Could Guillain-Barre syndrome be triggered by COVID-19 vaccination? Clin. Case Rep. 2022, 10, e05237. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, Y.G.; Park, Y.C.; Choi, S.; Lee, S.; Min, H.J.; Kim, M.J. Guillain-Barre Syndrome After Two COVID-19 Vaccinations: Two Case Reports With Follow-up Electrodiagnostic Study. J. Korean Med. Sci. 2022, 37, e58. [Google Scholar] [CrossRef]
- Zubair, A.S.; Bae, J.Y.; Desai, K. Facial Diplegia Variant of Guillain-Barre Syndrome in Pregnancy Following COVID-19 Vaccination: A Case Report. Cureus 2022, 14, e22341. [Google Scholar] [CrossRef]
- Silfat, A.; Abdalla, K.; Ahmad, T. Guillain-Barré syndrome in a 67-year-old male post COVID-19 vaccination (Astra Zeneca). Am. J. Med. Case Rep. 2021, 9, 424–427. [Google Scholar]
- Chang, Y.L.; Chang, S.T. The effects of intravascular photobiomodulation on sleep disturbance caused by Guillain-Barre syndrome after Astrazeneca vaccine inoculation: Case report and literature review. Medicine 2022, 101, e28758. [Google Scholar] [CrossRef]
- Prasad, A.; Hurlburt, G.; Podury, S.; Tandon, M.; Kingree, S.; Sriwastava, S. A Novel Case of Bifacial Diplegia Variant of Guillain-Barre Syndrome Following Janssen COVID-19 Vaccination. Neurol. Int. 2021, 13, 404–409. [Google Scholar] [CrossRef]
- Kanabar, G.; Wilkinson, P. Guillain-Barre syndrome presenting with facial diplegia following COVID-19 vaccination in two patients. BMJ Case Rep. 2021, 14, e244527. [Google Scholar] [CrossRef]
- Hilts, A.; Schreiber, A.; Singh, A. A Clinical Case of COVID-19 Vaccine-Associated Guillain-Barre Syndrome. Am. J. Case Rep. 2022, 23, e936896. [Google Scholar] [CrossRef]
- Hai, P.D.; Phuong, L.L.; Tot, N.H.; Anh, N.L.P.; Tung, N.D.; Quan, N.H. Guillain-Barre syndrome after COVID-19 vaccination: Report of two cases from Vietnam. J. Infect. Dev. Ctries. 2022, 16, 1703–1705. [Google Scholar] [CrossRef]
- Siddiqi, A.R.; Khan, T.; Tahir, M.J.; Asghar, M.S.; Islam, M.S.; Yousaf, Z. Miller Fisher syndrome after COVID-19 vaccination: Case report and review of literature. Medicine 2022, 101, e29333. [Google Scholar] [CrossRef]
- Shalash, A.; Belal, N.; Zaki, A.S.; Georgy, S.S.; Doheim, M.F.; Hazzou, A.; Abdelnasser, A. Guillain-Barre syndrome following different COVID-19 vaccines: A case series. Egypt. J. Neurol. Psychiatr. Neurosurg. 2022, 58, 153. [Google Scholar] [CrossRef]
- Carranza, O.; Babici, D.; Waheed, S.; Yousuf, F. Neurologic Sequela of COVID-19: Guillain-Barre Syndrome Following Johnson & Johnson COVID-19 Vaccination. Cureus 2022, 14, e24252. [Google Scholar] [CrossRef]
- Bazrafshan, H.; Mohamadi Jahromi, L.S.; Parvin, R.; Ashraf, A. A case of Guillain-Barre syndrome after the second dose of AstraZeneca COVID-19 vaccination. Turk. J. Phys. Med. Rehabil. 2022, 68, 295–299. [Google Scholar] [CrossRef]
- Prado, M.B., Jr.; Adiao, K.J.B. Facial Diplegia as the Sole Manifestation of Post-Vaccination Guillain-Barre Syndrome: A Case Report and Literature Review. Neurohospitalist 2022, 12, 508–511. [Google Scholar] [CrossRef]
- Donaldson, L.; Margolin, E. Variant Guillain-Barre Syndrome Following SARS-CoV-2 Vaccination: Case Report and Review of the Literature. Can. J. Neurol. Sci. 2023, 50, 138–140. [Google Scholar] [CrossRef]
- Wimmer Del-Solar, J.; Chavez-Martel, P.; Fontecilla-Villalobos, E.; Ibanez-Rodriguez, P.; Rozas-Vidal, J.P. Guillain Barre syndrome associated with coronavac vaccine. Report of one case. Rev. Med. Chil. 2022, 150, 125–130. [Google Scholar] [CrossRef]
- Lazaro, C.; Llaurado, A.; Sanchez-Tejerina, D.; Cabirta, A.; Carpio, C.; Sotoca, J.; Salvado, M.; Raguer, N.; Restrepo, J.; Juntas, R. Guillain-Barre syndrome and thrombocytopenia after SARS-CoV-2 vaccination with Moderna. A case report. Rev. Neurol. 2022, 75, 247–250. [Google Scholar] [CrossRef]
- Abicic, A.; Adamec, I.; Habek, M. Miller Fisher syndrome following Pfizer COVID-19 vaccine. Neurol. Sci. 2022, 43, 1495–1497. [Google Scholar] [CrossRef]
- Bijoy George, T.; Kainat, A.; Pachika, P.S.; Arnold, J. Rare occurrence of Guillain-Barre syndrome after Moderna vaccine. BMJ Case Rep. 2022, 15, e249749. [Google Scholar] [CrossRef]
- Katada, E.; Toyoda, T.; Yamada, G.; Morishima, A.; Matsukawa, N. A case of chronic inflammatory demyelinating polyneuropathy following COVID-19 vaccine. Neurol. Clin. Neurosci. 2022, 10, 223–225. [Google Scholar] [CrossRef]
- Hwang, B.W.; Bong, J.B. Two possible etiologies of Guillain-Barre syndrome: mRNA-1273 (Moderna) vaccination and scrub typhus: A case report. Medicine 2022, 101, e32140. [Google Scholar] [CrossRef]
- Oshibe, N.; Honda, M.; Koga, M.; Sato, R.; Oishi, M.; Kanda, T. A Patient Developing Guillain-Barre Syndrome After Receiving the BNT162b2 COVID-19 mRNA Vaccine. Brain Nerve 2022, 74, 1025–1030. [Google Scholar] [CrossRef]
- Kim, N.; Kim, J.H.; Park, J.S. Guillain-Barre syndrome associated with BNT162b2 COVID vaccination: A first case report from South Korea. Neurol. Sci. 2022, 43, 1491–1493. [Google Scholar] [CrossRef]
- Nanatsue, K.; Takahashi, M.; Itaya, S.; Abe, K.; Inaba, A. A case of Miller Fisher syndrome with delayed onset peripheral facial nerve palsy after COVID-19 vaccination: A case report. BMC Neurol. 2022, 22, 309. [Google Scholar] [CrossRef]
- Pirola, F.J.C.; Santos, B.A.M.; Sapienza, G.F.; Cetrangolo, L.Y.; Geranutti, C.; de Aguiar, P.H.P. Miller-Fisher syndrome after first dose of Oxford/AstraZeneca coronavirus disease 2019 vaccine: A case report. J. Med. Case Rep. 2022, 16, 437. [Google Scholar] [CrossRef]
- Chen, B.; Lopez, S.; Eggenberger, E. Miller Fisher syndrome after Pfizer BioNTech vaccine booster responsive to intravenous Ig treatment. BMJ Case Rep. 2022, 15, e251361. [Google Scholar] [CrossRef]
- Algahtani, H.A.; Shirah, B.H.; Albeladi, Y.K.; Albeladi, R.K. Guillain-Barre Syndrome Following the BNT162b2 mRNA COVID-19 Vaccine. Acta Neurol. Taiwan. 2023, 32, 82–85. [Google Scholar]
- Acharya, B.; Kc, S.; Karki, S.; Thapa, P.; Kc, P. Guillain-Barre syndrome following the second dose of COVID AstraZeneca vaccine in a 78-year-old male: A case report from Nepal. Ann. Med. Surg. 2023, 85, 466–469. [Google Scholar] [CrossRef]
