Abstract
Formaldehyde is a simple chemical compound that is used as a building block in obtaining a wide range of products. The versatility of formaldehyde in chemical synthesis becomes evident when it is reacted with N-alkylethylenediamines. Therefore, this paper reports the structure and reactivity of a series of compounds derived from easily accessible molecules, such as formaldehyde, sodium hydrosulphide, and N-alkylethylenediamines. The 1,3,5-triazines (1a-1d) and bis(3-alkyl-imidazolidin-1-yl)methanes (2a-2d) were obtained by simple reaction conditions. Additionally, different proportions of sodium hydrosulphide and formaldehyde were used with N-benzylamine to obtain N-benzyltriazinane (3), N-benzylthiadiazinane (4) and N-benzyldithiazinane (5). All these compounds were characterized by analytical, spectroscopic, and spectrometric techniques, such as melting point, solubility, one-dimensional and two-dimensional nuclear magnetic resonance (13C, 1H, 15N, COSY, HETCOR, NOESY, COLOC), elemental analysis, high- and low-resolution mass spectrometry, among others. The structures of compounds 4 and 5 were obtained by single-crystal X-ray diffraction. The results show that small variations in the stoichiometry and the reaction conditions significantly influence the products obtained.
1. Introduction
On the one hand, imidazolidines are of commercial interest due to their multiple biological activities related to ring substitution [1,2]. Bis(imidazolidinyl)methanes are derived from these heterocycles and have shown excellent antimicrobial, antiparasitic, and antitumor activity [3]. These properties could be related to its hydrophobic nature, which increases the bioavailability of its biologically active precursors (carbonyl and ethylenediamine) [4]. In addition, microbiological studies have shown a high activity of these derivatives against fungi, aerobic bacteria and anaerobic bacteria in the degradation of petroleum derivatives [5]. Artificial neural networks used in pharmacology identify them as potential new drugs against Chagas disease [6]. Some authors have even used them in the design of lithium carbanions and in the reduction with borane [7,8]. Therefore, new green synthesis techniques have been implemented to obtain them [9].
On the other hand, 1,3,5-triazinanes are exceptionally important because they have three nitrogen atoms with different degrees of hybridization and are good hydrogen-bond acceptors, which is why they are widely used by the pharmaceutical and biotechnological industries [10]. In the literature, 2,4,6-trichloro-1,3,5-triazine, also named as cyanuric chloride, and 1,3,5-trichloro-1,3,5-triazine-2,4,6(1H,3H,5H)-trione, also named as trichloroisocyanuric acid, symclosene or chloreal, are known [11,12]. Both commercial compounds are produced on an industrial scale and are economically accessible. The first is used in agricultural pesticides and photoactive compounds, while the second is used in the treatment and disinfection of swimming pools and food surfaces [13,14]. Additionally, it has been reported that 1,3,5-triazinanes have a wide variety of applications, such as in anticorrosives [15,16,17], explosives [18,19], precursors in the synthesis of insecticides [20] and organic compounds [21,22]. For example, in the synthesis of heterocycles, they are easy to prepare and low-cost substances, allowing other compounds of biological or technological interest to be obtained [23].
N-alkylethylenediamines react with formaldehyde to obtain different products (imidazolidines, 1,3,5-triazinanes or bis(imidazolidinyl)methanes), which depend on the nature of the reagents, the stoichiometry of the reaction, the solvent used, as well as the time and temperature of the reaction. In this work, a clear competition between 1,3,5-triazinanes and bis(imidazolidinyl)methanes was observed, testing various reaction conditions during the synthesis of the products. Due to the above and considering the chemical and biological importance of these compounds, there was an interest in understanding the structural and dynamic behaviour of the new heterocycles derived from N-alkylethylenediamines.
2. Materials and Methods
2.1. General Experimental Details
The reagents used in this investigation were purchased from Sigma-Aldrich Chemical, Fluka Chemika or Strem Chemical. Reactive-grade solvents, such as toluene, methylene chloride, chloroform and tetrahydrofuran were previously dried according to the procedures already established in the literature. NMR spectra in one and two dimensions were determined in the following equipment: Jeol GSX-DELTA 270 MHz [1H: 270.17 MHz, 13C: 67.94 MHz, 15N: 27.39 MHz], Jeol Eclipse GSX-DELTA 400 [1H: 399.78 MHz, 13C: 100.53 MHz, 15N: 40.52 MHz] and BRUKER-AVANCE 300 [1H: 300.13 MHz, 13C: 75.47 MHz, 15N: 30.42 MHz]. The 1H and 13C spectra were acquired with reference to Si(CH3)4 and the 15N spectra with reference to CH3NO2 in 5 mm diameter resonance tubes. Chemical shifts are expressed in parts per million (ppm) and were obtained at room temperature.
Melting points were measured in capillary tubes sealed in Gallemkamp Mel-Temp II Laboratory Devices equipment. The elemental analyses were performed on FLASH(EA) 1112 Series, Thermo Finnigan equipment. Mass spectra were acquired in HP-5989A MS Engine Hewlett-Packard equipment coupled to a gas chromatograph 5890 series II by direct insertion to 20 eV or by high resolution using Agilent Technologies LC/MCD TOF equipment with ESI and APCI ionization sources.
Crystallographic data were collected in Nonius Kappa CCD equipment with an area detector using monochromatic molybdenum Kα radiation (0.71073 Å), and intensities were measured using scans in φ and ω. The structures were solved using direct methods with SHELX-97, Sir 2002 and 2004. The refinement of all structures (F2 based on all data) was done with the least-squares technique of the complete matrix with Crystals-1287d-2009. All atoms except hydrogen were refined anisotropically.
2.2. Synthesis of 1,3,5-Triazinanes (1a-1d)
2.2.1. Synthesis of 2,2′,2″-(1,3,5-Triazinane-1,3,5-triyl)tris(N-methylethylenamine) (1a)
In a round-bottom flask provided with a magnetic stirrer, N-methylethylenediamine (5 mL, 53.9 mmol) was dissolved in tetrahydrofuran (50 mL), then placed at –78 °C in a dry ice–acetone bath, and aqueous formaldehyde (4.4 mL, 59.3 mmol) previously cooled in an ice bath was slowly added. The reaction mixture was stirred for 30 min at 5 °C and 24 h at room temperature, then tetrahydrofuran was evaporated. The reaction mixture was solubilized with methylene chloride, purified with activated carbon, and dried with sodium sulphate. After filtering and evaporating the solvent, the mixture of compounds 1a and 2a was obtained as a colourless liquid in a ratio of 40:60, respectively. Product 1a was identified by comparison with the NMR data of pure compound 2a obtained later. Compound 1a: 1H NMR (400 MHz, CDCl3) δ: 3.29 (s, 6H, H2, H4, H6), 2.96 (t, 6H, J = 7.0 Hz, H8), 2.50 (t, 6H, J = 7.0 Hz, H7), 3.40 (s br, 3H, H9), 2.25 (s, 9H, H10). 13C NMR (101 MHz, CDCl3) δ: 72.3 (C2, C4, C6), 54.2 (C7), 45.7 (C8), 39.5 (C10). 15N NMR (41 MHz, CDCl3) δ: −327.1 (N9), −340.0 (N1, N3, N5).
2.2.2. Synthesis of 2,2′,2″-(1,3,5-Triazinane-1,3,5-triyl)tris(N-ethylethylenamine) (1b)
Compound 1b was obtained in the same manner as compound 1a from N-ethylethylenediamine (5 mL, 46.5 mmol) and aqueous formaldehyde (3.8 mL, 51.2 mmol). The mixture of compounds 1b and 2b was obtained as a slightly yellow liquid in a ratio of 45:55, respectively. Product 1b was identified by comparison with the NMR data of pure compound 2b obtained later. Compound 1b: 1H NMR (400 MHz, CDCl3) δ: 3.24 (s, 6H, H2, H4, H6), 2.95 (s br, 3H, H9), 2.86 (t, 6H, J = 7.0 Hz, H8), 2.42 (t, 6H, J = 7.0 Hz, H7), 2.31 (q, 6H, J = 7.3 Hz, H10), 0.92 (t, 9H, J = 7.3 Hz, H11). (101 MHz, CDCl3) δ: 70.3 (C2, C4, C6), 51.9 (C7), 47.5 (C10), 45.2 (C8), 14.1 (C11). 15N NMR (41 MHz, CDCl3) δ: −326.1 (N9), −341.2 (N1, N3, N5).
