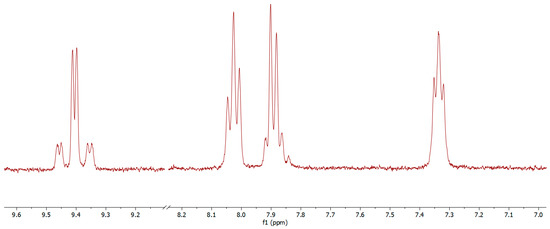
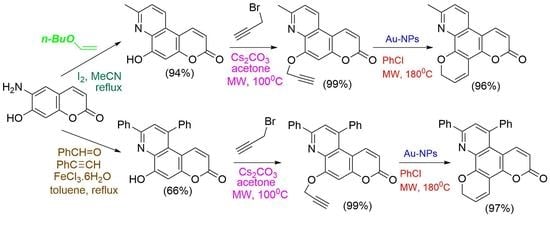
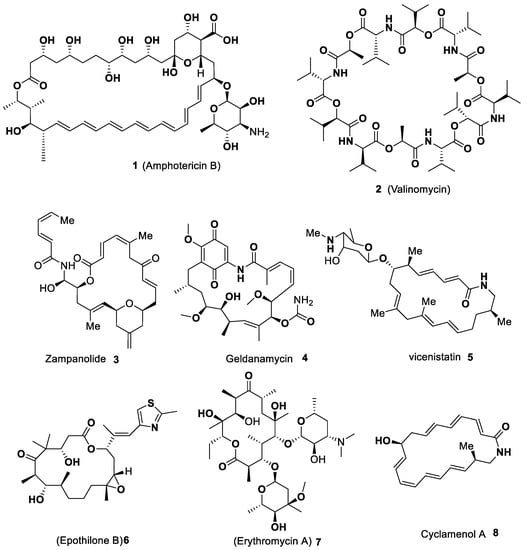
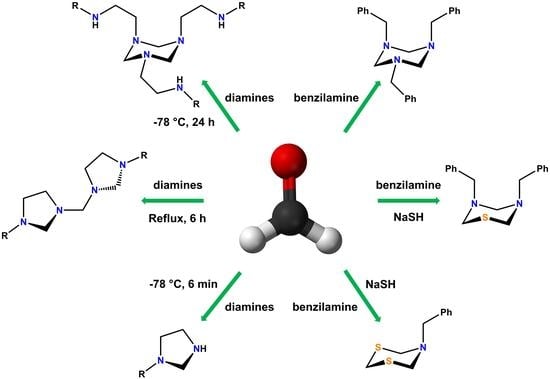
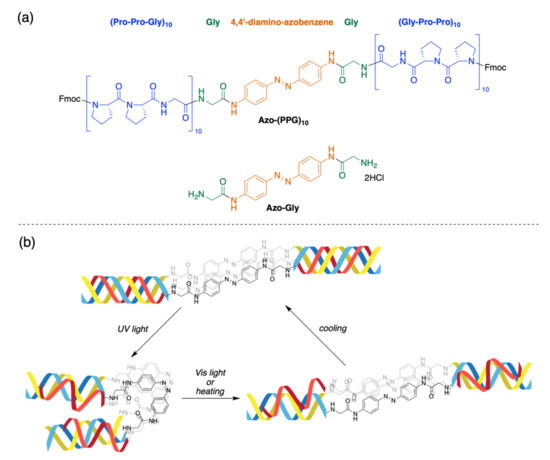
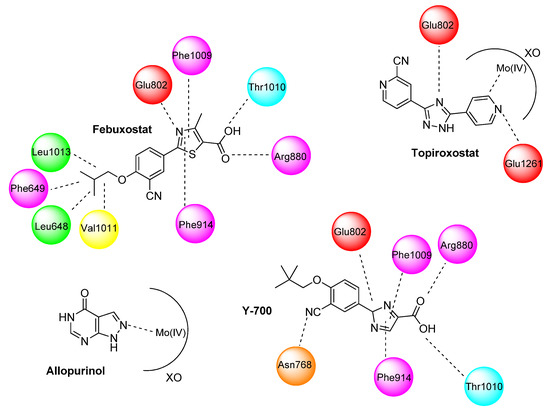
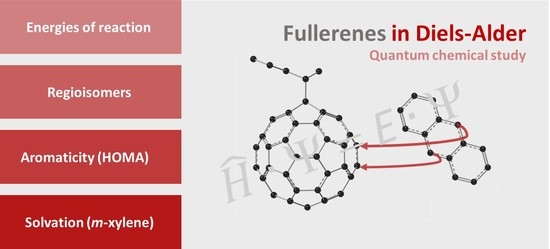
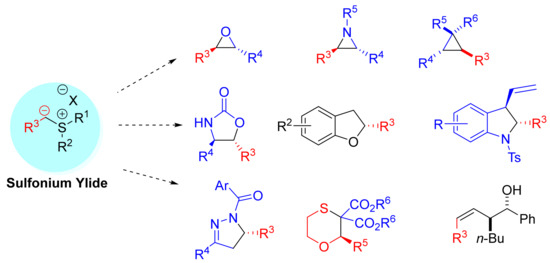
Advanced Research Papers in Organics
A topical collection in Organics (ISSN 2673-401X).
Viewed by 155156Editors
Interests: organic chemistry; heterocyclic chemistry; supramolecular chemistry
Special Issues, Collections and Topics in MDPI journals
Interests: amide bonds; N-heterocyclic carbenes; C-N activation; C-H activation; C-O activation; lanthanides; cross-coupling; catalysis; reductions; reductive couplings; radical chemistry; synthetic methodology; natural products
Special Issues, Collections and Topics in MDPI journals
Interests: redox responsive polymers
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
We are pleased to announce that the Special Issue entitled "Feature Papers in Organics" is now open for submissions, which will be published, free of charge, in open access format after a peer-review process. We welcome contributions of high-quality manuscripts from Editorial Board Members, and from outstanding scholars invited by the Academic Editors and the Editorial Office. There is flexibility in the types of manuscript we will accept, and they include original research articles, short communications, highlights of new developments, and insightful critical reviews. Detailed experimental procedures are required for research articles and communications.
As shown by the keywords list, the sample research topics included in this Special Issue represent the whole journal; this is intended to provide an opportunity for manuscripts related to organic chemistry around the world to be submitted.
Prof. Dr. Wim Dehaen
Prof. Dr. Michal Szostak
Prof. Dr. Huaping Xu
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Organics is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1200 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- organic synthesis
- development of synthetic methodology
- theoretical organic chemistry
- physical organic chemistry
- supramolecular and macromolecular chemistry
- heterocyclic chemistry
- organocatalysis
- bioorganic chemistry
- organometallic chemistry
- functional organic materials.