- Do, K.; Kawana, E.; Do, J.; Diaz, L. Onset of Guillain-Barre Syndrome and Transverse Myelitis Following COVID-19 Vaccination. Cureus 2023, 15, e41009. [Google Scholar] [CrossRef]
- Wijekoon, L.; Sarathchandra, C.; Siribaddana, S. Guillain-Barre Syndrome Following the First Dose of Inactivated SARS-CoV-2 Vaccine, BBIBP-CorV. Cureus 2023, 15, e33952. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Varela Ramos, P.; Durao-Carvalho, G.; Almeida, V.; Goncalves Pereira, J. Guillain-Barre Syndrome After a SARS-CoV-2 Vaccine. Cureus 2024, 16, e57705. [Google Scholar] [CrossRef]
- Hurtado, I.C.; Vallejo-Serna, R.; Hurtado-Zapata, J.S.; Misnaza, S.P. Guillain-Barre Syndrome Post COVID-19 Vaccination with ChAdOx1 nCoV-19 Vaccine: A Colombian Case Report. Case Rep. Infect. Dis. 2023, 2023, 3290956. [Google Scholar] [CrossRef]
- Berrim, K.; Lakhoua, G.; Zaiem, A.; Charfi, O.; Aouinti, I.; Kastalli, S.; Daghfous, R.; El Aidli, S. Guillain-Barre syndrome after COVID-19 vaccines: A Tunisian case series. Br. J. Clin. Pharmacol. 2023, 89, 574–578. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lien, W.C. Effects of COVID-19 vaccine type on Guillain-Barre syndrome: Two cases and a literature review. Hum. Vaccin. Immunother. 2023, 19, 2171231. [Google Scholar] [CrossRef]
- Lee, M.L.; Bautista, J.M.P. Guillain-Barre Syndrome Following the Administration of Adenovirus Vector-Based COVID-19 Vaccine. Cureus 2023, 15, e42316. [Google Scholar] [CrossRef]
- Sii, H.L.; Ng, S.H.; Wong, V.F.; Law, W.C. A Case Report of Guillain-Barre Syndrome In Association with SARS-CoV-2 Vaccination in Malaysia. Acta Neurol. Taiwan. 2023, 32, 207–211. [Google Scholar]
- Zhu, C.; Wang, H.; Zhang, Y.; Wang, W.; Wang, J.; Li, W. Experience of treatment in critical Guillain-Barre Syndrome case after COVID-19 vaccination. Int. J. Dev. Neurosci. 2024, 84, 342–348. [Google Scholar] [CrossRef]
- Duong-Quy, S.; Huynh-Truong-Anh, D.; Nguyen-Quang, T.; Nguyen-Thi-Kim, T.; Tran-Ngoc-Anh, T.; Nguyen-Van-Hoai, N.; Do-Thi-Thu, M.; Nguyen-Van, T.; Tang-Thi-Thao, T.; Nguyen-Tuan, A.; et al. Guillain-Barre Syndrome due to COVID-19 Vero Cell Vaccination Associated with Concomitant COVID-19 Infection-induced ARDS and Treated Successfully by Therapeutic Plasma Exchange: A First Case Report from Vietnam. Pulm. Ther. 2023, 9, 271–280. [Google Scholar] [CrossRef]
- Soh, Y.; Yoo, E.A.; Kim, E.S.; Kim, S.J. MRI Features of Multiple Cranial Neuropathies in Guillain-Barre Syndrome Occurring after COVID-19 Vaccination: A Case Report. J. Korean Soc. Radiol. 2023, 84, 964–969. [Google Scholar] [CrossRef]
- Kamar, S.B.; Bhatta, U.K.; Singh, R.; Paudel Timilsana, M. Guillain Barre Syndrome after vaccination against Corona Virus Disease19: Managed in limited resource setting. J. Nepal. Health Res. Counc. 2023, 20, 1013–1015. [Google Scholar] [CrossRef]
- Smaoui, E.; Moalla, K.S.; Bouattour, N.; Farhat, N.; Sakka, S.; Daoud, S.; Damak, M.; Mhiri, C. Acute-onset chronic inflammatory demyelinating polyneuropathy following AstraZeneca COVID-19 vaccine: A case report. Pan Afr. Med. J. 2024, 47, 46. [Google Scholar] [CrossRef]
- Sukockiene, E.; Breville, G.; Fayolle, D.; Nencha, U.; Uginet, M.; Hubers, A. Case Series of Acute Peripheral Neuropathies in Individuals Who Received COVID-19 Vaccination. Medicina 2023, 59, 501. [Google Scholar] [CrossRef]
- Dalakas, M.C. Guillain-Barre syndrome: The first documented COVID-19-triggered autoimmune neurologic disease: More to come with myositis in the offing. Neurol. Neuroimmunol. Neuroinflamm 2020, 7, e781. [Google Scholar] [CrossRef]
- Willison, A.G.; Pawlitzki, M.; Lunn, M.P.; Willison, H.J.; Hartung, H.P.; Meuth, S.G. SARS-CoV-2 Vaccination and Neuroimmunological Disease: A Review. JAMA Neurol. 2024, 81, 179–186. [Google Scholar] [CrossRef]
- Schmeltzer, P.A.; Russo, M.W. Clinical narrative: Autoimmune hepatitis. Am. J. Gastroenterol. 2018, 113, 951–958. [Google Scholar] [CrossRef]
- Mieli-Vergani, G.; Vergani, D.; Czaja, A.J.; Manns, M.P.; Krawitt, E.L.; Vierling, J.M.; Lohse, A.W.; Montano-Loza, A.J. Autoimmune hepatitis. Nat. Rev. Dis. Primers 2018, 4, 18017. [Google Scholar] [CrossRef]
- Clayton-Chubb, D.; Schneider, D.; Freeman, E.; Kemp, W.; Roberts, S.K. Autoimmune hepatitis developing after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. J. Hepatol. 2021, 75, 1249–1250. [Google Scholar] [CrossRef]
- Mekritthikrai, K.; Jaru-Ampornpan, P.; Komolmit, P.; Thanapirom, K. Autoimmune Hepatitis Triggered by COVID-19 Vaccine: The First Case From Inactivated Vaccine. ACG Case Rep. J. 2022, 9, e00811. [Google Scholar] [CrossRef]
- Bril, F.; Al Diffalha, S.; Dean, M.; Fettig, D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J. Hepatol. 2021, 75, 222–224. [Google Scholar] [CrossRef]
- Rela, M.; Jothimani, D.; Vij, M.; Rajakumar, A.; Rammohan, A. Auto-immune hepatitis following COVID vaccination. J. Autoimmun. 2021, 123, 102688. [Google Scholar] [CrossRef]
- Mathew, M.; John, S.B.; Sebastian, J.; Ravi, M.D. COVID-19 vaccine triggered autoimmune hepatitis: Case report. Eur. J. Hosp. Pharm. 2023, 30, e27. [Google Scholar] [CrossRef]
- Lopez Romero-Salazar, F.; Veras Lista, M.; Gomez-Dominguez, E.; Ibarrola-Andres, C.; Munoz Gomez, R.; Fernandez Vazquez, I. SARS-CoV-2 vaccine, a new autoimmune hepatitis trigger? Rev. Esp. Enferm. Dig. 2022, 114, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Diez Ruiz, S.; Quinones Castro, R.; Alcoba Vega, L.; Martin Izquierdo, A.; Gonzalez Puente, I.; Rodriguez Martin, L.; Diez Rodriguez, R.; Jorquera Plaza, F. Autoimmune hepatitis after SARS-CoV-2 vaccination. Rev. Esp. Enferm. Dig. 2023, 115, 406. [Google Scholar] [CrossRef]
- Yoshida, Y.; Iwata, N.; Ishii, Y.; Hinoda, Y.; Endo, T. Autoimmune Hepatitis Following mRNA COVID-19 Vaccination in a Very Old Patient With Preexisting Sjogren’s Syndrome: A Case Report. Cureus 2022, 14, e30896. [Google Scholar] [CrossRef]
