Palladium-Catalyzed Cross-Coupling Reactions of Borylated Alkenes for the Stereoselective Synthesis of Tetrasubstituted Double Bond
Abstract
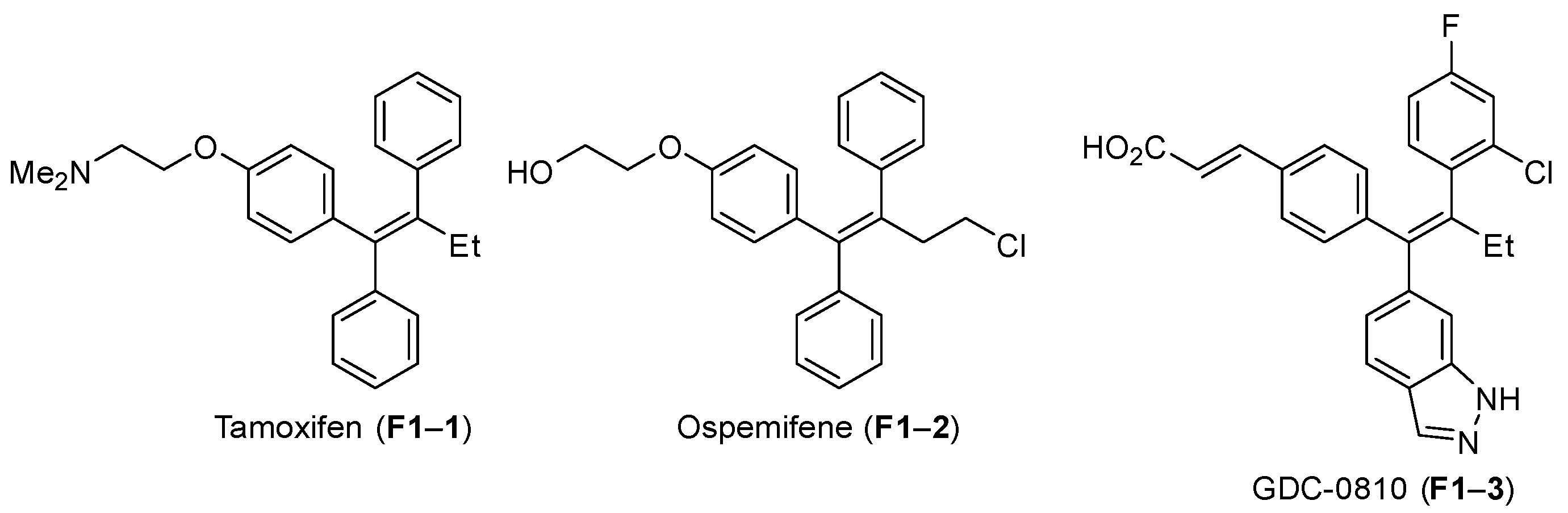