2.2.3. Synthesis of 2,2′,2″-(1,3,5-Triazinane-1,3,5-triyl)tris(N-benzylethylenmine) (1c)
Compound 1c was obtained in the same manner as compound 1a from N-benzylethylenediamine (5 mL, 32.3 mmol) and aqueous formaldehyde (2.7 mL, 35.5 mmol). The mixture of compounds 1c and 2c was obtained as a colourless liquid in a ratio of 50:50, respectively. Product 1c was identified by comparison with the NMR data of pure compound 2c obtained later. Compound 1c: 1H NMR (400 Mhz, CDCl3) δ: 7.35–7.15 (m, 15H, Har), 3.59 (s, 6H, H10), 3.42 (s, 6H, H2, H4, H6), 3.02 (t, 6H, J = 7.1 Hz, H8), 2.62 (t, 6H, J = 7.1 Hz, H7), 2.60 (s br, 3H, H9). 13C NMR (101 MHz, CDCl3) δ: 139.0 (Ci), 128.7 (Co), 128.5 (Cm), 127.2 (Cp), 70.7 (C2, C4, C6), 58.0 (C10), 52.3 (C7), 45.4 (C8). 15N NMR (41 MHz, CDCl3) δ: −318.8 (N9), −340.9 (N1, N3, N5).
2.2.4. Synthesis of 2,2′,2″-(1,3,5-Triazinane-1,3,5-triyl)tris(N,N-dimethylethylenamine) (1d)
Compound 1d was obtained in the same manner as compound 1a of N,N-dimethylethylenediamine (5 mL, 43.5 mmol) and aqueous formaldehyde (3.6 mL, 35.5 mmol). Compound 1d was obtained pure as a slightly yellow liquid (3.2 g, 73%). Compound 1d: 1H NMR (400 Mhz, CDCl3) δ: 3.12 (s, 6H, H2, H4, H6), 2.29 (t, 6H, J = 6.8 Hz, H7), 2.12 (t, 6H, J = 6.8 Hz, H8), 1.96 (s, 18H, H10). 13C NMR (101 MHz, CDCl3) δ: 74.7 (C2, C4, C6), 57.5 (C8), 50.4 (C7), 45.6 (C10). 15N NMR (41 MHz, CDCl3) δ: −355.6 (N9), −334.1 (N1, N3, N5). LRMS (EI, 20 eV), m/z (%): 301 (2), 242 (5), 201 (9), 158 (8), 142 (38), 130 (27), 101 (68), 72 (22), 58 (100), 42 (12); HRMS (ESI+) m/z calc. for (M+H)+: 301.3080, found: 301.3074. EA calc. for C15H36N6 ½CH2Cl2: C, 54.28; H, 10.87; N, 24.50. found: C, 54.01; H, 10.86; N, 25.01.
2.3. Synthesis of Bis(3-alkyl-imidazolidin-1-yl)methane (2a-2d)
2.3.1. Obtaining Bis(3-methylimidazolidin-1-yl)methane (2a)
In a round-bottom flask provided with a magnetic stirrer, N-methylethylenediamine (5 mL, 53.9 mmol) was dissolved in tetrahydrofuran (50 mL), then aqueous formaldehyde was added slowly (4.4 mL, 59.3 mmol). The reaction mixture was stirred for 10 min at 5 °C in a water ice bath and maintained at reflux for 6 h in tetrahydrofuran. The solvent was evaporated, and the reaction mixture was solubilized with methylene chloride, purified with activated carbon and dried with sodium sulphate. After filtering and evaporating the solvent, pure compound 2a was obtained as a colourless liquid (4.6 g, 92%). 1H NMR (400 Mhz, CDCl3) δ: 3.26 (s, 4H, H2), 3.23 (s, 2H, H6), 2.75 (t, 4H, J = 6.7 Hz, H5), 2.55 (t, 4H, J = 6.7 Hz, H4), 2.19 (s, 6H, H7). 13C NMR (101 MHz, CDCl3) δ: 77.0 (C6), 76.6 (C2), 54.1 (C4), 51.0 (C5), 40.6 (C7). 15N NMR (41 MHz, CDCl3) δ: −326.9 (N1), −340.7 (N3). LRMS (EI, 20 eV), m/z (%): 183 (2), 167 (2), 156 (3), 142 (2), 126 (2), 113 (9), 99 (31), 85 (45), 72 (22), 58 (54), 44 (100). EA calc. for C9H20N4 ⅓CH2Cl2: C, 52.73; H, 9.80; N, 26.35. found: C, 52.78; H, 10.09; N, 26.47.
2.3.2. Obtaining Bis(3-ethylimidazolidin-1-yl)methane (2b)
Compound 2b was obtained following the same procedure as compound 2a from N-ethylethylenediamine (5 mL, 46.5 mmol) and aqueous formaldehyde (3.8 mL, 51.2 mmol). After filtering and evaporating the solvent, pure compound 2b was obtained as a colourless liquid (4.5 g, 91%). 1H NMR (400 Mhz, CDCl3) δ: 3.09 (s, 4H, H2), 3.01 (s, 2H, H6), 2.53 (t, 4H, J = 6.6 Hz, H5), 2.36 (t, 4H, J = 6.6 Hz, H4), 2.14 (q, 4H, J = 7.2 Hz, H7), 0.73 (t, 6H, J = 7.2 Hz, H8). 13C NMR (101 MHz, CDCl3) δ: 76.4 (C6), 74.6 (C2), 51.7 (C4), 50.3 (C5), 48.5 (C7), 13.7 (C8). 15N NMR (41 MHz, CDCl3) δ: −327.2 (N1), −328.8 (N3). LRMS (EI, 20 eV), m/z (%): 211 (16), 197 (1), 154 (1), 127 (2), 113 (100), 99 (7), 84 (3), 72 (8), 58 (2), 56 (3), 42 (2). EA calc. C11H24N4 ⅑CH2Cl2: C, 60.18; H, 11.01; N, 25.26. found: C, 60.51; H, 10.86; N, 24.86.
2.3.3. Obtaining Bis(3-benzylimidazolidin-1-yl)methane (2c)
Compound 2c was obtained following the same procedure as compound 2a from N-benzylethylenediamine (5 mL, 32.3 mmol) and aqueous formaldehyde (2.7 mL, 35.5 mmol). After filtering and evaporating the solvent, pure compound 2c was obtained as a colourless liquid (4.8 g, 88%). 1H NMR (400 Mhz, CDCl3) δ: 7.35–7.15 (m, 15H, Har), 3.60 (s, 4H, H7), 3.42 (s, 4H, H2), 3.33 (s, 2H, H6), 2.86 (t, 4H, J = 7.2 Hz, H5), 2.70 (t, 4H, J = 7.2 Hz, H4). 13C NMR (101 MHz, CDCl3) δ: 139.1 (Ci), 128.7 (Co), 128.4 (Cm), 127.1 (Cp), 76.7 (C6), 75.2 (C2), 59.1 (C7), 52.3 (C4), 50.7 (C5). 15N NMR (41 MHz, CDCl3) δ: −327.6 (N1), −327.9 (N3). LRMS (EI, 20 eV), m/z (%): 335 (3), 292 (2), 251 (2), 220 (13), 205 (42), 175 (91), 161 (54), 146 (6), 132 (16), 119 (10), 99 (16), 91 (100), 83 (37), 72 (14), 57 (18), 42 (37). EA calc. C21H28N4: C (74.96), H (8.39), N (16.65). found: C (74.68), H (8.30), N (16.73).