- Gips, J.R.; Woreta, T.A. It Can’t Be a Coincidence: A Comparison of Cases of Autoimmune Hepatitis After Vaccination Against COVID-19. ACG Case Rep. J. 2023, 10, e00965. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Gordon, K.; Macken, L.; Parvin, J.; Heath, S.; Whibley, M.; Tibble, J. COVID-19 Vaccination-Induced Cholangiopathy and Autoimmune Hepatitis: A Series of Two Cases. Cureus 2022, 14, e30304. [Google Scholar] [CrossRef] [PubMed]
- Erard, D.; Villeret, F.; Lavrut, P.M.; Dumortier, J. Autoimmune hepatitis developing after COVID 19 vaccine: Presumed guilty? Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101841. [Google Scholar] [CrossRef]
- Fimiano, F.; D’Amato, D.; Gambella, A.; Marzano, A.; Saracco, G.M.; Morgando, A. Autoimmune hepatitis or drug-induced autoimmune hepatitis following COVID-19 vaccination? Liver Int. 2022, 42, 1204–1205. [Google Scholar] [CrossRef]
- Camacho-Dominguez, L.; Rodriguez, Y.; Polo, F.; Restrepo Gutierrez, J.C.; Zapata, E.; Rojas, M.; Anaya, J.M. COVID-19 vaccine and autoimmunity. A new case of autoimmune hepatitis and review of the literature. J. Transl. Autoimmun. 2022, 5, 100140. [Google Scholar] [CrossRef]
- Ghielmetti, M.; Schaufelberger, H.D.; Mieli-Vergani, G.; Cerny, A.; Dayer, E.; Vergani, D.; Terziroli Beretta-Piccoli, B. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: A novel clinical entity? J. Autoimmun. 2021, 123, 102706. [Google Scholar] [CrossRef]
- Vuille-Lessard, E.; Montani, M.; Bosch, J.; Semmo, N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J. Autoimmun. 2021, 123, 102710. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kakisaka, K.; Takikawa, Y. Letter to the editor: Autoimmune hepatitis after COVID-19 vaccination: Need for population-based epidemiological study. Hepatology 2022, 75, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Palla, P.; Vergadis, C.; Sakellariou, S.; Androutsakos, T. Letter to the editor: Autoimmune hepatitis after COVID-19 vaccination: A rare adverse effect? Hepatology 2022, 75, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Lopes, S.; Simoes, M.S.; Liberal, R.; Lopes, J.; Carneiro, F.; Macedo, G. Autoimmune hepatitis after COVID-19 vaccine—More than a coincidence. J. Autoimmun. 2021, 125, 102741. [Google Scholar] [CrossRef]
- Avci, E.; Abasiyanik, F. Autoimmune hepatitis after SARS-CoV-2 vaccine: New-onset or flare-up? J. Autoimmun. 2021, 125, 102745. [Google Scholar] [CrossRef]
- Cao, Z.; Gui, H.; Sheng, Z.; Xin, H.; Xie, Q. Letter to the editor: Exacerbation of autoimmune hepatitis after COVID-19 vaccination. Hepatology 2022, 75, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Lodato, F.; Larocca, A.; D’Errico, A.; Cennamo, V. An unusual case of acute cholestatic hepatitis after m-RNABNT162b2 (Comirnaty) SARS-CoV-2 vaccine: Coincidence, autoimmunity or drug-related liver injury. J. Hepatol. 2021, 75, 1254–1256. [Google Scholar] [CrossRef]
- Zhou, T.; Fronhoffs, F.; Dold, L.; Strassburg, C.P.; Weismuller, T.J. New-onset autoimmune hepatitis following mRNA COVID-19 vaccination in a 36-year-old woman with primary sclerosing cholangitis—Should we be more vigilant? J. Hepatol. 2022, 76, 218–220. [Google Scholar] [CrossRef]
- Zin Tun, G.S.; Gleeson, D.; Al-Joudeh, A.; Dube, A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J. Hepatol. 2022, 76, 747–749. [Google Scholar] [CrossRef]
- Rocco, A.; Sgamato, C.; Compare, D.; Nardone, G. Autoimmune hepatitis following SARS-CoV-2 vaccine: May not be a casuality. J. Hepatol. 2021, 75, 728–729. [Google Scholar] [CrossRef] [PubMed]
- McShane, C.; Kiat, C.; Rigby, J.; Crosbie, O. The mRNA COVID-19 vaccine—A rare trigger of autoimmune hepatitis? J. Hepatol. 2021, 75, 1252–1254. [Google Scholar] [CrossRef]
- Tan, C.K.; Wong, Y.J.; Wang, L.M.; Ang, T.L.; Kumar, R. Autoimmune hepatitis following COVID-19 vaccination: True causality or mere association? J. Hepatol. 2021, 75, 1250–1252. [Google Scholar] [CrossRef]
- Ghorbani, H.; Rouhi, T.; Vosough, Z.; Shokri-Shirvani, J. Drug-induced hepatitis after Sinopharm COVID-19 vaccination: A case study of a 62-year-old patient. Int. J. Surg. Case Rep. 2022, 93, 106926. [Google Scholar] [CrossRef]
- Londono, M.C.; Gratacos-Gines, J.; Saez-Penataro, J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination—Still casualty? J. Hepatol. 2021, 75, 1248–1249. [Google Scholar] [CrossRef]
- Izagirre, A.; Arzallus, T.; Garmendia, M.; Torrente, S.; Castiella, A.; Zapata, E.M. Autoimmune hepatitis following COVID-19 vaccination. J. Autoimmun. 2022, 132, 102874. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N.; Matsuoka, R.; Ishikawa, N.; Endo, M.; Terasaki, M.; Seo, E.; Tsuchiya, K. Autoimmune hepatitis with history of HCV treatment triggered by COVID-19 vaccination: Case report and literature review. Clin. J. Gastroenterol. 2022, 15, 791–795. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Lore, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef]
- McLean-Tooke, A.; Lucas, M.; French, M. Autoimmunity elicited by the chemokine response to adenovirus vector vaccines may underlie vaccine-induced immune thrombotic thrombocytopaenia: A hypothesis. Clin. Transl. Immunology 2021, 10, e1349. [Google Scholar] [CrossRef]
- Syed, F.Z. Type 1 Diabetes Mellitus. Ann. Intern. Med. 2022, 175, ITC33–ITC48. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Narita, D.; Saito, N.; Ueno, T.; Sato, R.; Niitsuma, S.; Takahashi, K.; Arihara, Z. Type 1 diabetes mellitus following COVID-19 RNA-based vaccine. J. Diabetes Investig. 2022, 13, 1290–1292. [Google Scholar] [CrossRef]
- Tang, X.; He, B.; Liu, Z.; Zhou, Z.; Li, X. Fulminant type 1 diabetes after COVID-19 vaccination. Diabetes Metab. 2022, 48, 101324. [Google Scholar] [CrossRef]
- Sato, T.; Kodama, S.; Kaneko, K.; Imai, J.; Katagiri, H. Type 1 Diabetes Mellitus Associated with Nivolumab after Second SARS-CoV-2 Vaccination, Japan. Emerg. Infect. Dis. 2022, 28, 1518–1520. [Google Scholar] [CrossRef]
- Tanaka, T.; Nagasu, S.; Furuta, T.; Gobaru, M.; Suzuki, H.; Shimotsuura, Y.; Akiba, J.; Nomura, M.; Fujita, F.; Kawaguchi, T.; et al. Case report: A case of fulminant type 1 diabetes mellitus after COVID-19 vaccination during treatment of advanced gastric cancer: Pitfall in managing immune-related adverse events. Front. Oncol. 2023, 13, 1264281. [Google Scholar] [CrossRef]