1. Introduction
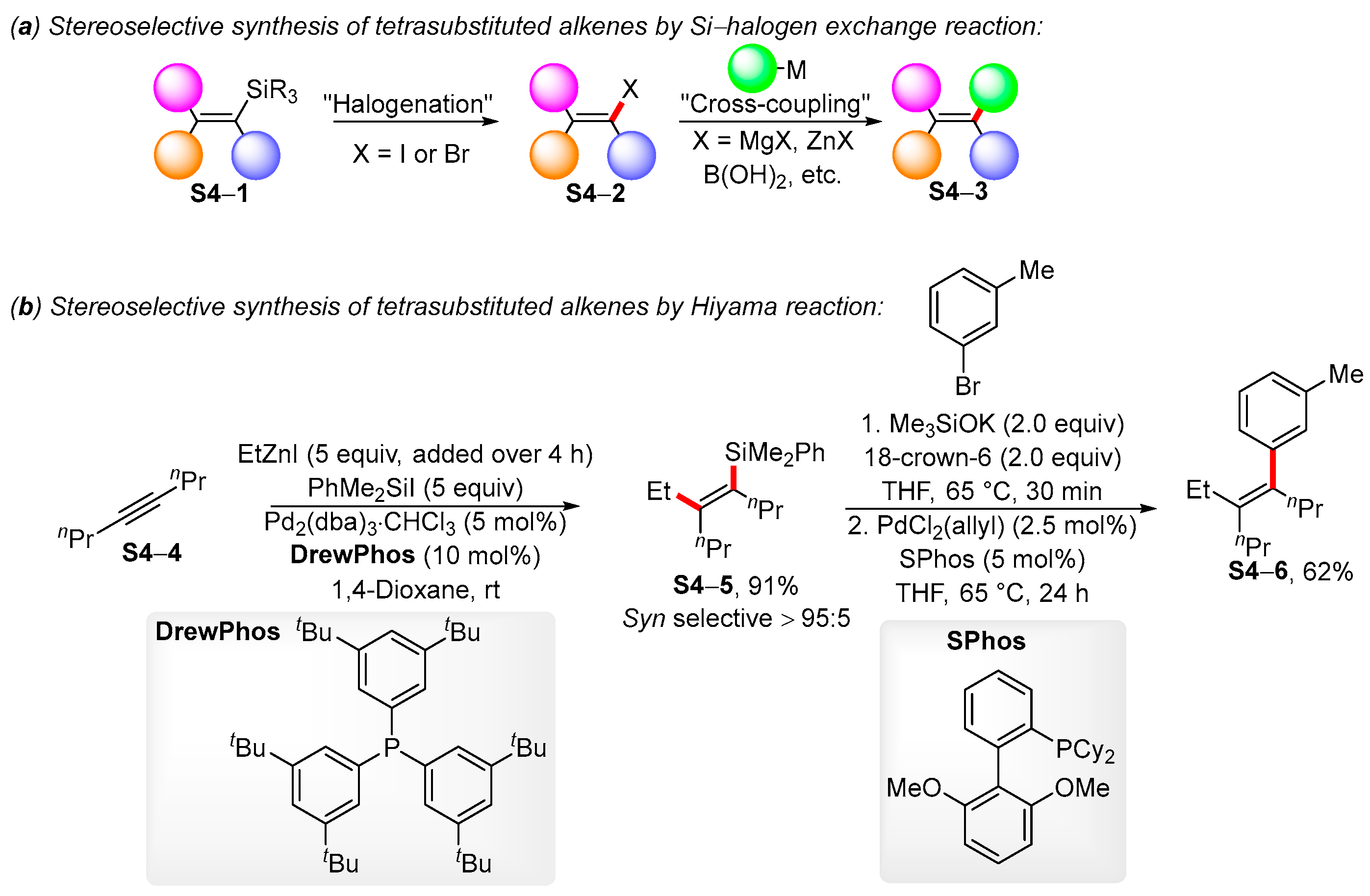
2. Recent Developments in Stereoselective Synthesis of Tetrasubstituted Alkenes by Palladium-Catalyzed Cross-Coupling Reactions