2.3.4. Obtaining Bis(3-phenylimidazolidin-1-yl)methane (2d)
Compound 2d was obtained following the same procedure as compound 2a from N-phenylethylenediamine (5 mL, 37.6 mmol) and aqueous formaldehyde (3.1 mL, 41.4 mmol). After filtering and evaporating the solvent, pure compound 2d was obtained as a white solid (4.8 g, 82%, Mp 142 °C). 1H NMR (400 Mhz, CDCl3) δ: 7.25 (dd, 4H, JHm-Hp = 7.3 Hz, JHm-Ho = 7.8 Hz, Hm), 6.72 (t, 2H, JHp-Hm = 7.3 Hz, Hp), 6.54 (d, 4H, JHo-Hm = 7.8 Hz, Ho), 4.17 (s, 4H, H2), 3.47 (s, 2H, H6), 3.42 (t, 4H, J = 6.6 Hz, H4), 3.13 (t, 4H, J = 6.6 Hz, H5). 13C NMR (101 MHz, CDCl3) δ: 146.7 (Ci), 129.4 (Cm), 116.5 (Cp), 111.8 (Co), 74.5 (C6), 69.1 (C2), 51.0 (C5), 45.8 (C4). 15N NMR (41 MHz, CDCl3) δ: −325.7 (N1), −309.4 (N3). LRMS (EI, 20 eV), m/z (%): 308 (1), 176 (1), 161 (100), 147 (47), 120 (5), 106 (48), 77 (6), 56 (20), 42 (5). EA calc. for C19H24N4 ⅟16CH2Cl2: C, 72.98; H, 7.75; N, 17.86. found: C, 72.77; H, 7.37; N, 17.51.
2.4. Synthesis of 1,3,5-Tribenzyl-1,3,5-triazinane (3)
Compound 3 is obtained from N-benzylamine (10 mL, 89.8 mmol) dissolved in distilled water (50 mL) and tetrahydrofuran (25 mL). The reaction mixture was placed in a water ice bath, and aqueous formaldehyde (7.4 mL, 98.8 mmol) was slowly added. The reaction was kept under stirring and after 24 h at room temperature the solvent was evaporated. Compound 3 was extracted with methylene chloride and dried with anhydrous sodium sulphate (10.5 g, 98%, Mp 50 °C). 1H NMR (400 Mhz, CDCl3) δ: 7.45–7.25 (m, 15H, Har), 3.76 (s, 6H, H7), 3.52 (s br, 6H, H2, H4, H6). 13C NMR (101 MHz, CDCl3) δ: 138.7 (Ci), 129.1 (Co), 128.4 (Cm), 127.2 (Cp), 74.0 (C2, C4, C6), 57.2 (C7). 15N NMR (41 MHz, CDCl3) δ: −329.7 (N1, N3, N5). LRMS (EI, 20 eV), m/z (%): 357 (10), 238 (14), 133 (19), 120 (72), 106 (12), 91 (100), 65 (6), 42 (9); HRMS (ESI+) m/z calc. for (M + Na)+: 380.2103, found: 380.2097. EA calc. for C24H27N3: C, 80.63; H, 7.61; N, 11.75. found: C, 80.28; H 7.42; N 11.45.
2.5. Synthesis of 3,5-Dibenzyl-1,3,5-thiadiazinane (4)
In a round-bottom flask provided with a magnetic stirrer, N-benzylamine (5 mL, 44.9 mmol) dissolved in distilled water (50 mL) was placed. The solution was cooled in an ice bath, and a cold solution (5 °C) of sodium hydrosulphide (7.6 g, 134.7 mmol) in aqueous formaldehyde (16.7 mL, 224.5 mmol) was slowly added. The reaction mixture was stirred for 30 min at 5 °C and 24 h at room temperature. Compound 4 was extracted with methylene chloride and dried with anhydrous sodium sulphate. Compound 4 was obtained mixed with compound 5. However, the slow evaporation of methylene chloride allowed the obtaining of colourless crystals of compound 4, which were separated by filtration. (3.0 g, 47%, Mp 96 °C). 1H NMR (400 Mhz, CDCl3) δ: 7.42–7.26 (m, 10H, Har), 4.26 (s br, 4H, H2, H6), 4.13 (s, 4H, H7), 4.08 (s br, 2H, H2). 13C NMR (101 MHz, CDCl3) δ: 138.7 (Ci), 128.9 (Co), 128.6 (Cm), 127.4 (Cp), 73.8 (C4), 57.3 (C2, C6), 56.2 (C7). 15N NMR (41 MHz, CDCl3) δ: −334.0 (N3, N5). MS (EI, 20 eV), m/z (%): 284 (2), 251 (6), 211 (1), 193 (2), 165 (28), 133 (31), 118 (38), 91 (100), 74 (2), 65 (13), 42 (17); HRMS (ESI+) m/z calc. for (M + H)+: 285.1425, found: 285.1419. EA calc. for C17H20N2S: C, 71.79; H, 7.09; N, 9.85. found: C, 71.84; H, 7.42; N, 9.73.
2.6. Synthesis of 5-Benzyl-1,3,5-dithiazinane (5)
Compound 5 was obtained from N-benzylamine (5 mL, 44.9 mmol) dissolved in distilled water (50 mL). The solution was cooled in an ice bath, and a cold solution (5 °C) of 17.6 g of sodium hydrosulphide (314.3 mmol) in aqueous formaldehyde (73.6 mL, 987.8 mmol) was slowly added. The reaction mixture was stirred for 30 min at 5 °C and 24 h at room temperature. Compound 5 was extracted with methylene chloride and dried with anhydrous sodium sulphate (6.6 g, 70%). 1H NMR (400 Mhz, CDCl3) δ: 7.42–7.22 (m, 5H, Har), 4.44 (s br, 4H, H4, H6), 4.24 (s, 2H, H7), 4.14 (s br, 2H, H2). 13C NMR (101 MHz, CDCl3) δ: 137.4 (Ci), 129.4 (Co), 128.7 (Cm), 127.6 (Cp), 58.0 (C4, C6), 53.4 (C7), 34.2 (C2). 15N NMR (41 MHz, CDCl3) δ: −344.8 (N5). LRMS (EI, 20 eV), m/z (%): 211 (13), 165 (15), 133 (45), 120 (10), 118 (30), 91 (100), 65 (12), 42 (72); HRMS m/z calc. for (M+H)+: 212.0568, found: 212.0562. EA calc. for C10H13NS2: C, 56.83; H, 6.20; N, 6.63. found: C, 56.96; H 6.27; N 6.67.
3. Results and Discussion
Condensation reactions between N-alkylethylenediamines, formaldehyde and sodium hydrosulphide in a stoichiometric ratio [1:5:3] led to the formation of N-alkyl-ethylene-dithiazinanes, which were previously reported by our research group [24]. However, by varying stoichiometry, by-products were obtained, such as N-alkylethylenethiadiazinanes, or N-alkylethylenetriazinanes, which made it difficult to isolate pure compounds. Therefore, we decided to explore the condensation reactions between N-alkylethylenediamines and formaldehyde in the absence of sodium hydrosulphide, favouring the formation of sulphur-free heterocycles, such as 1,3,5-triazinanes (1a-1d) and bis(imidazolidinyl)methanes (2a-2d).
3.1. Preparation of 1,3,5-Triazinanes (1a-1d)
To a solution of the N-alkylethylenediamines (R = Me, Et or Bn) in tetrahydrofuran at –78 °C was added dropwise an equivalent of formaldehyde, previously cooled. After twenty-four hours of stirring at room temperature, a mixture of 1,3,5-triazinanes (1a-1c) and bis(imidazolidinyl)methanes (2a-2c) was obtained in proportions of: 1a/2a 40:60, 1b/2b 45:55, and 1c/2c 50:50; see Scheme 1. The 1,3,5-triazinanes (1a-1c) could not be isolated pure by any of the known separation techniques, so they were only identified by NMR. However, reactions to tetrahydrofuran reflux for six hours and subsequent distillation led exclusively to the formation of bis(imidazolidinyl)methanes (2a-2c); see Section 3.2.
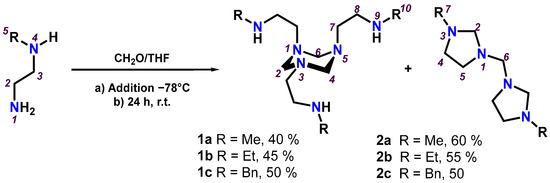
Scheme 1.
Preparation of 1,3,5-triazinanes (1a-1c) and bis(imidazolidinyl)methanes (2a-2c).
Under the same reaction conditions, the N,N-dimethylethylenediamine produced exclusively the 1,3,5-triazinane (1d), while N-phenylethylenediamine gave the bis(imidazolidinyl)methane (2d); see Scheme 2. The 1,3,5-triazinanes (1a-1c) discussed in this section had not previously been reported in the literature, except compound 1d, which had already been described under different reaction conditions [25].