- Moon, H.; Suh, S.; Park, M.K. Adult-Onset Type 1 Diabetes Development Following COVID-19 mRNA Vaccination. J. Korean Med. Sci. 2023, 38, e12. [Google Scholar] [CrossRef]
- Yano, M.; Morioka, T.; Natsuki, Y.; Sasaki, K.; Kakutani, Y.; Ochi, A.; Yamazaki, Y.; Shoji, T.; Emoto, M. New-onset Type 1 Diabetes after COVID-19 mRNA Vaccination. Intern. Med. 2022, 61, 1197–1200. [Google Scholar] [CrossRef]
- Bleve, E.; Venditti, V.; Lenzi, A.; Morano, S.; Filardi, T. COVID-19 vaccine and autoimmune diabetes in adults: Report of two cases. J. Endocrinol. Investig. 2022, 45, 1269–1270. [Google Scholar] [CrossRef]
- Sasaki, H.; Itoh, A.; Watanabe, Y.; Nakajima, Y.; Saisho, Y.; Irie, J.; Meguro, S.; Itoh, H. Newly developed type 1 diabetes after coronavirus disease 2019 vaccination: A case report. J. Diabetes Investig. 2022, 13, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Morioka, T.; Okada, N.; Natsuki, Y.; Kakutani, Y.; Ochi, A.; Yamazaki, Y.; Shoji, T.; Ohmura, T.; Emoto, M. New-onset fulminant type 1 diabetes after severe acute respiratory syndrome coronavirus 2 vaccination: A case report. J. Diabetes Investig. 2022, 13, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Patrizio, A.; Ferrari, S.M.; Antonelli, A.; Fallahi, P. A case of Graves’ disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J. Autoimmun. 2021, 125, 102738. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, B.I.; Unluturk, U.; Cesur, M. Type 1 diabetes mellitus following SARS-CoV-2 mRNA vaccination. Endocrine 2022, 78, 42–46. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yakou, F.; Saburi, M.; Hirose, A.; Akaoka, H.; Hirota, Y.; Yunaiyama, D.; Asahi, N.; Ohno, A.; Matsushita, T. New-onset atypical fulminant type 1 diabetes after COVID-19 vaccination: A case report. Clin. Case Rep. 2022, 10, e6473. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, K.; Amagai, R.; Tamabuchi, E.; Kambayashi, Y.; Fujimura, T. Fulminant type 1 diabetes mellitus triggered by coronavirus disease 2019 vaccination in an advanced melanoma patient given adjuvant nivolumab therapy. J. Dermatol. 2022, 49, e167–e168. [Google Scholar] [CrossRef]
- Huang, L.; Liang, M.; He, Y. New-Onset Fulminant Type 1 Diabetes Following SARS-CoV-2 Protein Subunit Vaccine: A Case Report and Literature Review. J. Korean Med. Sci. 2023, 38, e209. [Google Scholar] [CrossRef]
- Al-Jenaidi, F.A.; Wakim-Ghorayeb, S.F.; Al-Abbasi, A.; Arekat, M.R.; Irani-Hakime, N.; Najm, P.; Al-Ola, K.; Motala, A.A.; Almawi, W.Y. Contribution of selective HLA-DRB1/DQB1 alleles and haplotypes to the genetic susceptibility of type 1 diabetes among Lebanese and Bahraini Arabs. J. Clin. Endocrinol. Metab. 2005, 90, 5104–5109. [Google Scholar] [CrossRef]
- Beloor Suresh, A.; Asuncion, R.M.D. Myasthenia Gravis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chatterjee, A.; Chakravarty, A. Neurological Complications Following COVID-19 Vaccination. Curr. Neurol. Neurosci. Rep. 2023, 23, 1–14. [Google Scholar] [CrossRef]
- Huang, B.D.; Hsueh, H.W.; Yang, S.H.; Lin, C.W. New-Onset Myasthenia Gravis After ChAdOx1 nCOV-19 Vaccine Inoculation. J. Neuroophthalmol. 2023, 43, e298–e299. [Google Scholar] [CrossRef]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Poli, K.; Poli, S.; Ziemann, U. Multiple Autoimmune Syndromes Including Acute Disseminated Encephalomyelitis, Myasthenia Gravis, and Thyroiditis Following Messenger Ribonucleic Acid-Based COVID-19 Vaccination: A Case Report. Front. Neurol. 2022, 13, 913515. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.; Pougnier, C. A Case of COVID-19 Vaccine Associated New Diagnosis Myasthenia Gravis. J. Prim. Care Community Health 2021, 12, 21501327211051933. [Google Scholar] [CrossRef]
- Maher, D.I.; Hogarty, D.; Ben Artsi, E. Acute onset ocular myasthenia gravis after vaccination with the Oxford-AstraZeneca COVID-19 vaccine. Orbit 2023, 42, 630–634. [Google Scholar] [CrossRef]
- Chaturvedi, H.T.; Patel, V.; Vasava, R.; Chaturvedi, C. New acute onset of ocular myasthenia gravis after COVID-19 vaccine: A case report. J. Family Med. Prim. Care 2023, 12, 394–396. [Google Scholar] [CrossRef]
- Fanella, G.; Baiata, C.; Candeloro, E.; Toscano, G.; Colnaghi, S.; Mauri, M.; Cariddi, L.P.; Rebecchi, V.; Solazzo, F.; Banfi, P.; et al. New-onset myasthenia gravis after mRNA SARS-CoV-2 vaccination: A case series. Neurol. Sci. 2022, 43, 5799–5802. [Google Scholar] [CrossRef]
- Abicic, A.; Sitas, B.; Adamec, I.; Bilic, E.; Habek, M. New-Onset Ocular Myasthenia Gravis After Booster Dose of COVID-19 Vaccine. Cureus 2022, 14, e27213. [Google Scholar] [CrossRef]
- Su, W.Y.; Lu, C.J. The Clinical Course of New-Onset Ocular Myasthenia Gravis Caused by Pfizer-BioNTech COVID-19 Vaccine. Acta Neurol. Taiwan. 2023, 32, 218–222. [Google Scholar]
- Papadopoulou, M.; Stefanou, M.I.; Palaiodimou, L.; Tsivgoulis, G. Myasthenia Gravis Exacerbation Following Immunization With the BNT162b2 mRNA COVID-19 Vaccine: Report of a Case and Review of the Literature. Neurohospitalist 2023, 13, 303–307. [Google Scholar] [CrossRef]
- Lee, M.A.; Lee, C.; Park, J.H.; Lee, J.H. Early-Onset Myasthenia Gravis Following COVID-19 Vaccination. J. Korean Med. Sci. 2022, 37, e50. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Junior, J.W.L.; Sobreira-Neto, M.A.; Braga-Neto, P. Cogan’s sign in a patient with suspected post-COVID-19 vaccine-associated myasthenia gravis. Rev. Soc. Bras. Med. Trop. 2023, 56, e0007. [Google Scholar] [CrossRef] [PubMed]
- Slavin, E.; Fitzig, J.; Neubert, C.; Garcia-Lopez, F.; Cuevas-Trisan, R. New-Onset Myasthenia Gravis Confirmed by Electrodiagnostic Studies After a Third Dose of SARS-CoV-2 mRNA-1273 Vaccine. Am. J. Phys. Med. Rehabil. 2022, 101, e176–e179. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, C.G.; Cuciureanu, D.I.; Prutianu, I.; Cianga, P. Autoimmune myasthenia gravis after COVID-19 in a triple vaccinated patient. Arch. Clin. Cases 2022, 9, 104–107. [Google Scholar] [CrossRef]
- Galassi, G.; Rispoli, V.; Iori, E.; Ariatti, A.; Marchioni, A. Coincidental Onset of Ocular Myasthenia Gravis Following ChAdOx1 n-CoV-19 Vaccine against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Isr. Med. Assoc. J. 2022, 24, 9–10. [Google Scholar]