of Trisubstituted Alkenylstannanes and Alkenylsilanes
3. Acyclic Boron-Based Tetrasubstituted Double Bond Templates
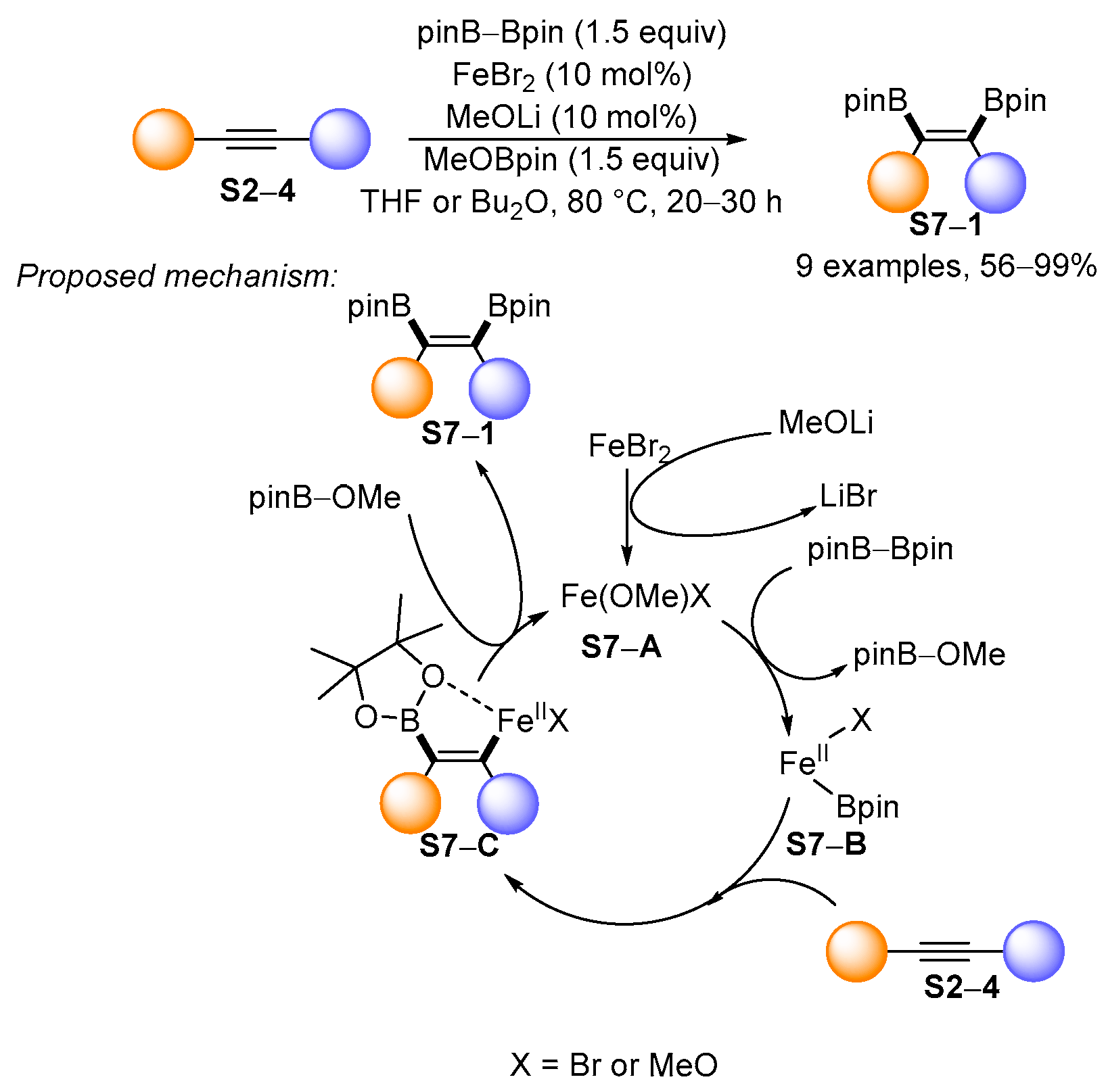
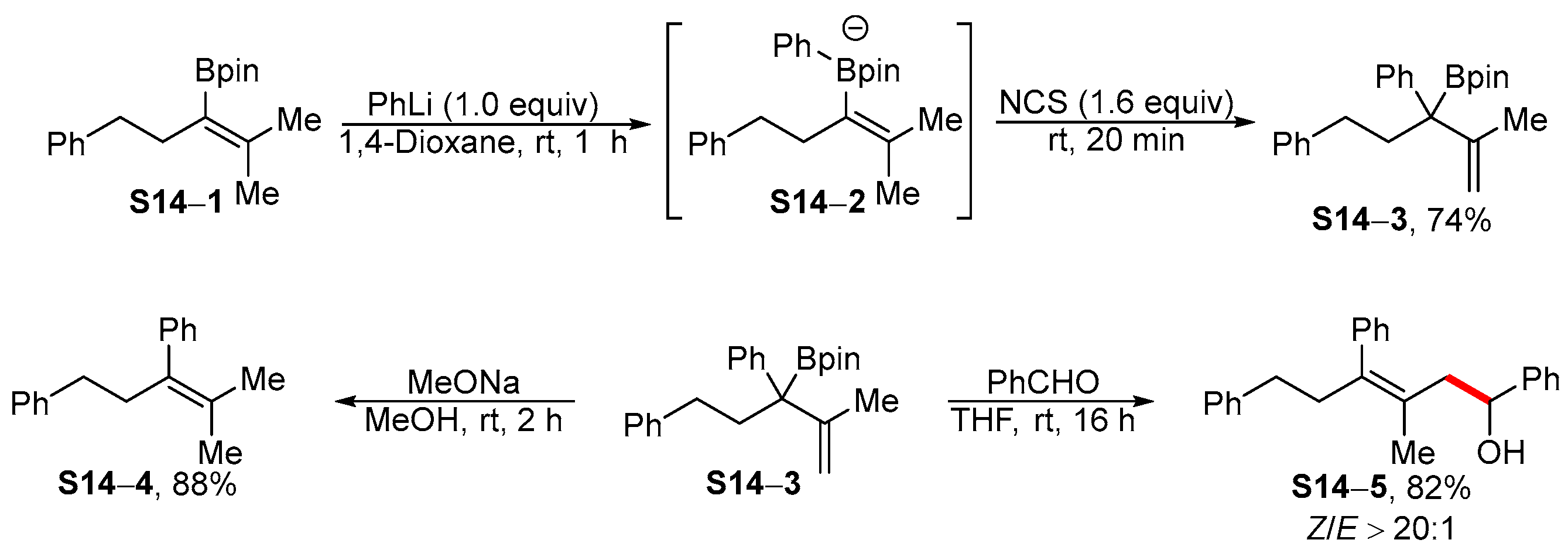