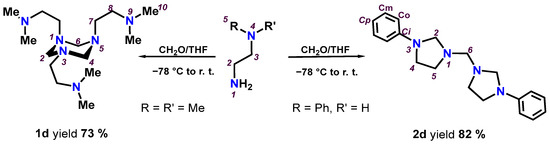
Scheme 2.
Preparation of 1d and 2d.
3.2. Preparation of Bis(imidazolidinyl)methanes (2a-2d)
Attempts to purify 1,3,5-triazinanes (1a-1c) by vacuum distillation (0.5 mmHg, +20 °C to +90 °C) showed that heating the reaction mixture transforms them into bis(imidazolidinyl)methanes (2a-2c); see Scheme 3.
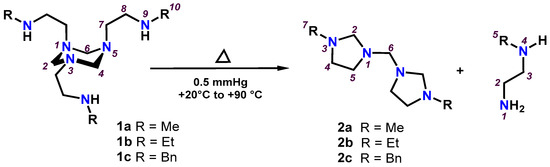
Scheme 3.
Preparation of the bis(imidazolidinyl)methanes (2a-2c) by heating the 1,3,5-triazinanes (1a-1c).
Bis(imidazolidinyl)methanes (2a-2d) are very stable compounds and were synthesized directly with good yields, from the equimolar reaction between formaldehyde and the corresponding N-alkylethylenediamine. The addition was made at room temperature, and the reaction mixture was subsequently maintained at reflux in tetrahydrofuran for six hours; see Scheme 4. The bis(imidazolidinyl)methanes (2a-2d) were obtained in good yields and are colourless liquids, soluble in polar organic solvents, such as tetrahydrofuran, dimethyl sulfoxide, chloroform, methylene chloride, methanol, ethanol, etc. The bis(imidazolidinyl)methanes 2a [5,8,9], 2c [5] and 2d [2,3,4,5,6] discussed in this section had previously been reported in the literature under different reaction conditions, except compound 2b, which had never been synthesized and characterized.
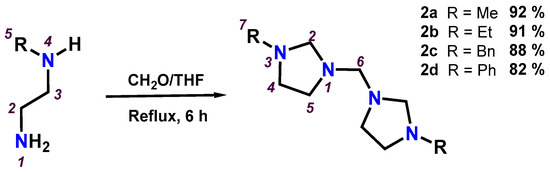
Scheme 4.
Preparation of bis(imidazolidinyl)methanes (2a-2d) by refluxing in tetrahydrofuran.
3.3. NMR Reaction Evaluation
The results in Section 3.1 showed that the reaction products depend on the amine structure, the reaction conditions and the stoichiometry. Therefore, to have more information about the evolution of different reactions, the equimolar reaction between N-methylethylenediamine and formaldehyde was monitored by 13C and 1H NMR in tubes at different acquisition temperatures; see Scheme 5. The experimental strategy was to evaluate the reaction in 1:1 stoichiometry using two solvents with a wide range between their boiling (Bp) and melting points (Mp). Tetrahydrofuran-d8 allowed the temperature to be lowered to −78 °C and dimethylsulfoxide-d6 allowed the temperature to be raised to 120 °C. Therefore, the experiments were carried out at −78, 25, 65 °C (for tetrahydrofuran-d8) and 5, 25, 120 °C (for dimethylsulfoxide-d6). The reaction times used were 0.1, 1, 6 and 24 h. In addition, the stoichiometric ratios 1:2 and 2:1 were analysed in order to verify their influence on the reaction. The intensity and integration of the signals assigned by NMR to the [N-CH2-N] fragment and to the N-CH3 group were used in the quantification of the ratio of compounds a, 1a and 2a. In an NMR tube at −78 °C, the spectra acquired immediately after mixing the reagents in tetrahydrofuran-d8 showed three compounds: imidazolidine (a, 42%), 1,3,5-triazinane (1a, 21%) and bis(imidazolidinyl)methane (2a, 37%). The spectra recorded after six hours of reaction at room temperature showed an increase in 1,3,5-triazinane (1a, 43%) and a decrease in imidazolidine (a, 21%) and bis(imidazolidinyl)methane (2a, 36%). When the reaction was monitored in dimethylsulfoxide-d6, initially at 5 °C and later at room temperature, results similar to those found with tetrahydrofuran-d8 were observed. Additionally, with this solvent the NMR tube was heated for one hour at 120 °C, changing the ratio between the products 1,3,5-triazinane (1a, 26%) and bis(imidazolidinyl)methane (2a, 74%); see Table 1.
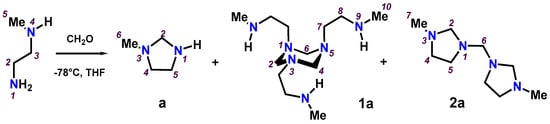
Scheme 5.
Condensation products of N-methyl-ethylenediamine and formaldehyde.

Table 1.
Proportion of the products obtained from the reaction between N-methylethylenediamine and formaldehyde by NMR.
When the reaction was carried out in dimethylsulfoxide-d6 in a 2:1 ratio between N-methylethylenediamine and formaldehyde, the spectra acquired a few minutes after mixing the reagents showed: imidazolidine (71%), 1,3,5-triazinane (1a, 17%) and bis(imidazolidinyl)methane (2a, 12%). The spectra of the solution after heating for three hours at 120 °C have a mixture of 1,3,5-triazinane (1a) and the free diamine. The spectra of the reaction carried out in dimethylsulfoxide-d6 in a 1:2 stoichiometric ratios between N-methylethylenediamine and formaldehyde showed the signals corresponding to bis(imidazolidinyl)methane (2a) as a majority product. The 1,3,5-triazinane (1a) and imidazolidine were observed in small amounts, even when the reaction was heated at 120 °C for two hours; see Table 1. From the results obtained, we can determine that in excess of formaldehyde, the main product is bis(imidazolidinyl)methane (2a), while in excess of N-methylethylenediamine, 1,3,5-triazinane (1a) is the predominant compound. It is interesting to note that imidazolidine is precursor to 1,3,5-triazinane (1a).
The previous results allow us to discuss the preliminary thermodynamic and kinetic stability of the products obtained from the equimolar reaction between N-methylethylenediamine and formaldehyde. The kinetically controlled product is 1,3,5-triazinane (1a), since it is the least stable product and is obtained by lowering the temperature in short reaction times. Additionally, the thermodynamically controlled product is bis(imidazolidinyl)methane (2a), since it is the most stable product and is obtained by increasing the temperature in long reaction times. That is, 1,3,5-triazinane (1a) is formed faster than bis(imidazolidinyl)methane (2a) because the activation energy for bis(imidazolidinyl)methane (2a) is lower than for bis(imidazolidinyl)methane (2a), even though bis(imidazolidinyl)methane (2a) is more stable. However, to confirm these assertions, a deeper study is necessary, gradually modifying the reaction conditions for a better comparison.
3.4. Spectroscopic Characterization of 1,3,5-Triazinanes (1a-1d)
The 13C NMR spectra confirmed the formation of 1,3,5-triazinanes, since the signal corresponding to the equivalent carbons (C2, C4 and C6) showed a characteristic displacement for this type of derivative at approximately 70 ppm. The C8 of compound 1d showed a different shift from that of 1,3,5-triazinanes (1a-1c), since it has a tertiary amine attached in that position. Table 2 summarizes the chemical shifts of 1H and 13C NMR (CDCl3, 25 °C) of 1,3,5-triazinanes (1a-1d); the protons of the heterocycle are characterized by a broad signal for equivalent hydrogens (H2, H4 and H6) that integrate for six protons. The unequivocal assignment of H7 and H8, as well as its correlation with 13C, was completed by experiments in two dimensions: COSY, NOESY and HETCOR.

Table 2.
NMR data of 13C and 1H (δ, J, CDCl3, 25 °C) for 1,3,5-triazinanes (1a-1d).