- Ramdas, S.; Hum, R.M.; Price, A.; Paul, A.; Bland, J.; Burke, G.; Farrugia, M.; Palace, J.; Storrie, A.; Ho, P.; et al. SARS-CoV-2 vaccination and new-onset myasthenia gravis: A report of 7 cases and review of the literature. Neuromuscul. Disord. 2022, 32, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Sansone, G.; Bonifati, D.M. Vaccines and myasthenia gravis: A comprehensive review and retrospective study of SARS-CoV-2 vaccination in a large cohort of myasthenic patients. J. Neurol. 2022, 269, 3965–3981. [Google Scholar] [CrossRef]
- Hoshina, Y.; Sowers, C.; Baker, V. Myasthenia Gravis Presenting after Administration of the mRNA-1273 Vaccine. Eur. J. Case Rep. Intern. Med. 2022, 9, 003439. [Google Scholar] [CrossRef]
- Kang, M.C.; Park, K.A.; Min, J.H.; Oh, S.Y. Myasthenia gravis with ocular symptoms following a ChAdOx1 nCoV-19 vaccination: A case report. Am. J. Ophthalmol. Case Rep. 2022, 27, 101620. [Google Scholar] [CrossRef] [PubMed]
- Ozenc, B.; Odabasi, Z. New-Onset Myasthenia Gravis Following COVID-19 Vaccination. Ann. Indian. Acad. Neurol. 2022, 25, 1224–1225. [Google Scholar] [CrossRef]
- Villasante Fricke, A.C.; Miteva, M. Epidemiology and burden of alopecia areata: A systematic review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 397–403. [Google Scholar] [CrossRef]
- Strazzulla, L.C.; Wang, E.H.C.; Avila, L.; Lo Sicco, K.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J. Am. Acad. Dermatol. 2018, 78, 1–12. [Google Scholar] [CrossRef]
- Vano-Galvan, S.; Saceda-Corralo, D.; Blume-Peytavi, U.; Cucchia, J.; Dlova, N.C.; Gavazzoni Dias, M.F.R.; Grimalt, R.; Guzman-Sanchez, D.; Harries, M.; Ho, A.; et al. Frequency of the Types of Alopecia at Twenty-Two Specialist Hair Clinics: A Multicenter Study. Skin. Appendage Disord. 2019, 5, 309–315. [Google Scholar] [CrossRef]
- May Lee, M.; Bertolani, M.; Pierobon, E.; Lotti, T.; Feliciani, C.; Satolli, F. Alopecia areata following COVID-19 vaccination: Vaccine-induced autoimmunity? Int. J. Dermatol. 2022, 61, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Fornaro, L.; Picone, V.; Marasca, D.; Gargiulo, M.; Annunziata, M.C.; Fabbrocini, G.; Marasca, C. Herpes zoster and alopecia areata following mRNA BNT162b2 COVID-19 vaccine: Controversial immune effects. J. Cosmet. Dermatol. 2023, 22, 36–38. [Google Scholar] [CrossRef]
- Gamonal, S.B.L.; Marques, N.C.V.; Pereira, H.M.B.; Gamonal, A.C.C. New-onset systemic lupus erythematosus after ChAdOX1 nCoV-19 and alopecia areata after BNT162b2 vaccination against SARS-CoV-2. Dermatol. Ther. 2022, 35, e15677. [Google Scholar] [CrossRef]
- Abdalla, H.; Ebrahim, E. Alopecia Areata Universalis Precipitated by SARS-CoV-2 Vaccine: A Case Report and Narrative Review. Cureus 2022, 14, e27953. [Google Scholar] [CrossRef]
- Scollan, M.E.; Breneman, A.; Kinariwalla, N.; Soliman, Y.; Youssef, S.; Bordone, L.A.; Gallitano, S.M. Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. 2022, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Genco, L.; Cantelli, M.; Noto, M.; Battista, T.; Patri, A.; Fabbrocini, G.; Vastarella, M. Alopecia Areata after COVID-19 Vaccines. Skin. Appendage Disord. 2023, 9, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Su, H.A.; Juan, C.K.; Chen, Y.C. Alopecia areata following ChAdOx1 nCoV-19 vaccination (Oxford/AstraZeneca). J. Formos. Med. Assoc. 2022, 121, 2138–2140. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Mastorino, L.; Tonella, L.; Ribero, S.; Quaglino, P. Alopecia areata after COVID-19 vaccination. Clin. Exp. Vaccine Res. 2022, 11, 129–132. [Google Scholar] [CrossRef]
- Ganjei, Z.; Yazdan Panah, M.; Rahmati, R.; Zari Meidani, F.; Mosavi, A. COVID-19 vaccination and alopecia areata: A case report and literature review. Clin. Case Rep. 2022, 10, e6039. [Google Scholar] [CrossRef]
- Wu, Y.; Dai, Y.; Peng, J.; Song, X. Alopecia areata induced by the booster shot of Sinovac COVID-19 vaccination: A case report. Eur. J. Dermatol. 2022, 32, 804–805. [Google Scholar] [CrossRef]
- Ho, J.D.; McNish, A.; McDonald, L.; Burrell, C.; Smith-Matthews, S. Alopecia universalis with unusual histopathologic features after vaccination with ChAdOx1 nCoV-19 (AZD1222). JAAD Case Rep. 2022, 25, 4–8. [Google Scholar] [CrossRef]
- Bardazzi, F.; Guglielmo, A.; Abbenante, D.; Sacchelli, L.; Sechi, A.; Starace, M.V.R. New insights into alopecia areata during COVID-19 pandemic: When infection or vaccination could play a role. J. Cosmet. Dermatol. 2022, 21, 1796–1798. [Google Scholar] [CrossRef]
- Matsuda, Y.; Kawakami, Y.; Kawamoto, M.; Hirai, Y.; Morizane, S. A case of extensive alopecia areata following Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine with favorable outcome. J. Cutan. Immunol. Allergy 2023, 6, 115–116. [Google Scholar] [CrossRef]
- Iwata, K.; Kunisada, M. Alopecia universalis after injection of messenger RNA COVID-19 vaccine. A case report. IDCases 2023, 33, e01830. [Google Scholar] [CrossRef]
- Hernandez Arroyo, J.; Izquierdo-Condoy, J.S.; Ortiz-Prado, E. A Case Series and Literature Review of Telogen Effluvium and Alopecia Universalis after the Administration of a Heterologous COVID-19 Vaccine Scheme. Vaccines 2023, 11, 444. [Google Scholar] [CrossRef]
- AlZahrani, F.; Perlau, M.J.; Fiorillo, L. Alopecia areata and subsequent Marie Antoinette syndrome following COVID-19 infection and vaccination: A case report. SAGE Open Med. Case Rep. 2023, 11, 2050313X231152065. [Google Scholar] [CrossRef]
- Teng, H.C.; Chen, H.H. Platelet-rich plasma in the treatment of alopecia areata after COVID-19 vaccination. Clin. Case Rep. 2023, 11, e7342. [Google Scholar] [CrossRef]
- Aristizabal, M.; Hsu, J.T.S.; Gold, M.H. Alopecia areata after CoronaVac vaccination. J. Cosmet. Dermatol. 2022, 21, 3675–3678. [Google Scholar] [CrossRef]
- Lo, A.; Mir, A.; Sami, N. Letter in Reply: Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. 2022, 25, 25–26. [Google Scholar] [CrossRef]