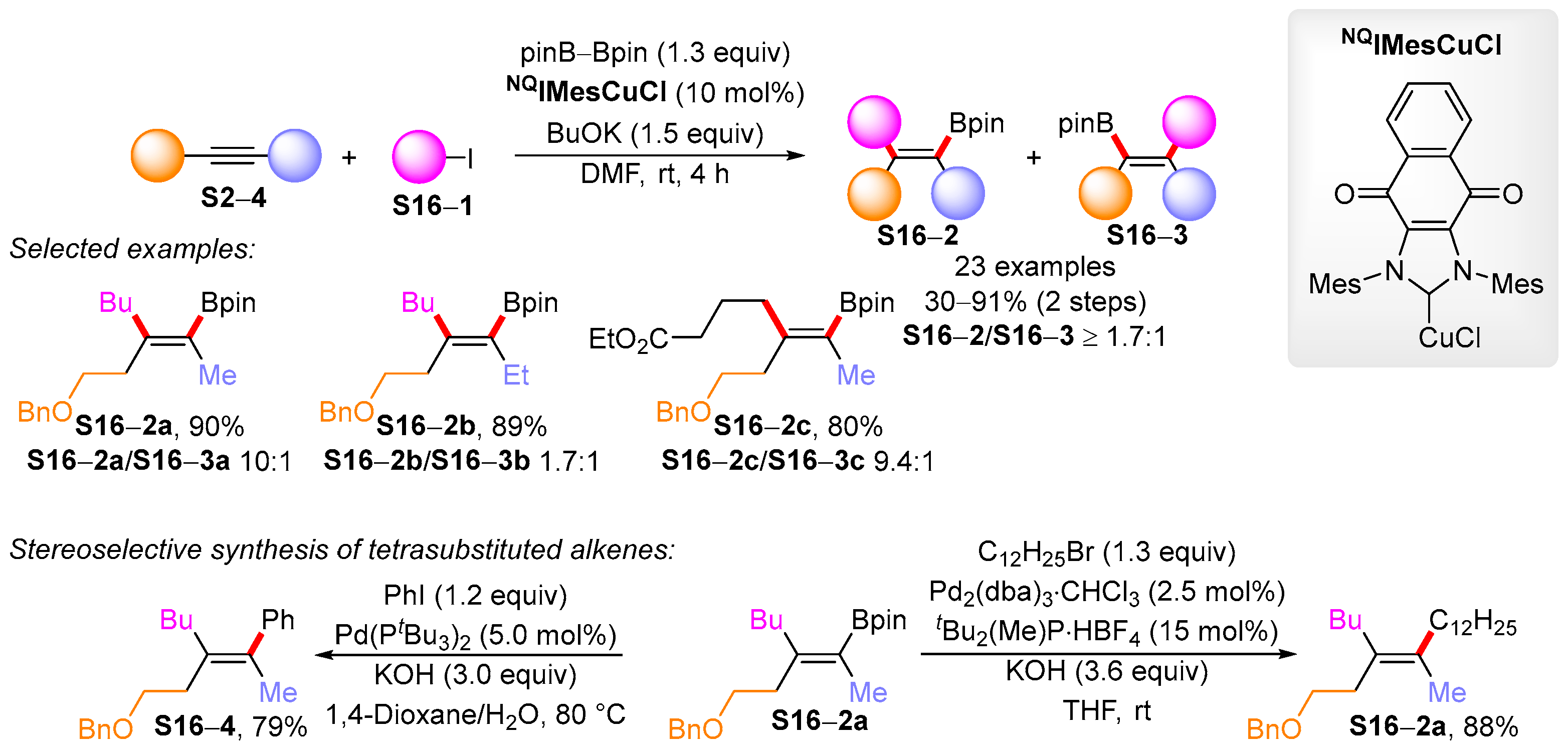
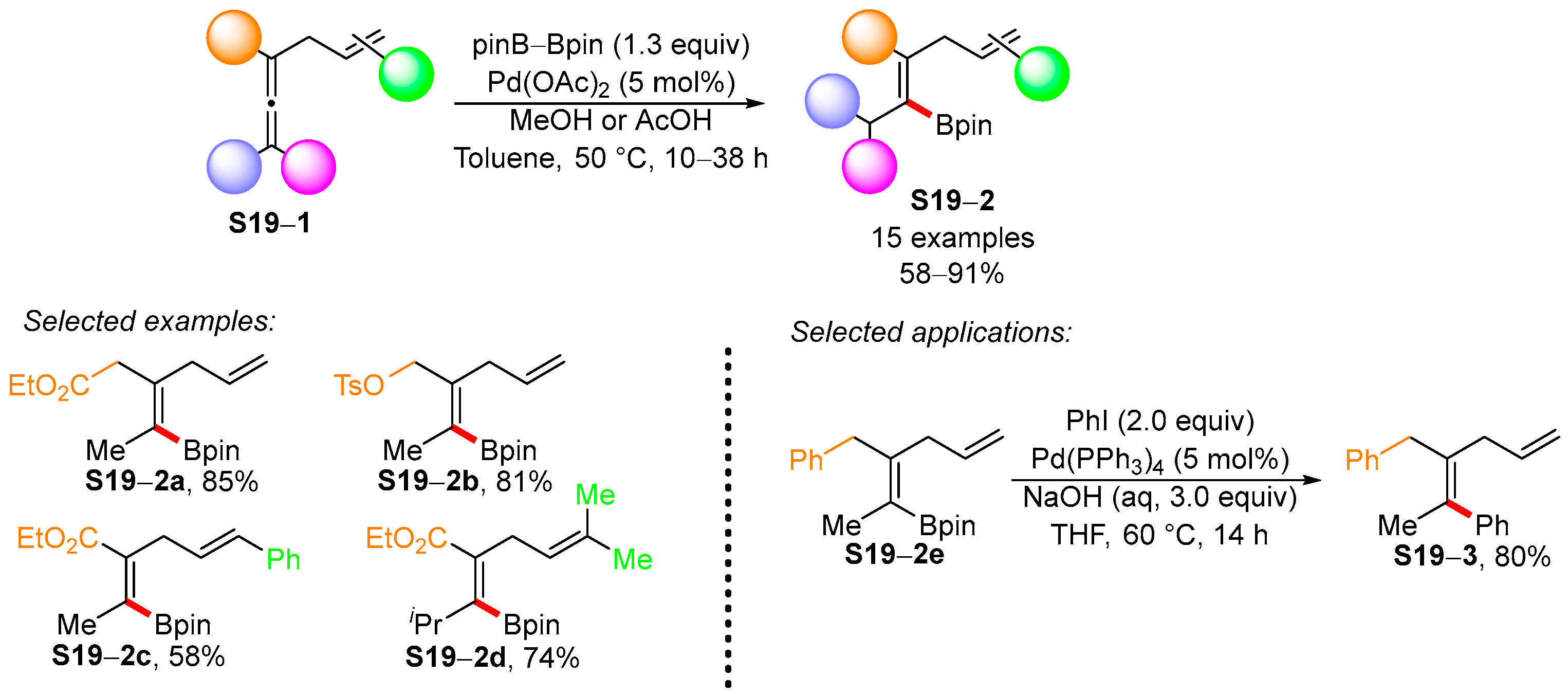
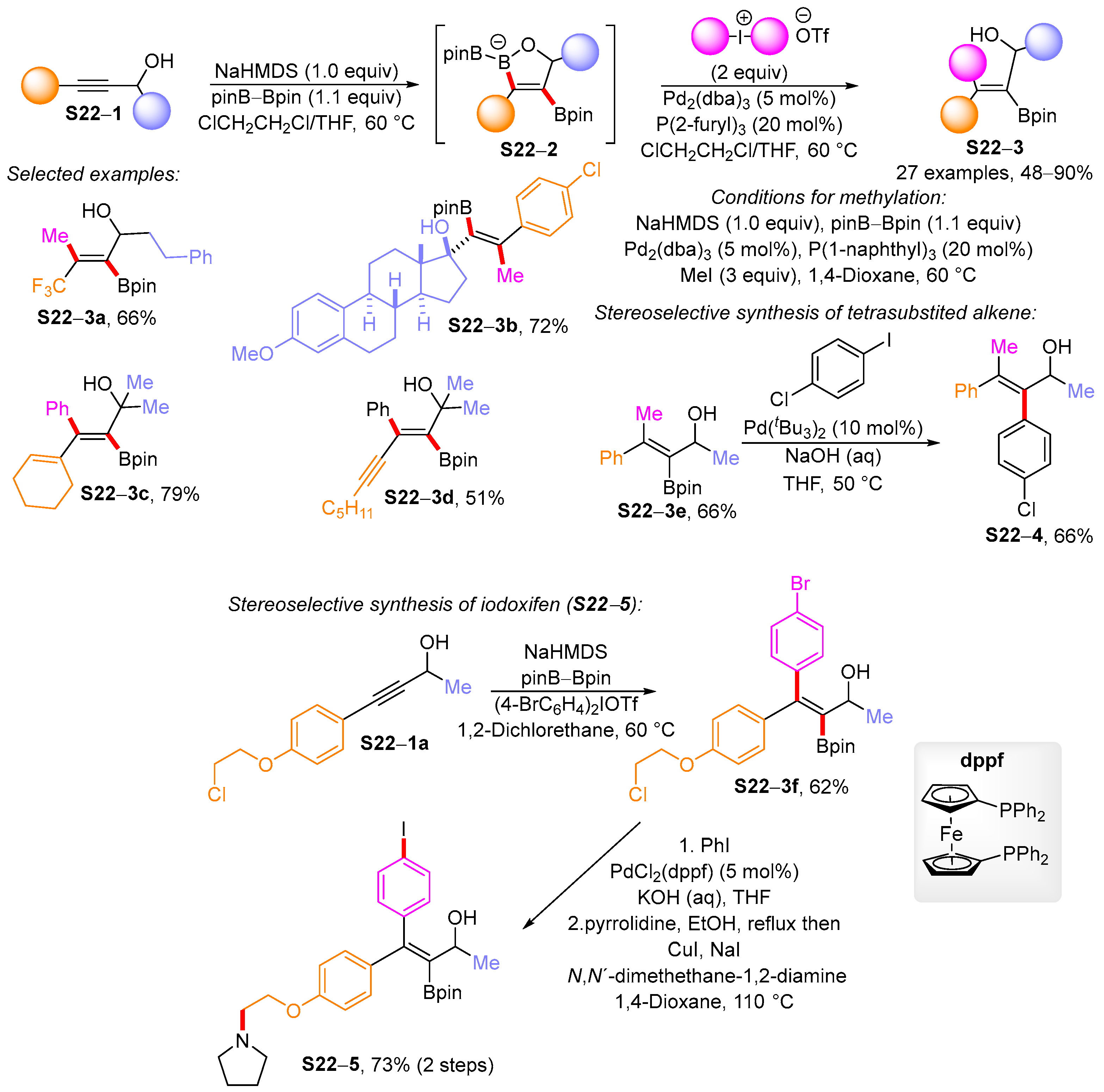
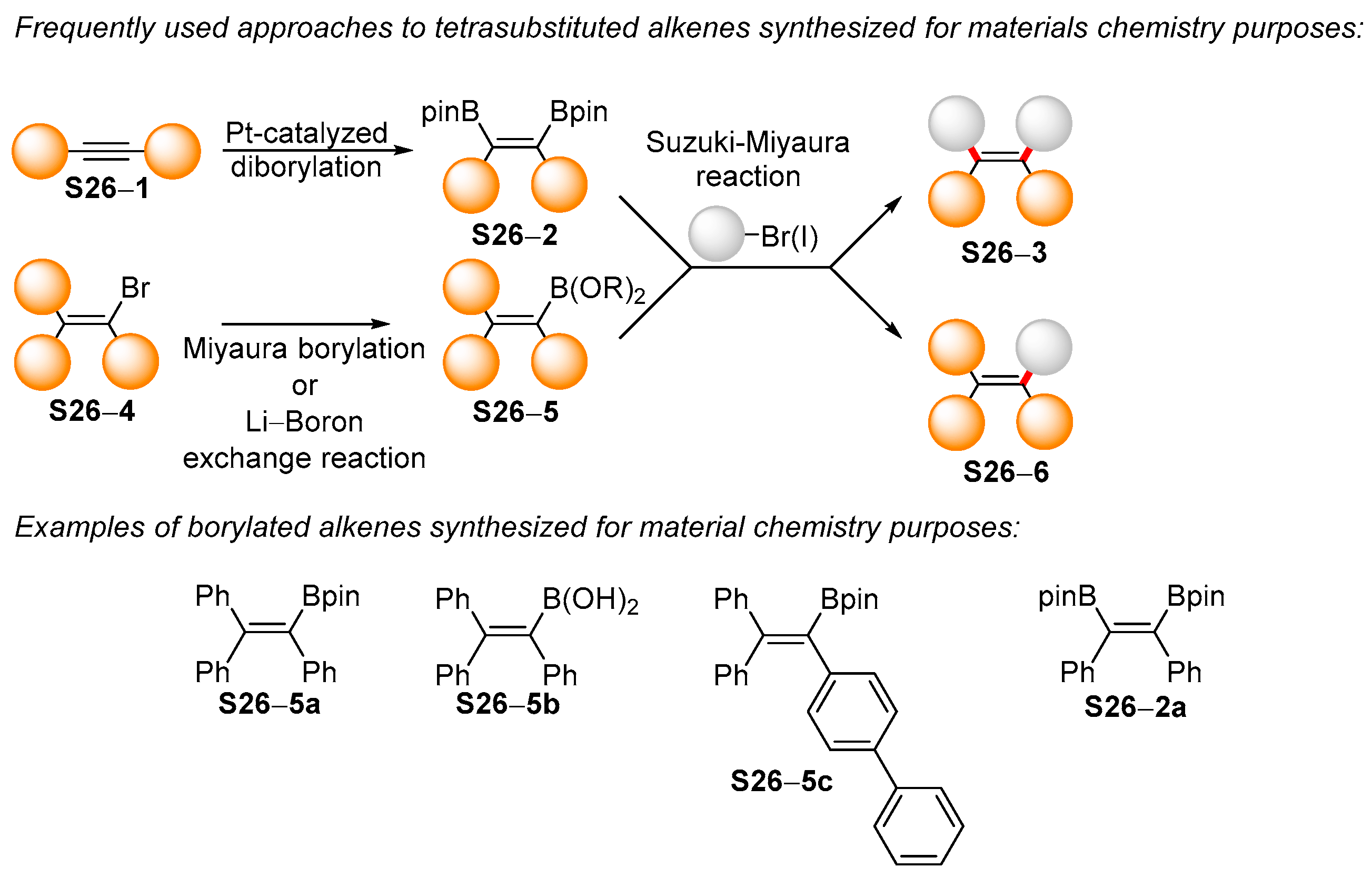