The 15N NMR spectroscopic data (CDCl3, 25 °C) showed the signal corresponding to the equivalent nitrogen atoms (N1, N3 and N5) at approximately −340 ppm. This displacement is characteristic for heterocyclic nitrogen at 1,3,5-triazinanes, as reported in the literature for similar compounds [26,27,28]. Table 3 compares the 15N NMR chemical shifts of 1,3,5-triazinanes (1a-1d) and the N-alkylethylenediamines (a-d) from which they come and shows that the primary amine (N1) in the N-alkylethylenediamine about 22 ppm was deprotected by forming the heterocycle and becoming a tertiary amine (N1, N3 and N5) in the new 1,3,5-triazinanes (1a-1d). The change in the nitrogen displacement of the secondary amine of the N-alkylethylenediamines (N4), by forming 1,3,5-triazinanes (N9), could be explained by the intramolecular hydrogen bridge N•••H-N that forms exocyclic N-H with endocyclic nitrogen in these compounds (1a-1c). This hydrogen bond cannot be formed in derivative 1d, so the shift does not change significantly with respect to that of the initial amine.

Table 3.
NMR data of 15N (δ, CDCl3, 25 °C) for 1,3,5-triazinanes (1a-1d) and their N-alkylethylenediamines (a-d).
3.5. Spectroscopic Characterization of Bis(imidazolidinyl)methanes (2a-2d)
The 13C NMR spectra showed the characteristic signals around 70 ppm for C2 and C6. The C2 integrates for two carbons and the C6 for one. Table 4 summarizes the chemical shifts of 13C and 1H (CDCl3, 25 °C) of bis(imidazolidinyl)methanes (2a-2d); protons H2 and H6 are characterized by being simple signals that integrate for four and two protons, respectively. The unambiguous allocation of H4 and H5, as well as the correlation between carbon and hydrogen, was completed by experiments in two dimensions: COSY, NOESY, HETCOR and COLOC. The coupling constants (3JH4-H5) and dihedral angles from the Karplus curve of compounds (2a-2d) confirm that the nitrogen is in the syn position: 2a (3JH4-H5 = 6.7) 34° and 137°, 2b (3JH4-H5 = 6.6) 34° and 136°, 2c (3JH4-H5 = 7.2) 30° and 140°, and 2d (3JH4-H5 = 6.6 Hz) 34° and 136°; see Figure 1.

Table 4.
NMR data of 13C and 1H (δ, J, CDCl3, 25 °C) for bis(imidizalodinyl)methanes (2a-2d).
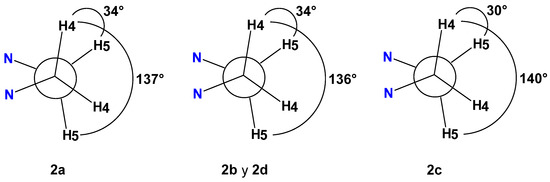
Figure 1.
Newman projections for compounds (2a-2d) showing dihedral angles according to coupling constants JH-H.
The 15N NMR spectroscopic data (CDCl3, 25 °C) showed a signal around −326 ppm, which corresponds to the bridging carbon between the two five-membered heterocycles, and their value is characteristic for nitrogen atoms in similar compounds reported in the literature [26,27,28]. Table 5 compares the 15N NMR chemical shifts of bis(imidazolidinyl)methanes (2a-2d) and the N-alkylethylenediamines (a-d) from which they come. It is observed that in the N-alkylethylenediamines, the nitrogen of the N1 and N4 positions was deprotected around 35 and 20 ppm, respectively, when forming the N1 and N3 positions of the bis(imidazolidinyl)methanes, both becoming tertiary amines.

Table 5.
NMR data of 15N (δ, CDCl3, 25 °C) for bis(imidizalodinyl)methanes (2a-2d) and their N-alkylethylenediamines (a-d).
3.6. Spectrometric Characterization of Bis(imidazolidinyl)methanes (2a-2d)
These derivatives were studied by electronic impact mass spectrometry at 20 eV. This technique allowed us to observe odd molecular ions in molecules with an even number of nitrogen, m/z(%): 183(2) (2a), 191(4) (2b), 335(4) (2c) and 307(1) (2d). The four compounds have several possibilities of rupture. The bis(imidazolidyl)methanes (2a-2d) had the same fragmentation pattern, so that the compound 2a is analysed by way of example. The molecular weight of the compound is 184 g/mol, but it has an even number of nitrogen, so the molecular ion found is 183 and is observed with a 2% abundance; the base peak 44(100) corresponds to the fragment [(Me)2N]+; see Figure 2.
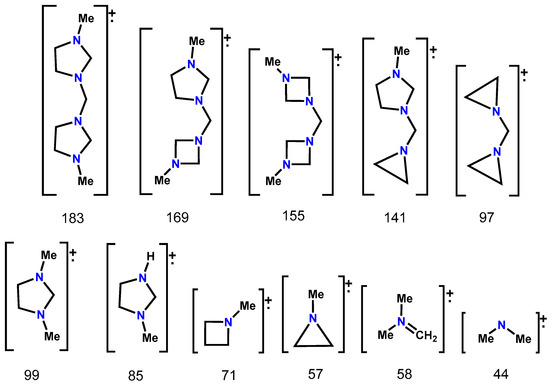
Figure 2.
Fragments observed in the ionization process of compound 2a by electronic impact mass spectrometry.
These derivatives were also studied by high-resolution mass spectrometry (MS-TOF), using electrospray ionization (ESI). Although this technique did not allow us to observe the characteristic molecular ions (M + H)+, despite the mild ionization and high sensitivity, this offered us valuable information about the stability of the methylene bridge in C6, which fragments easily during ionization. Fracture of the C-N bond in C6 produces characteristic fragments in bis(imidazolidyl)methanes (2a-2d). Compounds 2a and 2b, after breaking the bond, react with the methanol in the medium and form fragments at 131.1197 and 145.1382, respectively; see Figure 3.
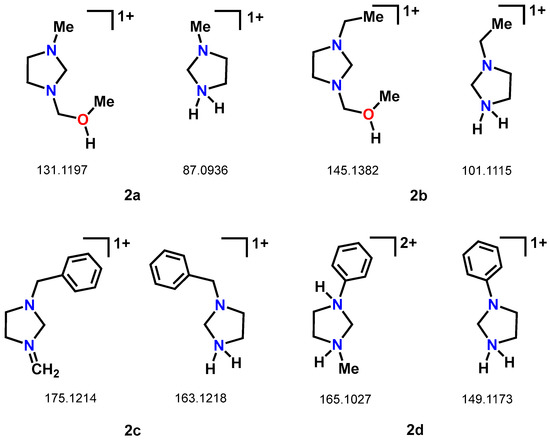
Figure 3.
Fragments observed in the ionization process of compounds (2a-2d) by electrospray (ESI).
3.7. Versatility of Formaldehyde in the Synthesis of Six-Member Heterocycles
In order to explore the versatility of formaldehyde in the synthesis of other six-membered heterocycles, the reactions of N-benzyl-amine in the presence of formaldehyde and sodium hydrosulphide in different proportions were explored. The equimolar reaction between N-benzylamine and formaldehyde led exclusively to the formation of N-benzyl-triazinane (3, 98% yield), which has already been previously reported by other research groups under different reaction conditions [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. The reaction of one equivalent of N-benzylamine with five of formaldehyde and three of sodium hydrosulphide was explored, obtaining a mixture of products in 50:50 ratios: N-benzyl-thiadiazinane (4) and N-benzyl-dithiazinane (5). Compound 4 is a colourless crystalline solid while compound 5 is a yellow liquid, so they were easily separated by filtration. The N-benzyl-dithiazinane was also obtained pure (5, 70% yield) of the condensation of one equivalent of N-benzylamine with twenty-two of formaldehyde and seven of sodium hydrosulphide; this compound had already been reported by other authors [45,46,47]; see Scheme 6.
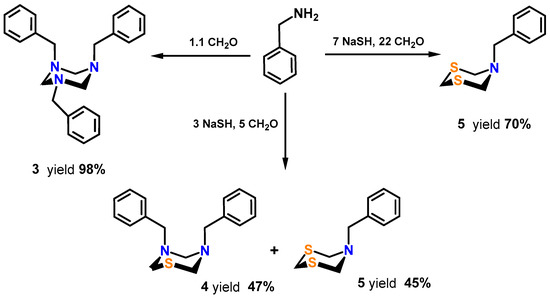
Scheme 6.
Preparation of triazinane, thiadiazinane and dithiazinane by condensation.