- Shakoei, S.; Kalantari, Y.; Nasimi, M.; Tootoonchi, N.; Ansari, M.S.; Razavi, Z.; Etesami, I. Cutaneous manifestations following COVID-19 vaccination: A report of 25 cases. Dermatol. Ther. 2022, 35, e15651. [Google Scholar] [CrossRef]
- Bigini, P.; Gobbi, M.; Bonati, M.; Clavenna, A.; Zucchetti, M.; Garattini, S.; Pasut, G. The role and impact of polyethylene glycol on anaphylactic reactions to COVID-19 nano-vaccines. Nat. Nanotechnol. 2021, 16, 1169–1171. [Google Scholar] [CrossRef]
- Murray, S.M.; Ansari, A.M.; Frater, J.; Klenerman, P.; Dunachie, S.; Barnes, E.; Ogbe, A. The impact of pre-existing cross-reactive immunity on SARS-CoV-2 infection and vaccine responses. Nat. Rev. Immunol. 2023, 23, 304–316. [Google Scholar] [CrossRef]
- Richardson, C.T.; Hayden, M.S.; Gilmore, E.S.; Poligone, B. Evaluation of the Relationship between Alopecia Areata and Viral Antigen Exposure. Am. J. Clin. Dermatol. 2018, 19, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; PG, D.E.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Kumar, M.A. Chapter 15—Hematology and Coagulation. In Monitoring in Neurocritical Care; Le Roux, P.D., Levine, J.M., Kofke, W.A., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 131–147. [Google Scholar]
- Vandevelde, A.; Devreese, K.M.J. Laboratory Diagnosis of Antiphospholipid Syndrome: Insights and Hindrances. J. Clin. Med. 2022, 11, 2164. [Google Scholar] [CrossRef]
- Moreno-Torres, V.; Gutierrez, A.; Valdenebro, M.; Ortega, A.; Citores, M.J.; Montero, E. Catastrophic antiphospholipid syndrome triggered by mRNA COVID-19 vaccine. Clin. Exp. Rheumatol. 2022, 40, 1054–1055. [Google Scholar] [CrossRef]
- Jinno, S.; Naka, I.; Nakazawa, T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: In search of the underlying mechanism. Rheumatol. Adv. Pract. 2021, 5, rkab096. [Google Scholar] [CrossRef]
- Bryan, P.; Williams, N.; Haebich, G. P09 A case of suspected COVID-19 vaccine-induced antiphospholipid syndrome. Br. J. Dermatol. 2023, 188, ljad113.037. [Google Scholar] [CrossRef]
- Siagian, S.N.; Christianto, C. A young woman with acute coronary syndrome and antiphospholipid syndrome. Is it the antiphospholipid syndrome or COVID-19 vaccination or classical risk as the risk factor? A case report. J. Med. Case Rep. 2024, 18, 47. [Google Scholar] [CrossRef]
- Watts, R.A.; Hatemi, G.; Burns, J.C.; Mohammad, A.J. Global epidemiology of vasculitis. Nat. Rev. Rheumatol. 2022, 18, 22–34. [Google Scholar] [CrossRef]
- Suzuki, M.; Sekiguchi, Y.; Sasaki, M.; Inaba, S.; Oyama, S.; Inoue, Y.; Warabi, M.; Ohashi, K.; Inoshita, S. Antineutrophil Cytoplasmic Antibody-associated Vasculitis after COVID-19 Vaccination with Pfizer-BioNTech. Intern. Med. 2022, 61, 2925–2929. [Google Scholar] [CrossRef] [PubMed]
- Dourado, E.; Silva, S.R.; Dias, F.; Duarte, J.; Inacio, A.; Abrantes, C.; Cunha, L.; Costa, A.S.; Ponte, C.; Valido, A. ANCA-associated vasculitis after Pfizer-BioNTech COVID-19 vaccination: Two case reports. ARP Rheumatol. 2023, 2, 173–174. [Google Scholar]
- Campos, M.A.G.; Valois, T.O.; Magalhaes, L.E.; Vasques, L.F.; de Medeiros, R.G.; Costa, D.; Salgado Filho, N.; Nogueira, R.; Neves, P.; Silva, G.E.B. ANCA-associated glomerulonephritis and lupus nephritis following COVID-19 vaccination: A case report and literature review. Front. Immunol. 2023, 14, 1298622. [Google Scholar] [CrossRef]
- Marouço, C.; Pereira, T.A.; Fonseca, N.M.; Menezes, M.D.M.; Carvalho, D.; Magriço, R.; Nolasco, F. #6178 ANCA-Associated Vasculitis Following Pfizer-Biontech COVID-19 Vaccine: True Association or Circumstantial? Nephrol. Dial. Transplant. 2023, 38, gfad063d_6178. [Google Scholar]
- Qaisar, I.; Sunmboye, K. AB1516 A Case of Severe Anca Associated Vasculitis after COVID-19 Vaccination. Ann. Rheum. Dis. 2022, 81, 1860. [Google Scholar] [CrossRef]
- Al-Yafeai, Z.; Horn, B.J.M.; Terraccaine, W.; Jose, A.; Krishnan, P. A Case of Antineutrophil Cytoplasmic Antibodies (ANCA)-Associated Vasculitis Post COVID-19 Vaccination. Cureus 2022, 14, e23162. [Google Scholar] [CrossRef]
- Kawamura, T.; Nakazawa, D.; Nishio, S.; Isozaki, T.; Komatsumoto, M.; Atsumi, T. Development of ANCA-associated vasculitis followed by SARS-CoV-2 vaccination in a patient with HLA-DRB1*09:01 allele. Mod. Rheumatol. Case Rep. 2023, 7, 426–430. [Google Scholar] [CrossRef]
- Uddin, K.; Mohamed, K.H.; Agboola, A.A.; Naqvi, W.A.; Hussaini, H.; Mohamed, A.S.; Haseeb, M.; Nasir, H.; Hussaini, H., Jr. Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Renal Vasculitis Following COVID-19 Vaccination: A Case Report and Literature Review. Cureus 2022, 14, e30206. [Google Scholar] [CrossRef]
- Villa, M.; Diaz-Crespo, F.; Perez de Jose, A.; Verdalles, U.; Verde, E.; Almeida Ruiz, F.; Acosta, A.; Mijaylova, A.; Goicoechea, M. A case of ANCA-associated vasculitis after AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 vaccination: Casualty or causality? Kidney Int. 2021, 100, 937–938. [Google Scholar] [CrossRef]
- Zamoner, W.; Scardini, J.B.; De Dio, B.J.; Marques, A.M.; Silva, V.D.S.; Garcia, A.L.; Dos Santos, D.C.; Viero, R.M. ANCA-associated vasculitis following Oxford-AstraZeneca COVID-19 vaccine in Brazil: Is there a causal relationship? A case report. Front. Med. 2022, 9, 1003332. [Google Scholar] [CrossRef]
- Gen, S.; Iwai, T.; Ohnari, S.; Nobe, K.; Ikeda, N. ANCA-Associated Vasculitis after Moderna COVID-19 Vaccination. Case Rep. Nephrol. 2023, 2023, 4906876. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huang, T.; Xu, G. ANCA-associated vasculitis following the CoronaVac vaccination. Ther. Adv. Chronic Dis. 2022, 13, 20406223221125708. [Google Scholar] [CrossRef]
- Yadav, R.; Shah, S.; Chhetri, S. ANCA-associated vasculitis following Johnson and Johnson COVID-19 vaccine. Ann. Med. Surg. 2022, 79, 104123. [Google Scholar] [CrossRef]
- Kim, B.C.; Kim, H.S.; Han, K.H.; Han, S.Y.; Jo, H.A. A Case Report of MPO-ANCA-Associated Vasculitis Following Heterologous mRNA1273 COVID-19 Booster Vaccination. J. Korean Med. Sci. 2022, 37, e204. [Google Scholar] [CrossRef]
- El Hasbani, G.; Uthman, I. ANCA-Associated Vasculitis following the First Dose of Pfizer-BioNTech COVID-19 Vaccine. Nephron 2023, 147, 103–107. [Google Scholar] [CrossRef]