3.1. Synthetic Approaches to Acyclic Borylated Alkenes
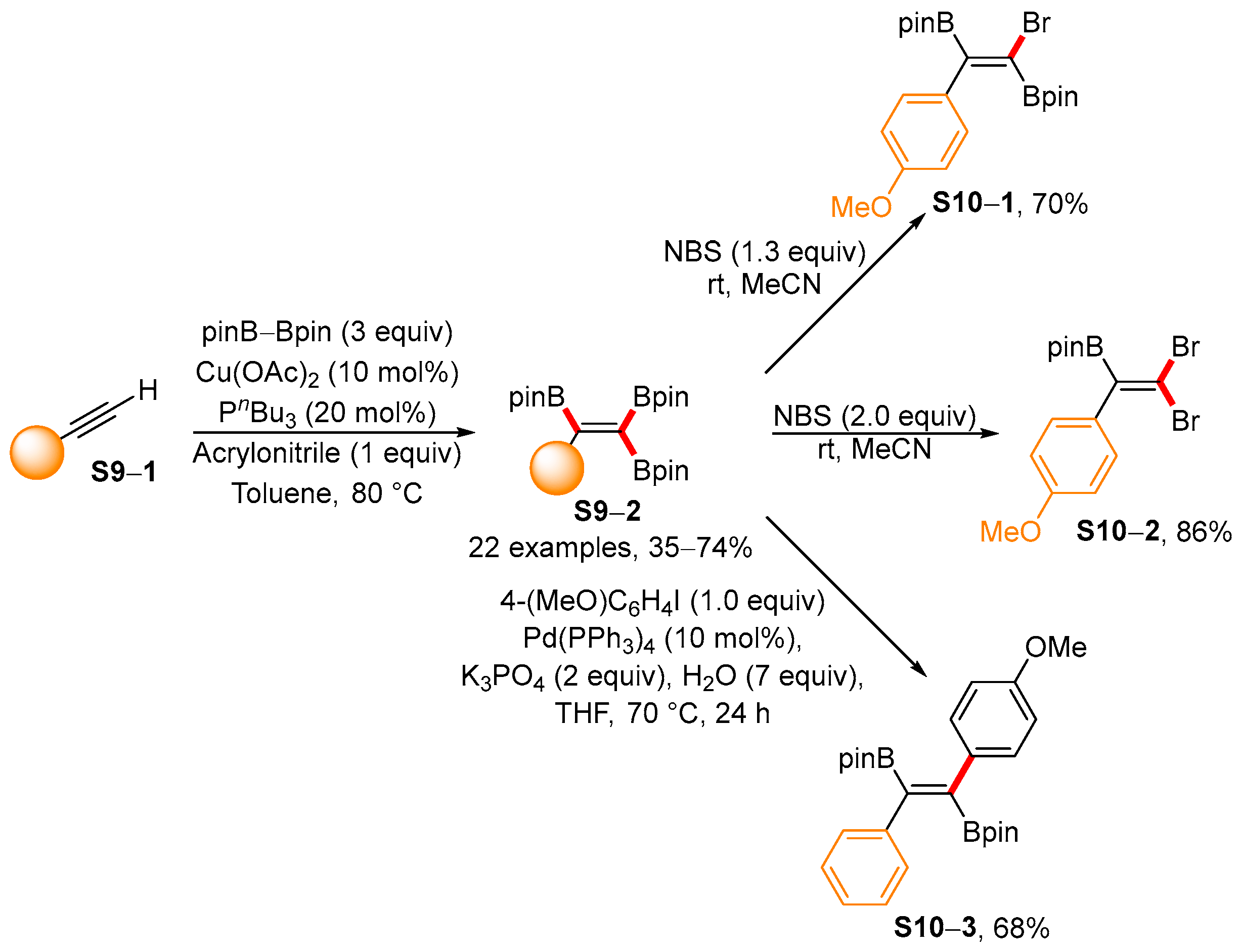
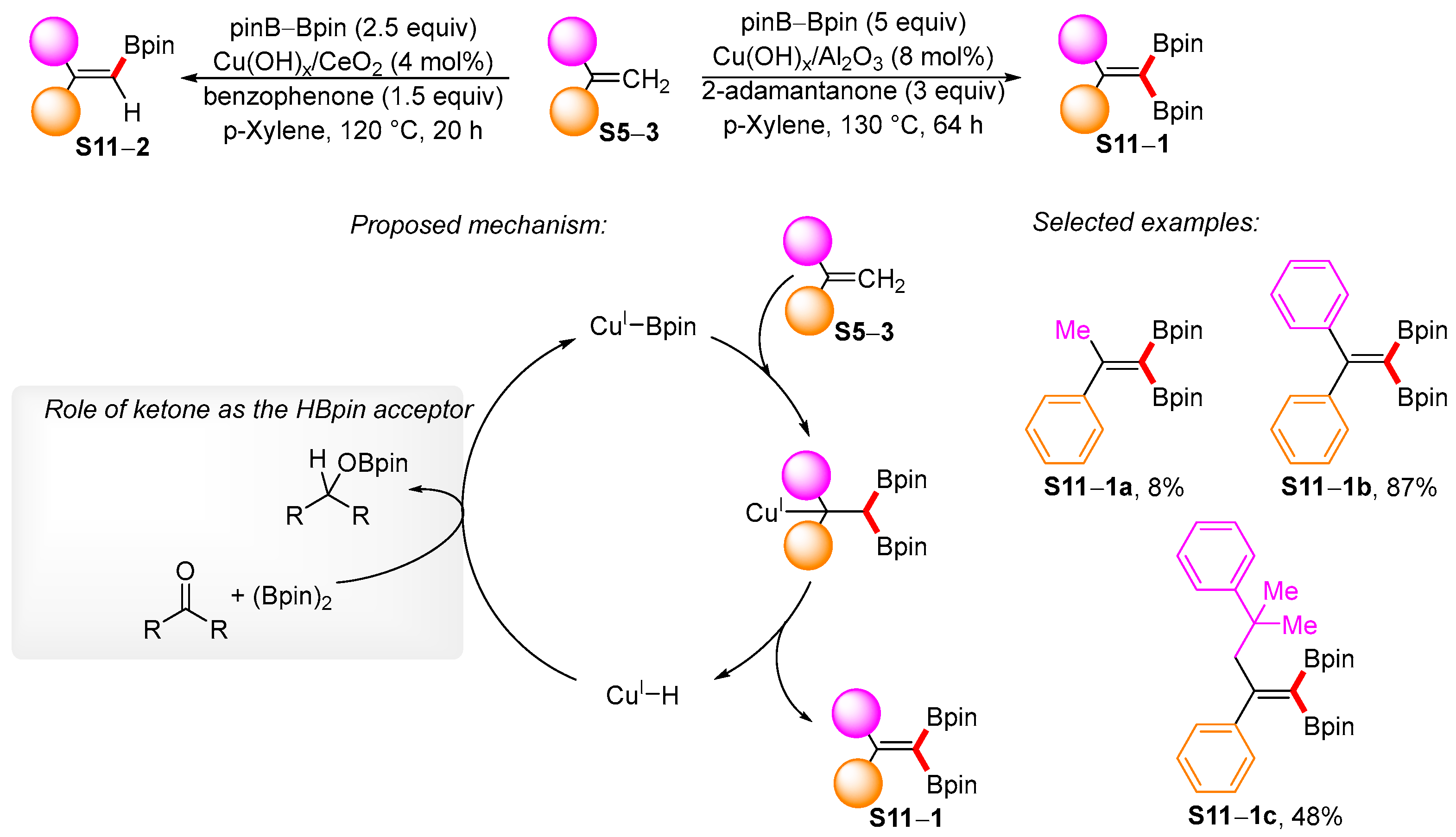
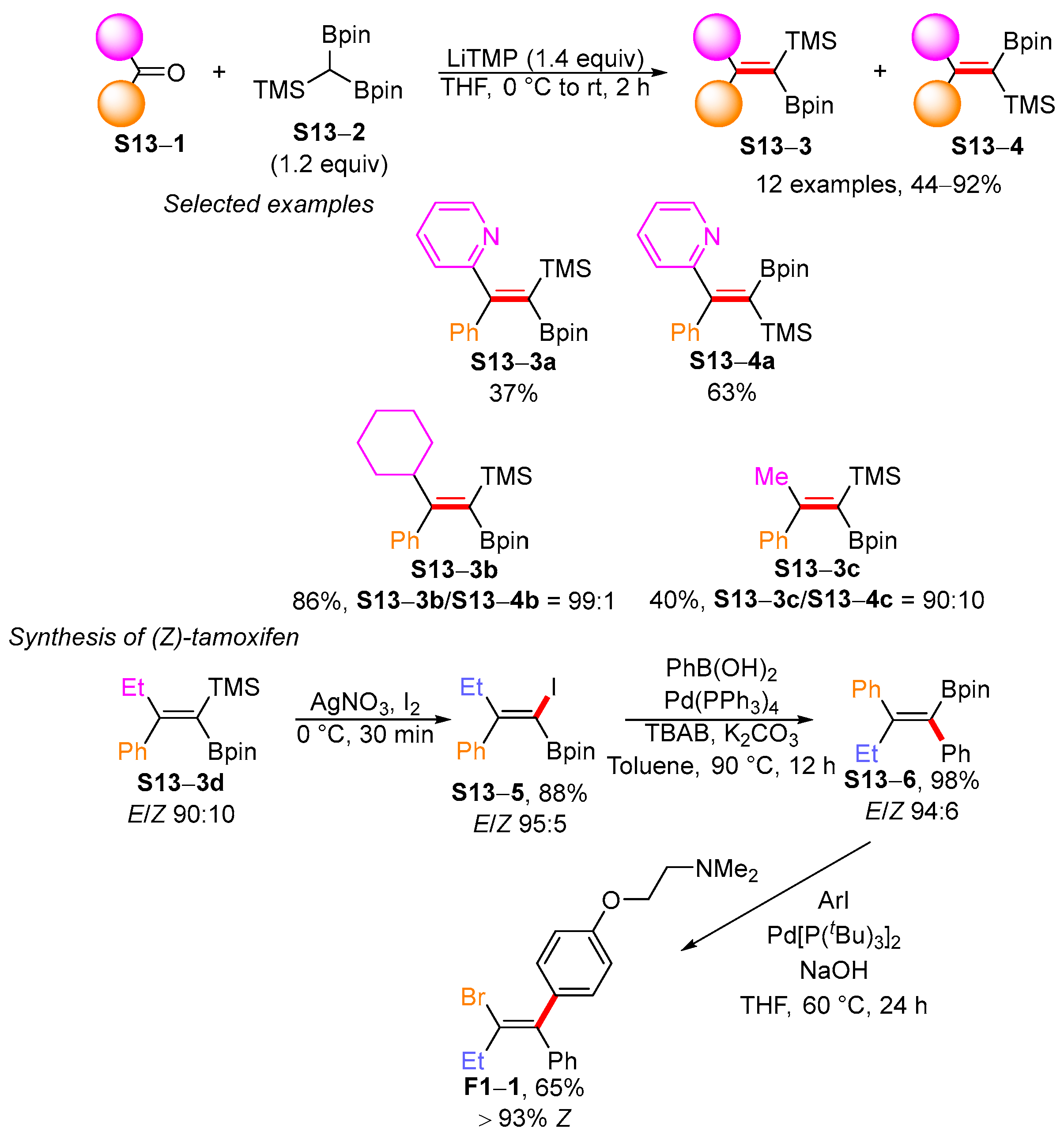