3.8. Crystallographic Characterization of N-Benzyl-triazinane (3) and N-Benzyl-thiadiazinane (4)
The N-benzyl-triazinane 3 is a crystalline solid that was obtained by slow evaporation of the solution obtained from the equimolar reaction between formaldehyde and N-benzylamine. The crystals were studied by DRX (monoclinic P 21/n). Crystallographic data and structure refinement are summarized in Table S1. The lengths and angles of the bonds are presented in Table S2. In compound 3, the heterocycle has a chair conformation; in addition, according to the NMR data obtained in solution [13C 57.2 ppm (C7), 1H 3.76 (s) (H7), benzyl carbon], the three substituents would be expected to be in equilibrium between the axial and equatorial positions. However, in the solid state it is observed that the first substituent is placed in an equatorial position, the second in an axial position, and the third is in equilibrium between both positions. For this reason, the disorder of the third benzyl substituent was modelled; see Figure 4. The nitrogen atoms N1 and N5 have a trigonal pyramidal geometry: C2-N1-C7 115.1(4)°, C2-N1-C6 102.9(5)°, C6-N1-C7 110.7(4)° [Σangles for N1(equatorial) = 328.7°, 100% sp3 character], C4-N5-C6 110.9(4)°, C6-N5-C21 115.0(5)°, C4-N5-C21 116.3(4)° [Σangles for N5(axial) = 342.2°, 49.7% sp3 character]. The percentage sp3 hybridization of N3 atoms was not determined due to the disorder of the benzyl group at that position. The conformational behaviour in the solid state and in solution of some other triazinanes analogous to compound 3 has been widely discussed by our working group [48,49,50,51].
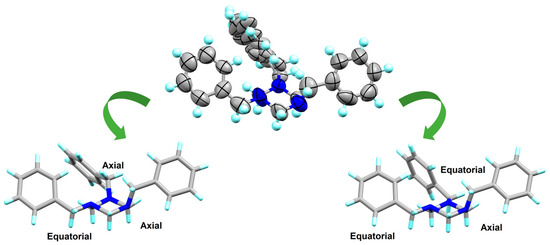
Figure 4.
ORTEP representation of compound 3 showing the location of the three benzyl substituents.
In the same way, the N-benzyl-thiadiazinane 4 is a crystalline solid that was obtained by slow evaporation of the solution containing it, mixed with compound 5. The crystals were studied by single-crystal X-ray diffraction (orthorhombic P 212121). Crystallographic data and structure refinement are summarized in Table S3. The lengths and angles of the bonds are presented in Table S4. Compound 4 is in a chair conformation; it would be expected that, according to the data obtained from NMR in solution [13C 56.2 ppm (C7), 1H 4.13 (s) (H7), benzyl carbon], one substituent will be placed in axial position and the other in the equatorial position. However, in the solid state, the two substituents were placed in axial position; see Figure 5. The nitrogen atoms N3 and N5 have a trigonal pyramidal geometry: C4-N3-C8 115.4(4)°, C2-N3-C8 113.4(3)°, C2-N3-C4 111.3(4)° [Σangles for N3 = 340.1°, 49.5% sp3 character], C4-N5-C6 112.0(4)°, C4-N5-C7 116.9(4)°, C6-N5-C7 113.5(3)° [Σangles for N5 = 342.4°, 49.7% sp3 character]. The bond angles around sulphur are C2-S1-C6 93.3(3)°, which indicates only 86.3% sp2 hybridization, leaving the free electron pairs in one sp2 and other p pure orbitals. Two hydrogen atoms in the benzyl groups have a shorter distance than the sum of the van der Waals radii (ΣrvdW [H•••H] = 2.40 Å). The above indicates that the steric interaction in axial is of lower energy than the electronic repulsion between the free electron pairs of the nitrogen and sulphur atoms when the substituents are equatorial. This behaviour is similar to that found for other thiadiazinanes reported by our work group [48,49,50,51]. Additionally, a polymorphic structure of compound 4 is reported, which was obtained by slow evaporation of the crude reaction. The structure crystallized in a monoclinic space group, P 21. Crystallographic data and structure refinement are summarized in Table S5. The bond lengths and angles are presented in Table S6. Both polymorphs showed intra- and intermolecular S•••H interactions smaller than the sum of van der Waals radii (ΣrvdW [S•••H] = 3.00 Å).
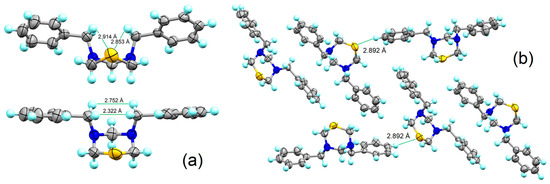
Figure 5.
(a) ORTEP representation and intramolecular interactions (H•••H and S•••H) of compound 4 (orthorhombic P 212121). (b) Intermolecular interactions (S•••H) of compound 4 (monoclinic P 21).
4. Conclusions
Formaldehyde, a basic but highly reactive compound, is widely used in the chemical industry due to its versatility, low cost, and easy production. This compound is also known as methanal and is mainly used in the production of phenolic and amino resins, acetal resins, polyhydric alcohols, fertilizers, and paraformaldehyde. Furthermore, this compound reacts with various organic derivatives including amines, forming heterocycles with one or more C-N bonds. Therefore, in this work, the reactivity of formaldehyde against N-alkylethylenediamines was explored using different reaction conditions, such as variations in reaction time and temperature, and modifications in the stoichiometric ratio, among others. Under mild conditions (addition at −78 °C and stirring for 24 h), mixtures were obtained between the products bis(imidazolidinyl)methanes (2a-2c) and 1,3,5-triazinanes (1a-1c), when the alkyl substituents were methyl, ethyl or benzyl. However, when the substituent was phenyl, the reaction led exclusively to bis(imidazolidinyl)methane 2d. When more severe conditions were used (addition at room temperature and reflux for 6 h) it exclusively led to the formation in good yields of the bis(imidazolidinyl)methanes (2a-2d). In the case of N,N-dimethylethylenediamine, when it reacts with formaldehyde it exclusively leads to the formation of 1,3,5-triazinane 1d under both mild and severe reaction conditions. To confirm the versatility of formaldehyde, its reaction with N-benzylamine was explored in the absence and presence of sodium hydrosulphide in different stoichiometric ratios. The manipulation of these three reagents led to the obtaining of three 1,3,5-heterocyclohexanes (triazinane, thiadiazinane and dithiazinane) with an interesting conformational behaviour.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org4020024/s1, Table S1: Crystallographic data for compound 3; Table S2: Selected bond distances (Å) and bond angles (°) for the compound 3; Table S3: Crystallographic data for compound 4 (orthorhombic polymorph); Table S4: Selected bond distances (Å) and bond angles (°) for the compound 4 (orthorhombic polymorph); Table S5: Crystallographic data for compound 4 (monoclinic polymorph); Table S6: Selected bond distances (Å) and bond angles (°) for the compound 3 (monoclinic polymorph). Crystallographic data have been deposited at the Cambridge Crystallographic Data Center as numbers (CCDC): 2251551–2251553 for compound 3 and 4. Copies can be obtained, free of charge, on applications to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [fax: +44-(0)1223-336033 or https://www.ccdc.cam.ac.uk/structures/ (accessed on 24 March 2023).
Author Contributions
R.C.-P. investigation and writing the paper; S.A.S.-R. performed the experiments; A.F.-P. conceptualization, formal analysis, supervision, writing and editing review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CINVESTAV-IPN and CONACyT, grant number 195554.
Data Availability Statement
The data reported in this paper will be available on request.
Acknowledgments
We greatly appreciate the academic support of our institutions: CINVESTAV-IPN and FCQ-UV. We also appreciate the technical support of Marco A. Leyva-Ramírez, Víctor M. González-Díaz and Ma. Luisa Rodríguez-Pérez.
Conflicts of Interest
The authors declare no conflict of interest.