- Christodoulou, M.; Iatridi, F.; Chalkidis, G.; Lioulios, G.; Nikolaidou, C.; Badis, K.; Fylaktou, A.; Papagianni, A.; Stangou, M. ANCA-Associated Vasculitis May Result as a Complication to Both SARS-CoV-2 Infection and Vaccination. Life 2022, 12, 1072. [Google Scholar] [CrossRef]
- Feghali, E.J.; Zafar, M.; Abid, S.; Santoriello, D.; Mehta, S. De-novo Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Following the mRNA-1273 (Moderna) Vaccine for COVID-19. Cureus 2021, 13, e19616. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Ishida, T. Anti-neutrophil Cytoplasmic Antibody-Associated Vasculitis With Periaortitis That Developed After mRNA COVID-19 Vaccination. Cureus 2023, 15, e37480. [Google Scholar] [CrossRef]
- Tonutti, A.; Simonetta, E.; Stainer, A.; Suigo, G.; De Santis, M.; Selmi, C.; Masetti, C.; Lleo, A.; Terracciano, L.M.; Aliberti, S.; et al. Hepatic and pulmonary involvement in a patient with PR3-ANCA vasculitis following SARS-CoV-2 vaccination: A case report. Mod. Rheumatol. Case Rep. 2023, 7, 440–443. [Google Scholar] [CrossRef]
- Anderegg, M.A.; Liu, M.; Saganas, C.; Montani, M.; Vogt, B.; Huynh-Do, U.; Fuster, D.G. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021, 100, 474–476. [Google Scholar] [CrossRef]
- Sekar, A.; Campbell, R.; Tabbara, J.; Rastogi, P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. 2021, 100, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.T.; Birkenbach, M.P.; Lynch, M. ANCA-Associated Vasculitis Following Pfizer-BioNTech COVID-19 Vaccine. Am. J. Kidney Dis. 2021, 78, 611–613. [Google Scholar] [CrossRef]
- Dube, G.K.; Benvenuto, L.J.; Batal, I. Antineutrophil Cytoplasmic Autoantibody-Associated Glomerulonephritis Following the Pfizer-BioNTech COVID-19 Vaccine. Kidney Int. Rep. 2021, 6, 3087–3089. [Google Scholar] [CrossRef]
- Takenaka, T.; Matsuzaki, M.; Fujiwara, S.; Hayashida, M.; Suyama, H.; Kawamoto, M. Myeloperoxidase anti-neutrophil cytoplasmic antibody positive optic perineuritis after mRNA coronavirus disease-19 vaccine. QJM 2021, 114, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Ellis, B.K. Concurrent Antiglomerular Basement Membrane Nephritis and Antineutrophil Cytoplasmic Autoantibody-Mediated Glomerulonephritis After Second Dose of SARS-CoV-2 mRNA Vaccination. Kidney Int. Rep. 2022, 7, 127–128. [Google Scholar] [CrossRef]
- Hakroush, S.; Tampe, B. Case Report: ANCA-Associated Vasculitis Presenting With Rhabdomyolysis and Pauci-Immune Crescentic Glomerulonephritis After Pfizer-BioNTech COVID-19 mRNA Vaccination. Front. Immunol. 2021, 12, 762006. [Google Scholar] [CrossRef] [PubMed]
- Klomjit, N.; Alexander, M.P.; Fervenza, F.C.; Zoghby, Z.; Garg, A.; Hogan, M.C.; Nasr, S.H.; Minshar, M.A.; Zand, L. COVID-19 Vaccination and Glomerulonephritis. Kidney Int. Rep. 2021, 6, 2969–2978. [Google Scholar] [CrossRef] [PubMed]
- Prema, J.; Muthukumaran, A.; Haridas, N.; Fernando, E.; Seshadri, J.; Kurien, A.A. Two Cases of Double-Positive Antineutrophil Cytoplasmic Autoantibody and Antiglomerular Basement Membrane Disease After BBV152/Covaxin Vaccination. Kidney Int. Rep. 2021, 6, 3090–3091. [Google Scholar] [CrossRef]
- Caza, T.N.; Cassol, C.A.; Messias, N.; Hannoudi, A.; Haun, R.S.; Walker, P.D.; May, R.M.; Seipp, R.M.; Betchick, E.J.; Amin, H.; et al. Glomerular Disease in Temporal Association with SARS-CoV-2 Vaccination: A Series of 29 Cases. Kidney360 2021, 2, 1770–1780. [Google Scholar] [CrossRef]
- Davidovic, T.; Schimpf, J.; Sprenger-Mähr, H.; Abbassi-Nik, A.; Soleiman, A.; Zitt, E.; Lhotta, K. De Novo and Relapsing Glomerulonephritis following SARS-CoV-2 mRNA Vaccination in Microscopic Polyangiitis. Case Rep. Nephrol. 2021, 2021, 8400842. [Google Scholar] [CrossRef]
- Obata, S.; Hidaka, S.; Yamano, M.; Yanai, M.; Ishioka, K.; Kobayashi, S. MPO-ANCA-associated vasculitis after the Pfizer/BioNTech SARS-CoV-2 vaccination. Clin. Kidney J. 2022, 15, 357–359. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, H.Y.; Lu, C.C.; Lin, S.H. Case Report: Anti-neutrophil Cytoplasmic Antibody-Associated Vasculitis With Acute Renal Failure and Pulmonary Hemorrhage May Occur After COVID-19 Vaccination. Front. Med. 2021, 8, 765447. [Google Scholar] [CrossRef]
- Cohen Tervaert, J.W.; Martinez-Lavin, M.; Jara, L.J.; Halpert, G.; Watad, A.; Amital, H.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) in 2023. Autoimmun. Rev. 2023, 22, 103287. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef]
- Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Kharrazian, D. Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins With Tissue Antigens: Implications for Autoimmune Diseases. Front. Immunol. 2020, 11, 617089. [Google Scholar] [CrossRef]
- Kanduc, D.; Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: Implications for the vaccine. Immunol. Res. 2020, 68, 310–313. [Google Scholar] [CrossRef]
- Wucherpfennig, K.W.; Strominger, J.L. Molecular mimicry in T cell-mediated autoimmunity: Viral peptides activate human T cell clones specific for myelin basic protein. Cell 1995, 80, 695–705. [Google Scholar] [CrossRef]
- Pender, M.P. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol. 2003, 24, 584–588. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Fox, C.B. New generation adjuvants--from empiricism to rational design. Vaccine 2015, 33, B14–B20. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Soullie, T.; van Nimwegen, M.; Willart, M.A.; Muskens, F.; Jung, S.; Hoogsteden, H.C.; Hammad, H.; Lambrecht, B.N. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008, 205, 869–882. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pasare, C.; Medzhitov, R. Control of B-cell responses by Toll-like receptors. Nature 2005, 438, 364–368. [Google Scholar] [CrossRef]
- Perricone, C.; Colafrancesco, S.; Mazor, R.D.; Soriano, A.; Agmon-Levin, N.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: Unveiling the pathogenic, clinical and diagnostic aspects. J. Autoimmun. 2013, 47, 1–16. [Google Scholar] [CrossRef]