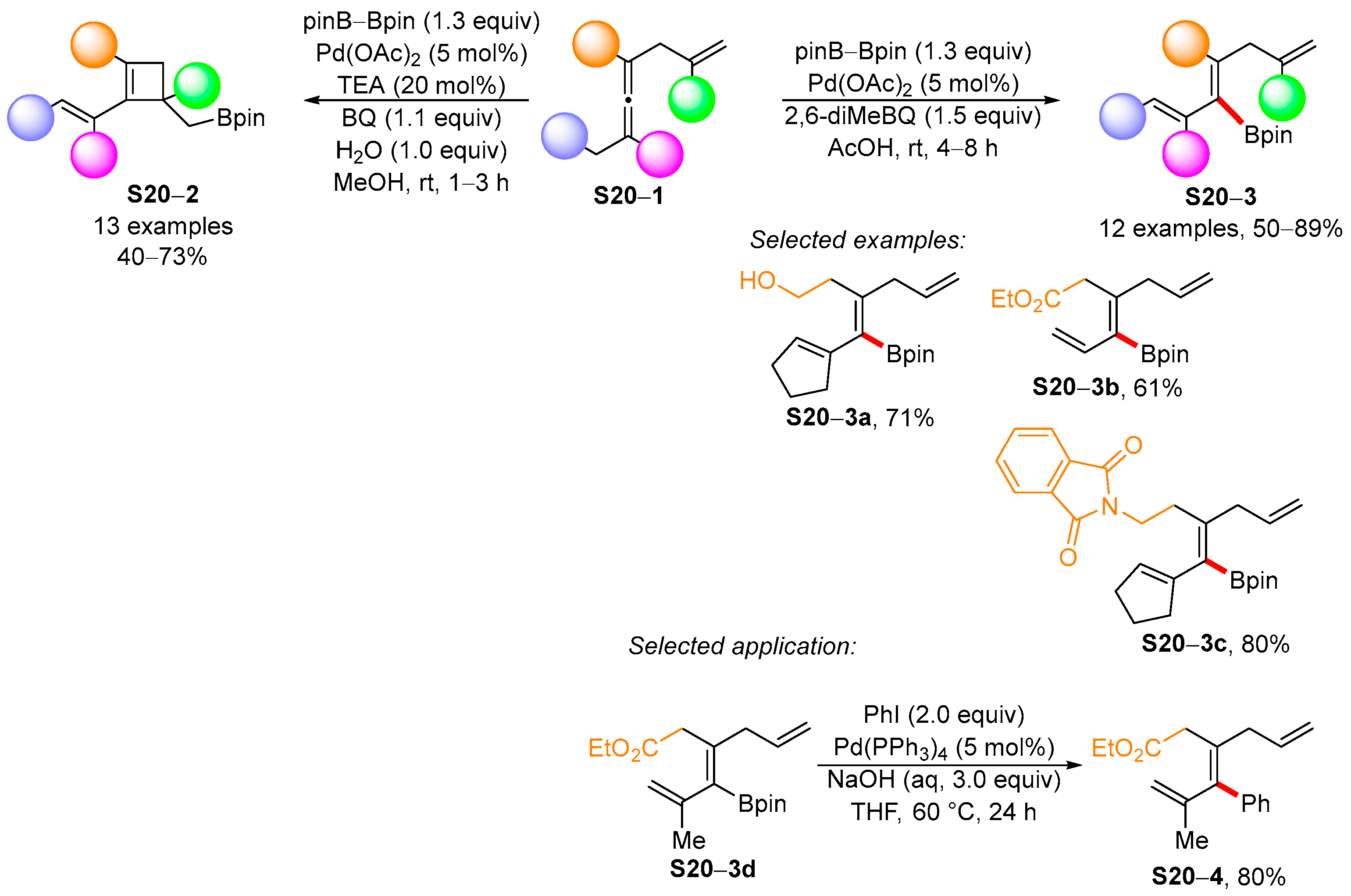
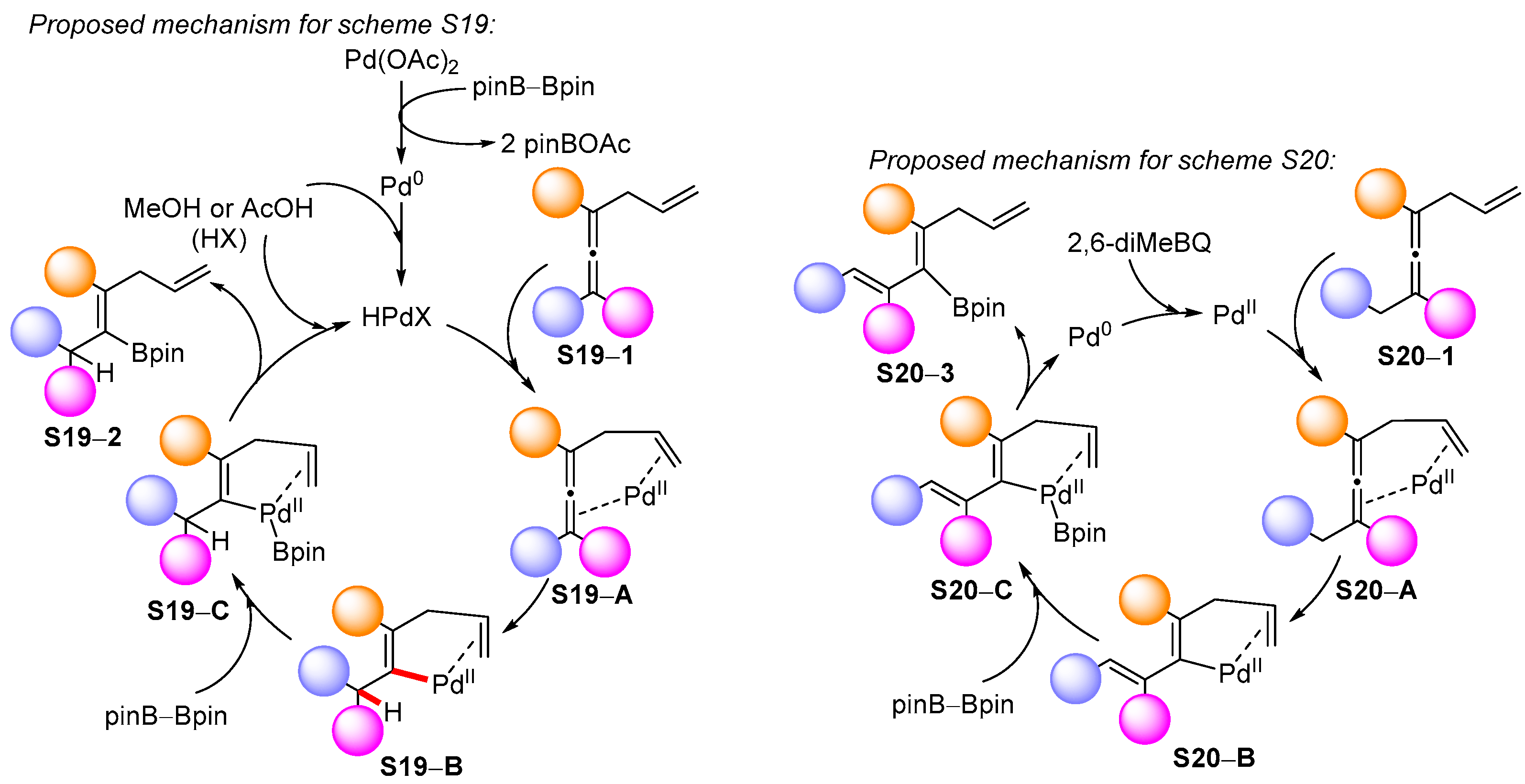
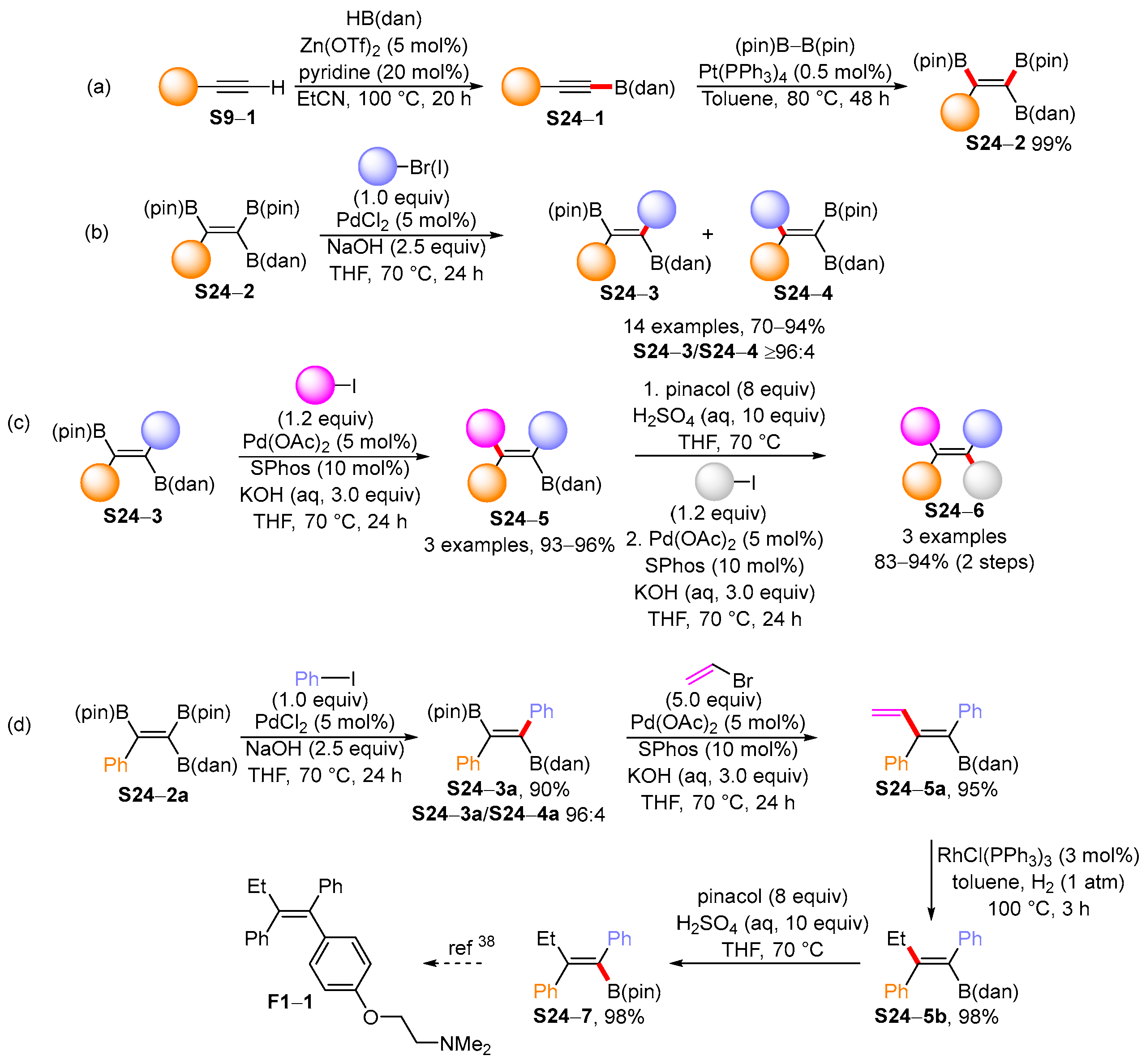
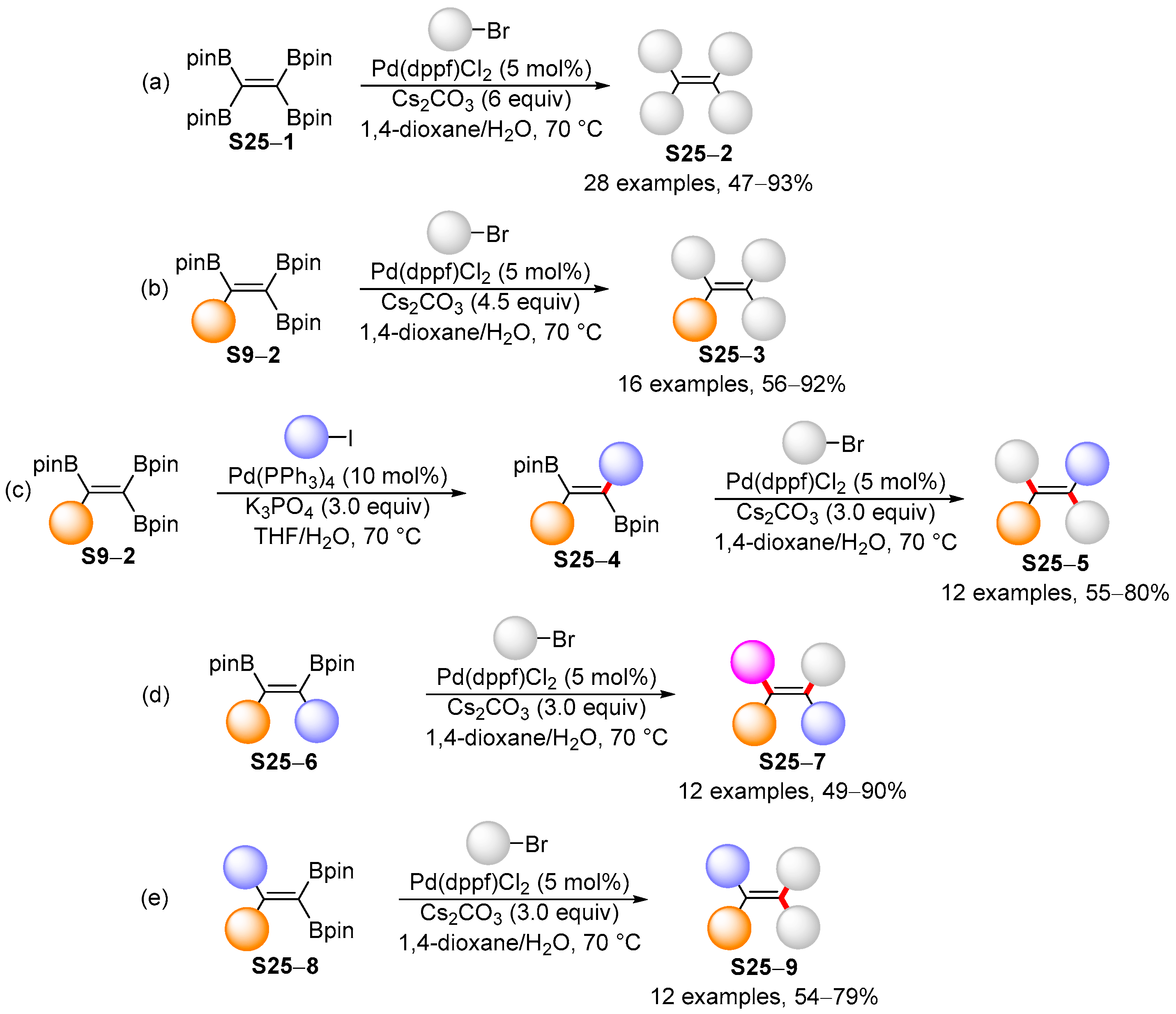
3.2. Applications of the Borylated