References
- da Silva Junior, A.A.; da Rocha Pitta, M.G.; de Oliveira Chagas, M.B.; Barreto de Melo Rêgo, M.J.; da Rosa, M.M.; da Rocha Pitta, M.G. Imidazolidine derivatives in cancer research: What is known? Anticancer. Agents Med. Chem. 2022, 22, 1272–1277. [Google Scholar] [CrossRef]
- Swain, S.P.; Mohanty, S. Imidazolidinones and imidazolidine-2,4-diones as antiviral agents. ChemMedChem 2019, 14, 291–302. [Google Scholar] [CrossRef]
- Perillo, I.; Repetto, E.; Caterina, M.C.; Massa, R.; Gutkind, G.; Salerno, A. Synthesis, spectroscopic and biological properties of bis(3-arylimidazolidinyl-1)methanes. A novel family of antimicrobial agents. Eur. J. Med. Chem. 2005, 40, 811–815. [Google Scholar] [CrossRef]
- Caterina, M.C.; Perillo, I.A.; Boiani, L.; Pezaroglo, H.; Cerecetto, H.; Gonzalez, M.; Salerno, A. Imidazolidines as new anti-Trypanosoma cruzi agents: Biological evaluation and structure-activity relationships. Bioorganic Med. Chem. 2008, 16, 2226–2234. [Google Scholar] [CrossRef]
- Farzaliev, V.M.; Babaeva, G.B.; Abbasova, M.T.; Nabiev, O.G.; Soltanova, Z.K.; Kerimova, Y.M. Derivatives of N-alkyl(aryl)-1,2(1,3)-diazacycloalkanes. Antimicrobial properties. Chem. Technol. Fuels Oil 2009, 45, 98–102. [Google Scholar] [CrossRef]
- Guerra, A.; Gonzalez-Naranjo, P.; Campillo, N.E.; Cerecetto, H.; Gonzalez, M.; Paez, J.A. Artificial neural networks based on CODES descriptors in pharmacology: Identification of novel trypanocidal drugs against Chagas disease. Curr. Comput.-Aided Drug Des. 2013, 9, 130–140. [Google Scholar] [CrossRef]
- Caterina, M.C.; Perillo, I.A.; Villalonga, X.; Amiano, N.; Payés, C.; Sanchez, M.L.; Salerno, A. New green synthesis and antineoplastic activity of bis(3-arylimidazolidinyl-1)methane. Open J. Med. Chem. 2013, 3, 121–127. [Google Scholar] [CrossRef]
- Kamps, I.; Mix, A.; Berger, R.J.F.; Neumann, B.; Stammler, H.-G.; Mitzel, N.W. Two diamino-substituted lithiocarbanions in one molecule. Chem. Commun. 2009, 5558–5560. [Google Scholar] [CrossRef] [PubMed]
- Salas-Coronado, R.; Galvez-Ruiz, J.C.; Jaen-Gaspar, J.G.; Noth, H.; Flores-Parra, A. 3-(1,3-Heterazolidin-3-yl-methyl)-1,3-oxazolidines and their reduction with borane-THF. J. Mol. Struct. THEOCHEM 2003, 640, 95–108. [Google Scholar] [CrossRef]
- Reis, M.I.P.; Romeiro, G.A.; Damasceno, R.; da Silva, F.d.C.; Ferreira, V.F. Synthesis and applications of 1,3,5-triazinanes. Rev. Virt. Quim. 2013, 5, 283–299. [Google Scholar] [CrossRef]
- Yan, Z.; Xue, W.-L.; Zeng, Z.-X.; Gu, M.-R. Kinetics of cyanuric chloride hydrolysis in aqueous solution. Ind. Eng. Chem. Res. 2008, 47, 5318–5322. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Kim, S.-S.; Ahn, W.-S. Covalent triazine polymers using a cyanuric chloride precursor via Friedel-Crafts reaction for CO2 adsorption/separation. Chem. Eng. J. 2016, 283, 184–192. [Google Scholar] [CrossRef]
- Basu, N.; Maity, S.K.; Chaudhury, A.; Ghosh, R. Trichloroisocyanuric acid (TCCA): An efficient green reagent for activation of thioglycosides toward hydrolysis. Carbohydr. Res. 2013, 369, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Combe, S.H.; Hosseini, A.; Parra, A.; Schreiner, P.R. Mild aliphatic and benzylic hydrocarbon C-H bond chlorination using trichloroisocyanuric acid. J. Org. Chem. 2017, 82, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Kukharev, B.F.; Stankevich, V.K.; Klimenko, G.R.; Lobanova, N.A.; Kovalyuk, E.N.; Negoda, A.Y.; Stankevich, V.V.; Bragin, E.V. Anticorrosion properties of products of N-(2-vinyloxyethyl)-1,2-ethylenediamine condensation with carbonyl compounds. Russ. J. Appl. Chem. 2010, 83, 1666–1667. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Chauhan, D.S.; Quraishi, M.A.; Obot, I.B. Influence of hydrodynamic condition on 1,3,5-tris(4-methoxyphenyl)-1,3,5-triazinane as a novel corrosion inhibitor formulation for oil and gas industry. Corros. Eng. Sci. Technol. 2021, 56, 154–161. [Google Scholar] [CrossRef]
- Divya, R.; Nair, L.P.; Bijini, B.R.; Nair, C.M.K.; Babu, K.R. Growth and characterization of barium complex of 1,3,5-triazinane-2,4,6-trione in gel: A corrosion inhibiting material. Appl. Phys. A Mater. Sci. Process. 2018, 124, 399. [Google Scholar] [CrossRef]
- He, Z.; Meng, T.; Wang, Y.; Guo, Z.; Liu, F.; Liu, Z. Effect of 2,4,6-triamino-3,5-dinitropyridine-1-oxide on the properties of 1,3,5-trinitro-1,3,5-triazinane-based PBX explosives. Propellants Explos. Pyrotech. 2021, 46, 530–536. [Google Scholar] [CrossRef]
- Morrison, K.A.; Denis, E.H.; Nims, M.K.; Broderick, A.M.; Fausey, R.C.; Rose, H.J.; Gongwer, P.E.; Ewing, R.G. Vapor pressures of RDX and HMX explosives measured at and near room temperature: 1,3,5-trinitro-1,3,5-triazinane and 1,3,5,7-tetranitro-1,3,5,7-tetrazocane. J. Phys. Chem. A 2021, 125, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, Y.; Wang, K.; Sun, Y.; Zhu, H.; Yu, J.; Xu, C. Process improvements for the preparation of insecticide clothianidin. Asian J. Chem. 2014, 26, 2815–2819. [Google Scholar] [CrossRef]
- Liang, D.; Xiao, W.-J.; Chen, J.-R. Recent advances of 1,3,5-triazinanes in aminomethylation and cycloaddition reactions. Synthesis 2020, 52, 2469–2482. [Google Scholar] [CrossRef]
- Gong, J.; Li, S.-W.; Qurban, S.; Kang, Q. Enantioselective Mannich reaction employing 1,3,5-triaryl-1,3,5-triazinanes catalyzed by chiral-at-metal rhodium complexes. Eur. J. Org. Chem. 2017, 2017, 3584–3593. [Google Scholar] [CrossRef]
- Kickelbick, G.; Gallauner, T. A Mixed copper(I)/copper(II) complex coordinated by a multidentate amidato ligand. Acta Crystallogr. E 2002, 58, m102–m104. [Google Scholar] [CrossRef]
- Colorado-Peralta, R.; Lopez-Rocha, C.A.; Sanchez-Ruiz, S.A.; Contreras, R.; Flores-Parra, A. New dithiazinanes and bis-dithiazinanes-bearing pendant ethylamines: Structure and reactivity. Heteroatom Chem. 2011, 22, 59–71. [Google Scholar] [CrossRef]
- Kickelbick, G.; Rutzinger, D.; Gallauner, T. Synthesis of hexadentate hexahydro-1,3,5-triazine-based ligands and their copper(I) complexes. Monatsh. Chem. 2002, 133, 1157–1164. [Google Scholar] [CrossRef]
- Witanowski, M.; Stefaniak, L.; Januszewski, H. Nitrogen Chemical Shifts in Organic Compounds; Nitrogen, N.M.R., Witanowski, M., Webb, G.A., Eds.; Springer: Boston, MA, USA, 1973; pp. 163–260. [Google Scholar] [CrossRef]
- Webb, G.A.; Nitrogen, N.M.R. Encyclopedia of Spectroscopy and Spectrometry; Lindon, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1504–1514. [Google Scholar] [CrossRef]