- Jara, L.J.; Garcia-Collinot, G.; Medina, G.; Cruz-Dominguez, M.D.P.; Vera-Lastra, O.; Carranza-Muleiro, R.A.; Saavedra, M.A. Severe manifestations of autoimmune syndrome induced by adjuvants (Shoenfeld’s syndrome). Immunol. Res. 2017, 65, 8–16. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. Author Correction: mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 880. [Google Scholar] [CrossRef]
- Yang, G.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Inflammasomes and their roles in arthritic disease pathogenesis. Front. Mol. Biosci. 2022, 9, 1027917. [Google Scholar] [CrossRef]
- Tough, D.F.; Borrow, P.; Sprent, J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 1996, 272, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Bevan, M.J. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 2003, 300, 339–342. [Google Scholar] [CrossRef]
- Boyman, O. Bystander activation of CD4+ T cells. Eur. J. Immunol. 2010, 40, 936–939. [Google Scholar] [CrossRef]
- Mannie, M.D.; DeOca, K.B.; Bastian, A.G.; Moorman, C.D. Tolerogenic vaccines: Targeting the antigenic and cytokine niches of FOXP3(+) regulatory T cells. Cell Immunol. 2020, 355, 104173. [Google Scholar] [CrossRef]
- Lehmann, P.V.; Forsthuber, T.; Miller, A.; Sercarz, E.E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature 1992, 358, 155–157. [Google Scholar] [CrossRef]
- Cornaby, C.; Gibbons, L.; Mayhew, V.; Sloan, C.S.; Welling, A.; Poole, B.D. B cell epitope spreading: Mechanisms and contribution to autoimmune diseases. Immunol. Lett. 2015, 163, 56–68. [Google Scholar] [CrossRef]
- Gulley, J.L. Therapeutic vaccines: The ultimate personalized therapy? Hum. Vaccin. Immunother. 2013, 9, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M.; Steinberg, A.D. Systemic autoimmune disease arises from polyclonal B cell activation. J. Exp. Med. 1987, 165, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Bucala, R. Polyclonal activation of B lymphocytes by lipopolysaccharide requires macrophage-derived interleukin-1. Immunology 1992, 77, 477–482. [Google Scholar] [PubMed]
- Pruett, S.B. Polyclonal Activators. In Encyclopedic Reference of Immunotoxicology; Vohr, H.-W., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 514–515. [Google Scholar]
- Toussirot, E.; Bereau, M. Vaccination and Induction of Autoimmune Diseases. Inflamm. Allergy Drug Targets 2015, 14, 94–98. [Google Scholar] [CrossRef]
- Mackay, I.R.; Leskovsek, N.V.; Rose, N.R. Cell damage and autoimmunity: A critical appraisal. J. Autoimmun. 2008, 30, 5–11. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.E.; Feng, A.; Meng, W.; Apostolidis, S.A.; Mack, E.; Artandi, M.; Barman, L.; Bennett, K.; Chakraborty, S.; Chang, I.; et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 2021, 12, 5417. [Google Scholar] [CrossRef] [PubMed]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Agmon-Levin, N. ‘ASIA’—Autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011, 36, 4–8. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, B.; Koeken, V.; Gupta, M.K.; Li, Y. Multi-Omics Approaches in Immunological Research. Front. Immunol. 2021, 12, 668045. [Google Scholar] [CrossRef]
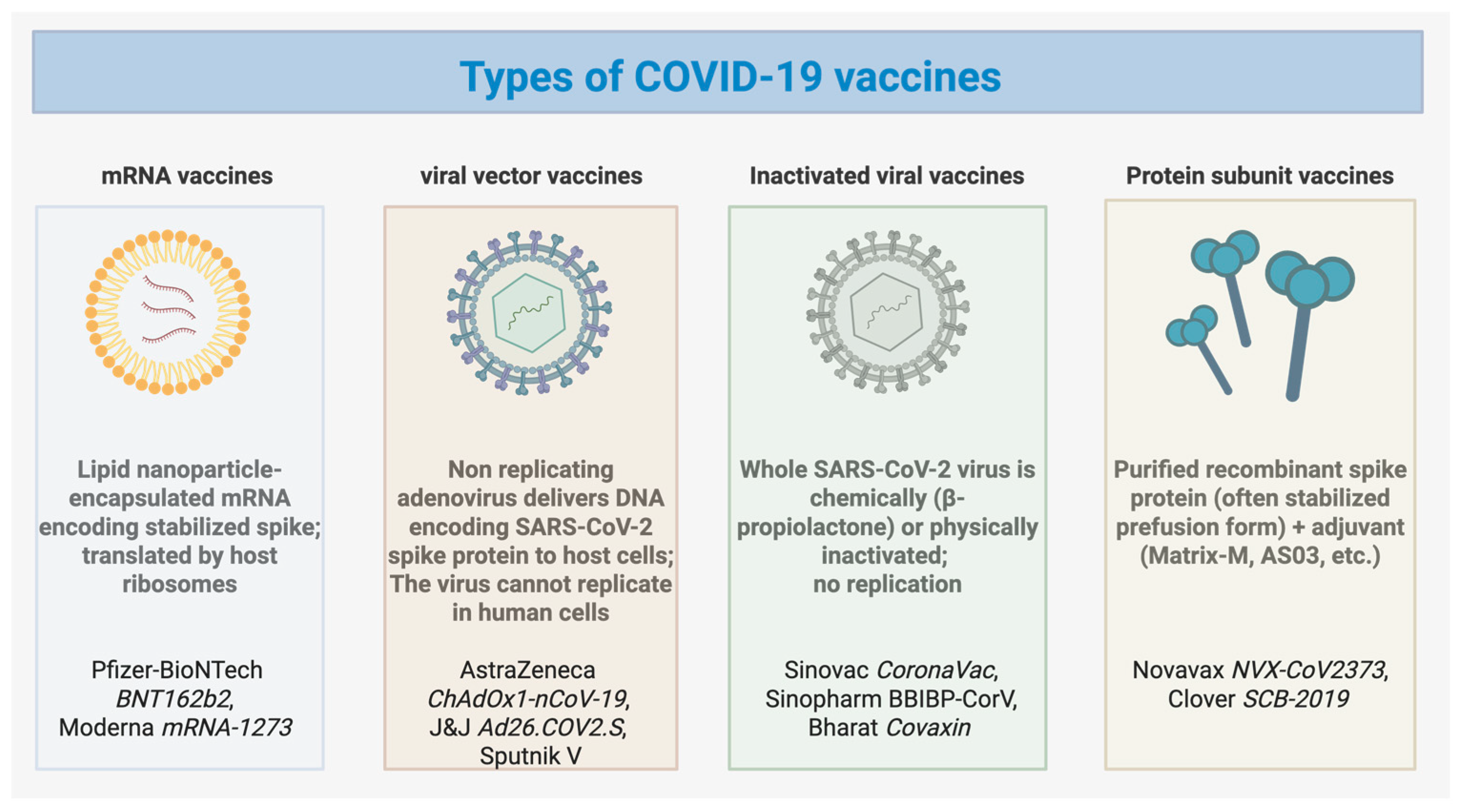

| Autoimmune Disease | Common Vaccine Type(s) Implicated | Median Age (Years) | Sex Ratio (F:M) | Typical Onset (After Vaccination) | Hallmark Laboratory Findings | Common Treatment | Overall Outcome |
|---|---|---|---|---|---|---|---|
| Systemic Lupus Erythematosus (SLE) | mRNA (majority), Adenoviral vector | 22–79 | ~2:1 | 2–14 days (median ≈ 7 days) | ANA+, anti-dsDNA+, low C3/C4, ↑ ESR/CRP | Prednisone ± HCQ ± MMF | Most improved; few lupus nephritis cases |
| Rheumatoid Arthritis (RA) | mRNA, Adenoviral vector, Inactivated | 32–88 | ~3:1 | 2–20 days | ↑ CRP/ESR, RF+, anti-CCP+, ANA+ | MTX, HCQ, steroids | Majority improved; rare recurrence |
| Vaccine-Induced Thrombotic Thrombocytopenia (VITT) | Adenoviral vector (dominant) | 25–75 | 1:1 | 5–21 days | ↓ Platelets, ↑ D-dimer, anti-PF4+ | IVIG, anticoagulants, steroids | ~25% mortality reported |
| Guillain–Barré Syndrome (GBS) | Adenoviral vector > mRNA > Inactivated | 20–90 | ~1.5:1 | 5–28 days | ↑ CSF protein (albuminocytologic dissociation) | IVIG ± plasma exchange | Mostly recovered; some partial |
| Autoimmune Hepatitis (AIH) | mRNA > Adenoviral vector > Inactivated | 35–85 | ~3:1 | 7–28 days | ↑ AST/ALT/bilirubin, ANA+, ASMA+, high IgG | Prednisone ± azathioprine | Most recovered with treatment |
| Type 1 Diabetes Mellitus (T1DM) | mRNA (predominant), Adenoviral vector, Inactivated | 36–73 | ~1.5:1 | 3–28 days | Hyperglycemia, ketoacidosis, ↓ C-peptide, anti-GAD+ | Insulin ± supportive therapy | Majority improved |
| Proposed Mechanism | Representative Diseases | Supporting Clinical Patterns | Key Immunological Features |
|---|---|---|---|
| Molecular mimicry | GBS, AIH, RA | Autoantibody overlap (anti-PF4, anti-CCP) | Cross-reactive epitopes between Spike and host proteins |
| Bystander activation | Thyroiditis, SLE | Occurs after strong innate cytokine response | ↑IL-6, IFN-α, TLR7/9 activation |
| Epitope spreading | SLE, RA | Expansion of antibody repertoire after initial trigger | Secondary autoantibody diversification |
| Polyclonal B-cell activation | SLE, AIH | Broad ANA/anti-dsDNA/anti-Sm induction | Excessive IL-6 and IFN signatures |
| Adjuvant effects (ASIA) | AIH, arthritis, myositis | Delayed onset, strong adjuvant formulations | Innate immune overactivation by squalene/alum/Matrix-M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, M.; Wang, Z. Vaccine-Associated Autoimmunity: From Clinical Signals to Immune Pathways. Vaccines 2025, 13, 1112. https://doi.org/10.3390/vaccines13111112
Peng M, Wang Z. Vaccine-Associated Autoimmunity: From Clinical Signals to Immune Pathways. Vaccines. 2025; 13(11):1112. https://doi.org/10.3390/vaccines13111112
Chicago/Turabian StylePeng, Mou, and Zijun Wang. 2025. "Vaccine-Associated Autoimmunity: From Clinical Signals to Immune Pathways" Vaccines 13, no. 11: 1112. https://doi.org/10.3390/vaccines13111112
APA StylePeng, M., & Wang, Z. (2025). Vaccine-Associated Autoimmunity: From Clinical Signals to Immune Pathways. Vaccines, 13(11), 1112. https://doi.org/10.3390/vaccines13111112