Acyclic Double Bond for Stereoselective Synthesis of Tetrasubstituted Alkenes
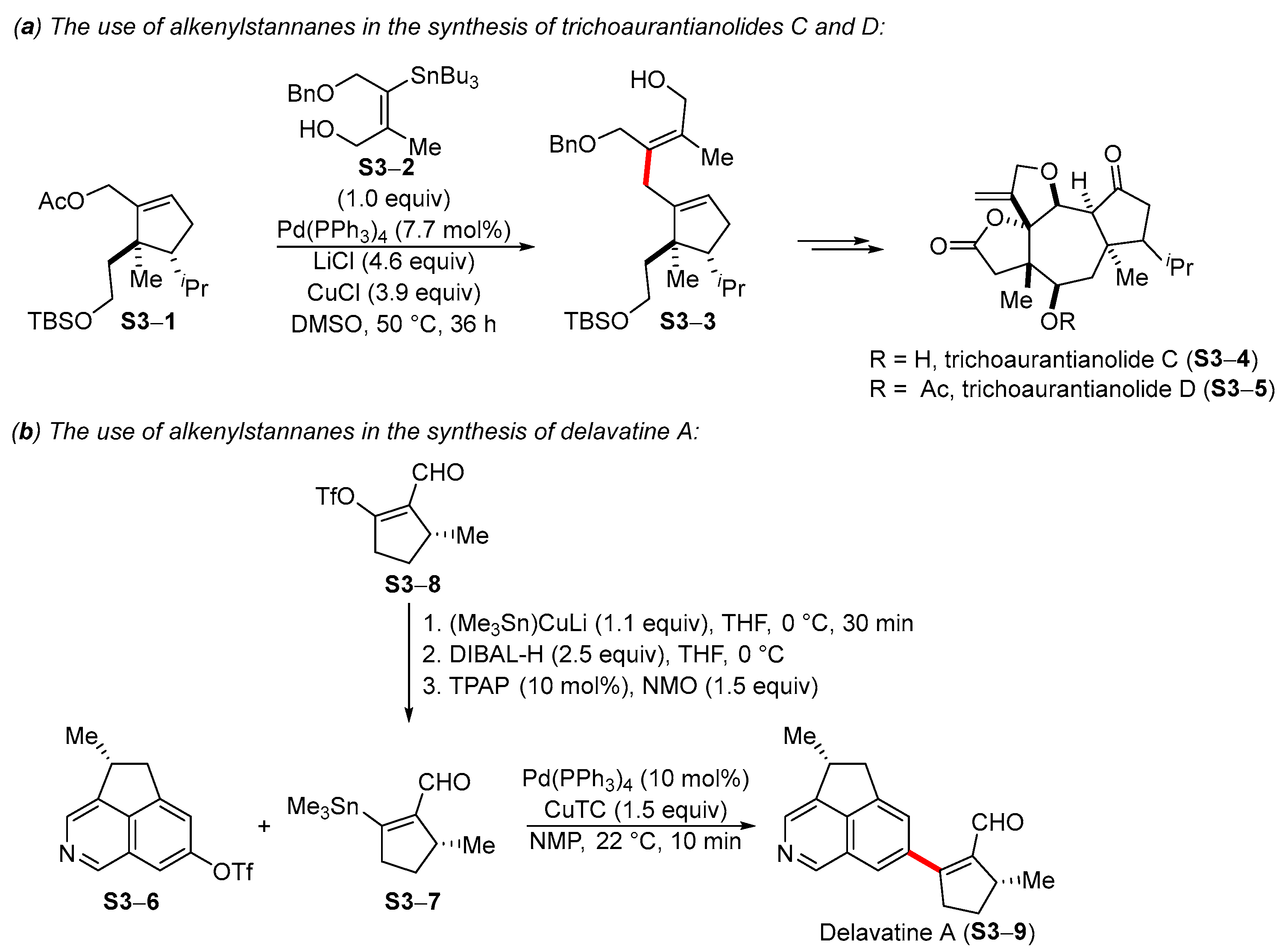
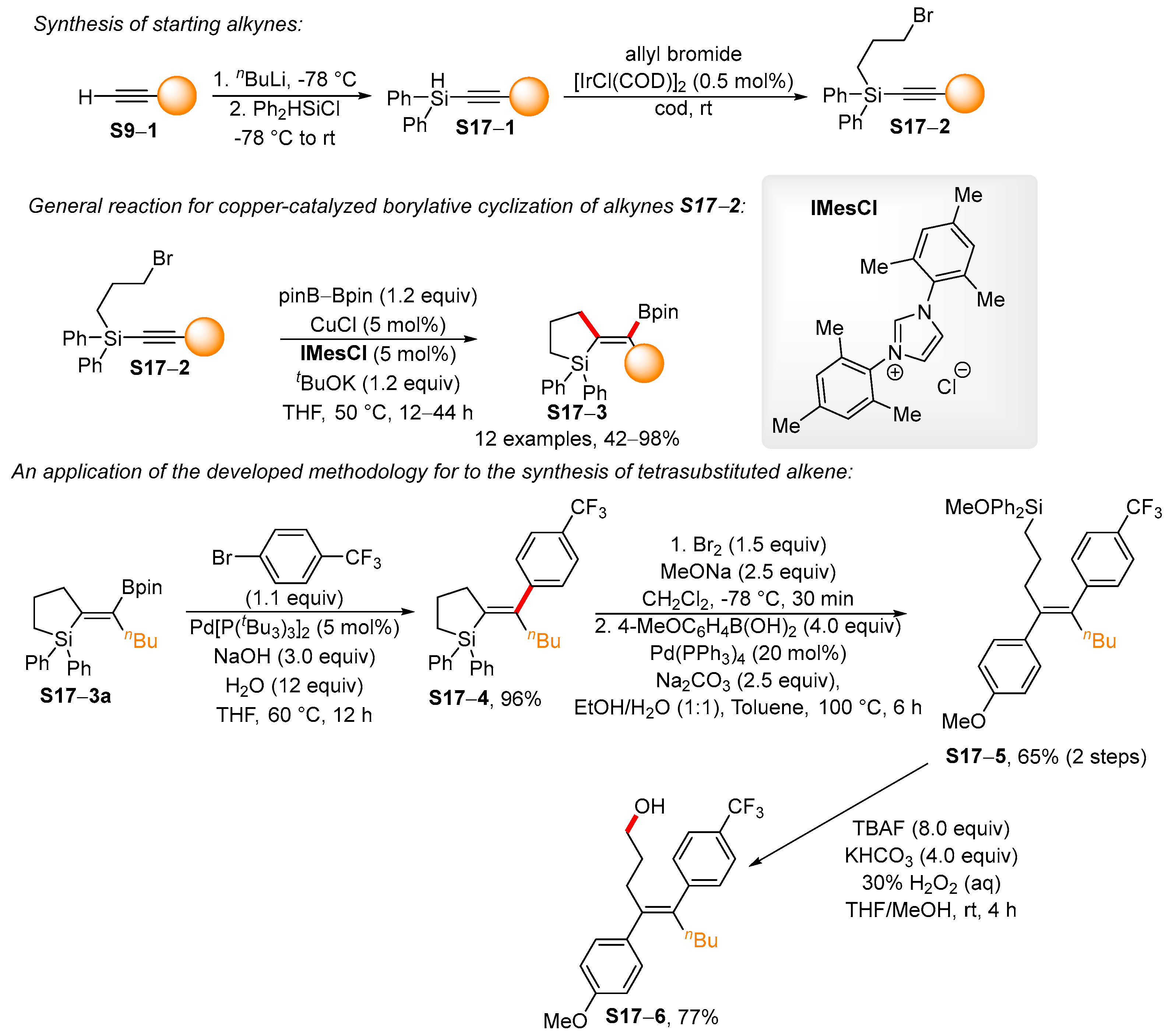
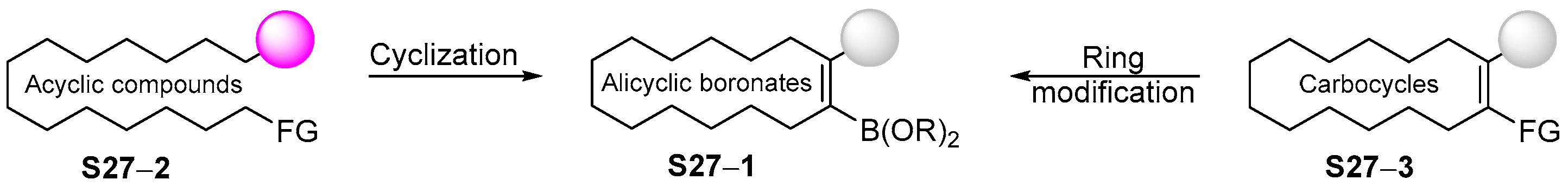