- Gao, P.; Wang, X.; Yu, H. Towards an accurate prediction of nitrogen chemical shifts by density functional theory and gauge-including atomic orbital. Adv. Theory Simul. 2019, 2, 1800148. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, S.-J.; Xu, G.-Y.; Wang, L.; Yang, Q.-Q.; Xuan, J. [4 + 2]-Cycloaddition of para-quinone methides with hexahydro-1,3,5-triazines: Access to 1,3-benzoxazine derivatives. Adv. Synth. Catal. 2020, 362, 523–527. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, B.; Li, H.; He, Y.; Xu, W.; Duan, X.; Sun, H.; Wang, T.; Zhai, H. Synthesis of hydrobenzoimidazoles from para-quinamines and 1,3,5-triazinanes via a Formal [3+2] annulation reaction. Adv. Synth. Catal. 2021, 363, 565–569. [Google Scholar] [CrossRef]
- Liang, D.; Tan, L.-P.; Xiao, W.-J.; Chen, J.-R. Inverse-electron-demand [4 + 2] cycloaddition of photogenerated aza-ortho-quinone methides with 1,3,5-triazinanes: Access to perfluoroalkylated tetrahydroquinazolines. Chem. Commun. 2020, 56, 3777–3780. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, X.; Zhai, S.; He, Y.; Tao, Q.; Li, H.; Wei, J.; Sun, H.; Wang, T.; Zhai, H. Synthesis of 1,2,3,4-tetrahydrobenzofuro[3,2-d]pyrimidines via [4 + 2] annulation reaction of 1,3,5-triazinanes and aurone-derived α,β-unsaturated imines. Adv. Synth. Catal. 2020, 362, 3836–3840. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, B.; Hu, L.; Sun, H.; Wang, Y.; Zhai, H.; Cheng, B. Synthesis of chromeno[2,3-d]pyrimidin-5-one derivatives from 1,3,5-triazinanes via two different reaction pathways. J. Org. Chem. 2022, 87, 1348–1356. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zheng, H.; Huang, J.; Su, Z.; Zhao, L.; Cao, H. Construction of diverse N-heterocycles by formal (3 + 3) cycloaddition of naphthol/thionaphthol/naphthylamine and 1,3,5-triazinanes. J. Org. Chem. 2023, 88, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhong, Q.; Tang, S.; Wang, L.; Li, P.; Li, H. Electrochemical formal [3 + 2] cycloaddition of azobenzenes with hexahydro-1,3,5-triazines. Org. Chem. Front. 2022, 9, 3769–3774. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, A.; Liu, X.; Yu, Y.; Zhu, B.; Cao, H. Lewis acid-catalyzed synthesis of polysubstituted furans from conjugated ene-yne-ketones and 1,3,5-triazinanes. J. Org. Chem. 2022, 87, 7056–7063. [Google Scholar] [CrossRef]
- Kireeva, D.R.; Sadretdinov, S.S.; Musina, A.I.; Ishmetova, D.V.; Vakhitov, V.A.; Murinov, Y.I.; Dokichev, V.A. Synthesis and cytotoxic activity of 1,3,5-triazinane derivatives based on primary amines and amino acids esters. Russ. J. Gen. Chem. 2022, 92, 24–28. [Google Scholar] [CrossRef]
- Fang, Z.; Jin, Q.; Wang, X.; Ning, Y. Metal-free [2 + 1 + 3] Cycloaddition of trifluoroacetaldehyde N-sulfonylhydrazones with hexahydro-1,3,5-triazines leading to trifluoromethylated 2,3,4,5-tetrahydro-1,2,4-triazines. J. Org. Chem. 2022, 87, 2966–2974. [Google Scholar] [CrossRef]
- Ruan, P.; Tang, Q.; Yang, Z.; Liu, X.; Feng, X. Enantioselective formal [2 + 2 + 2] cycloaddition of 1,3,5-triazinanes to construct tetrahydropyrimidin-4-one derivatives. Chem. Commun. 2022, 58, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Gao, L.; Chen, W.; Shi, Y.; Cao, Z.; Zheng, Y.; Liu, J. Formal [2+2+2] cycloaddition reaction of 1,3,5-triazinanes with diethyl acetylenedicarboxylate: Approach to tetrahydropyrimidines. Eur. J. Org. Chem. 2021, 2021, 5941–5945. [Google Scholar] [CrossRef]
- Wang, C.; Fang, L.; Wang, Z. Base-induced inverse-electron-demand aza-Diels-Alder reaction of azoalkenes and 1,3,5-triazinanes: Facile approaches to tetrahydro-1,2,4-triazines. Tetrahedron Lett. 2021, 79, 153303. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Z.; Zhang, X.; Zhan, F.; Lin, J.-S.; Jiang, Y. Synthesis of α-amino tertiary alkylperoxides by Lewis acid-catalyzed peroxidation of 1,3,5-triazines. Chem. Asian J. 2021, 16, 3487–3491. [Google Scholar] [CrossRef]
- Duan, S.; Meng, H.; Jablasone, S.T., Jr.; Luo, H.; Xu, Z.-F.; Li, C.-Y. Rhodium(II)-catalyzed [4 + 2] annulation of ester-tethered 1-sulfonyl-1,2,3-triazoles and hexahydro-1,3,5-triazines. Asian J. Org. Chem. 2021, 10, 1076–1080. [Google Scholar] [CrossRef]
- Galvez-Ruiz, J.C.; Jaen-Gaspar, J.C.; Castellanos-Arzola, I.G.; Contreras, R.; Flores-Parra, A. 2-(1,3,5-Dithiazinan-5-yl)ethanol heterocycles, structure and reactivity. Heterocycles 2004, 63, 2269–2285. [Google Scholar] [CrossRef]
- Winfield, L.; Zhang, C.; Reid, C.A.; Stevens, E.D.; Trudell, M.L.; Izenwasser, S.; Wade, D. Synthesis, lipophilicity and structure of 2,5-disubstituted 1,3,5-dithiazine derivatives. J. Heterocycl. Chem. 2003, 40, 827–832. [Google Scholar] [CrossRef]
- Khabibullina, G.R.; Yapparova, D.K.; Ibragimov, A.G.; Akhmetova, V.R. Sodium sulfide in the synthesis of N-Alkyl-1,3,5-dithiazinanes and 1,3,5-thiadiazinanes. Russ. J. Gen. Chem. 2021, 91, 1453–1458. [Google Scholar] [CrossRef]
- Peerzada, N.; Neely, I. Benzotriazole mediated synthesis of some 5-alkyldihydro-4H-1,3,5-dithiazines. Synth. Commun. 2000, 30, 779–788. [Google Scholar] [CrossRef]
- Xotlanihua-Flores, A.; Montes-Tolentino, P.; Sánchez-Ruiz, S.A.; Suárez-Moreno, G.V.; Gálvez-Ruiz, J.C.; Contreras, R.; Flores-Parra, A. New N-[2-chloropropyl]-heterocyclohexanes. NMR long range shielding effects of chlorine substituent. Use of BH3 as freezing conformational agent. J. Mol. Struct. 2016, 1106, 322–330. [Google Scholar] [CrossRef]
- Xotlanihua-Flores, A.; Villaseñor-Granados, T.O.; Montes-Tolentino, P.; Flores-Parra, A. New 1,3,5-heterocyclohexanes bearing pendant phosphorus groups. Structure and N→P pnicogen interactions. J. Mol. Struct. 2022, 1252, 131916. [Google Scholar] [CrossRef]
- Xotlanihua-Flores, A.; Villaseñor-Granados, T.O.; Colorado-Peralta, R.; Sánchez-Ruiz, S.A.; Montes-Tolentino, P.; Flores-Parra, A. Tin complexes derived from nitrogen-based 1,3,5-heterocyclohexanes bearing 2-hydroxypropan-1-yl, 2-diphenylphosphitepropan-1-yl and 2-diphenylphosphinepropan-1-yl as pendant N-substituents. J. Mol. Struct. 2022, 1254, 132368. [Google Scholar] [CrossRef]
- Suarez-Moreno, G.V.; Xotlanihua-Flores, A.; Vela, A.; Contreras, R.; Flores-Parra, A. Theoretical approach to the conformational analyses of dithiazinane, thiadiazinane and triazinane, their N-borane adducts and N-H cations. J. Mol. Struct. 2016, 1113, 112–126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).