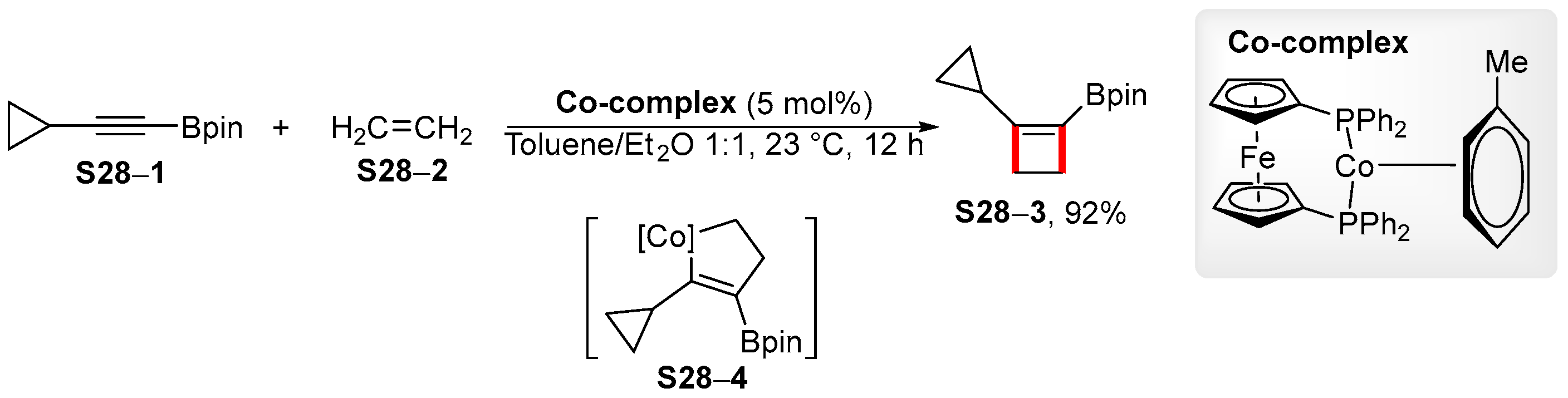
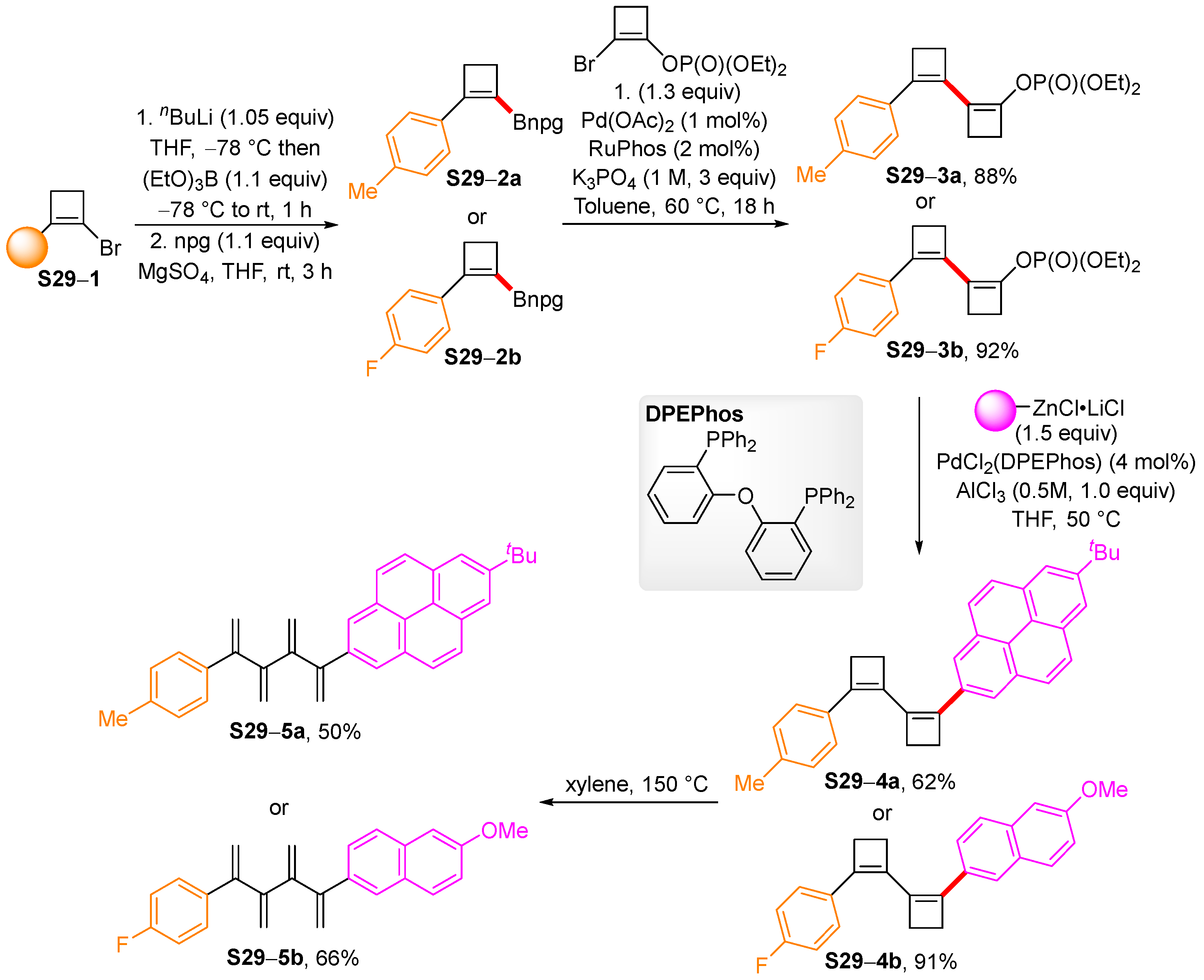
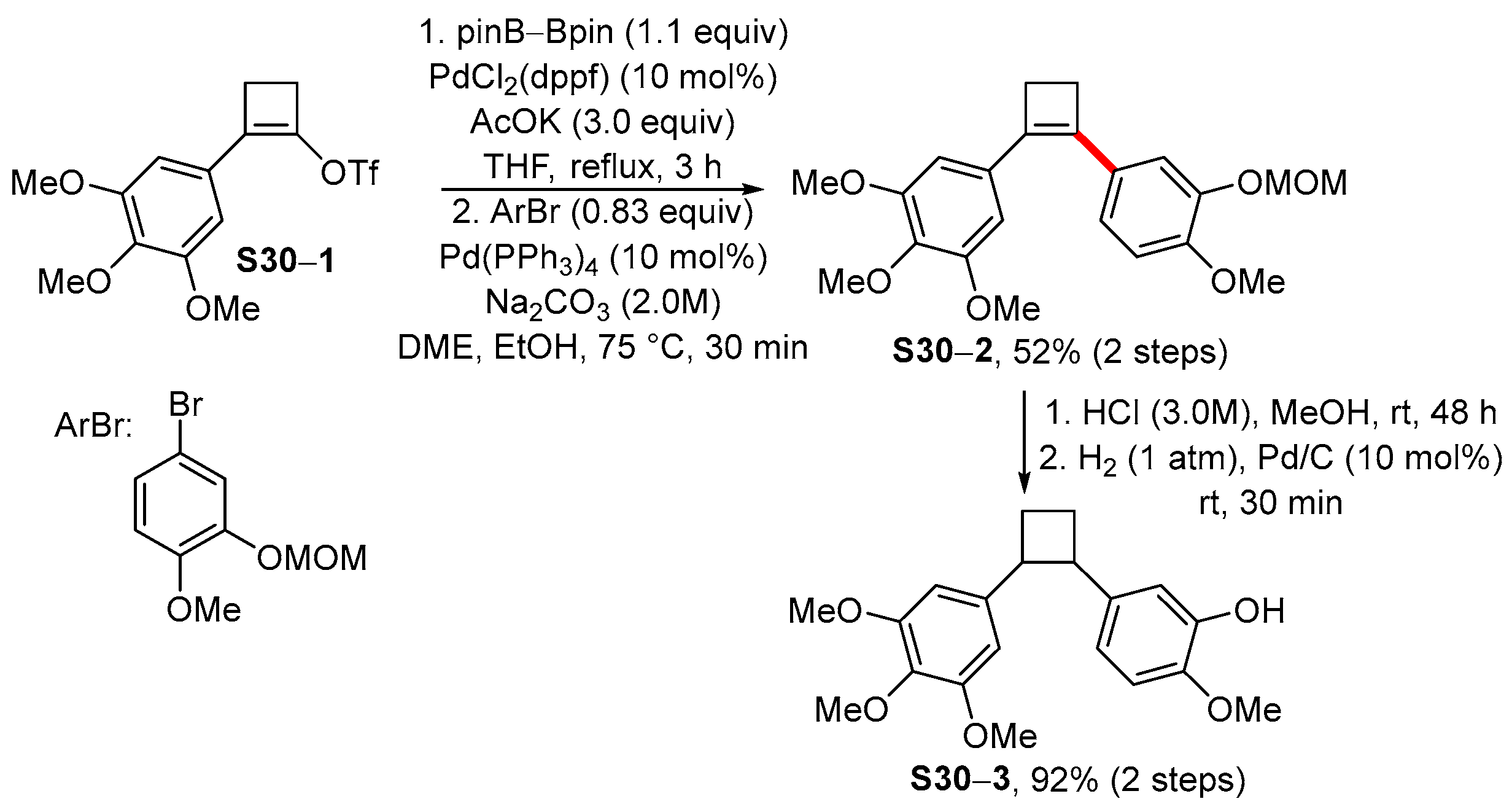
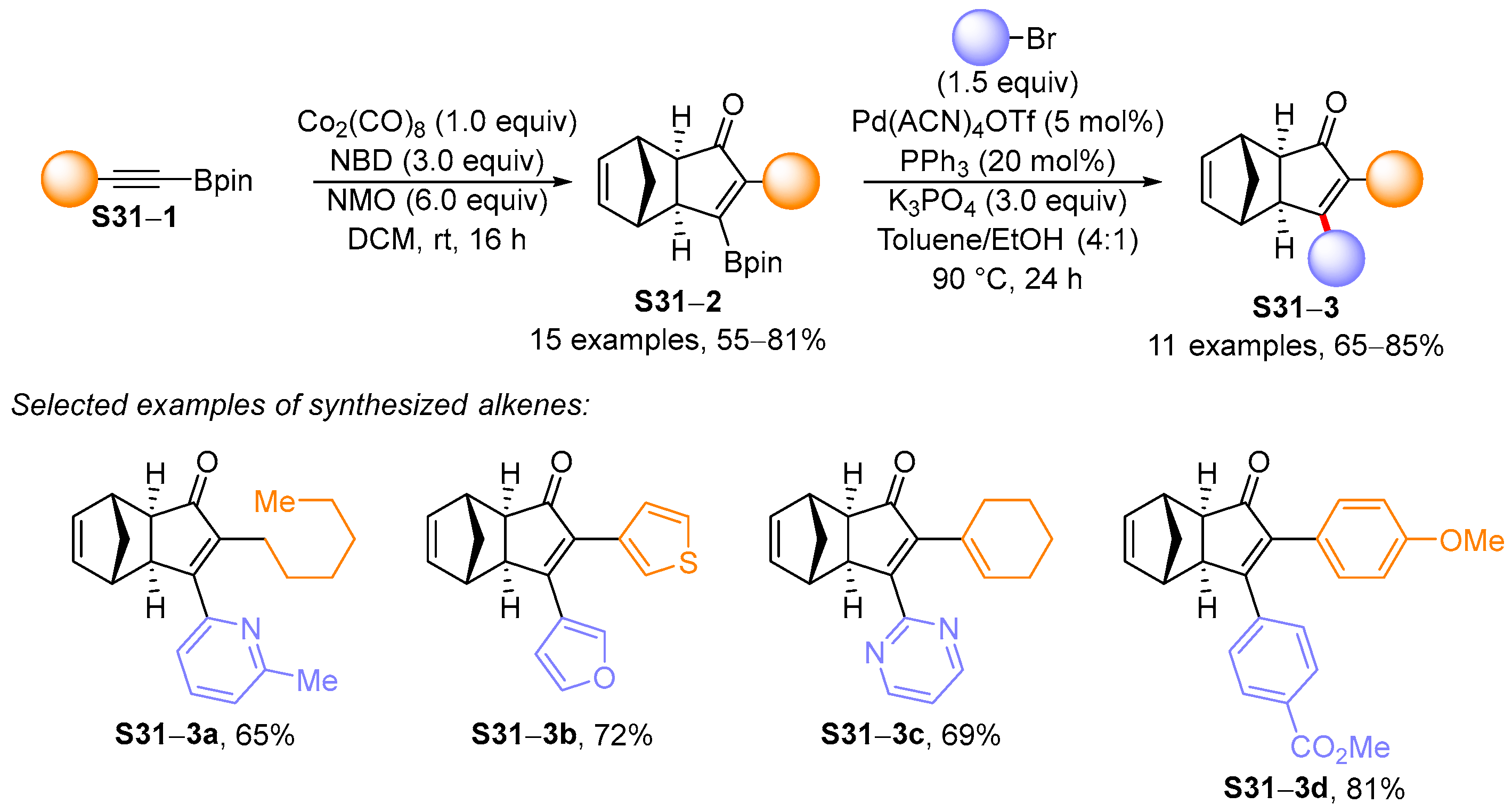
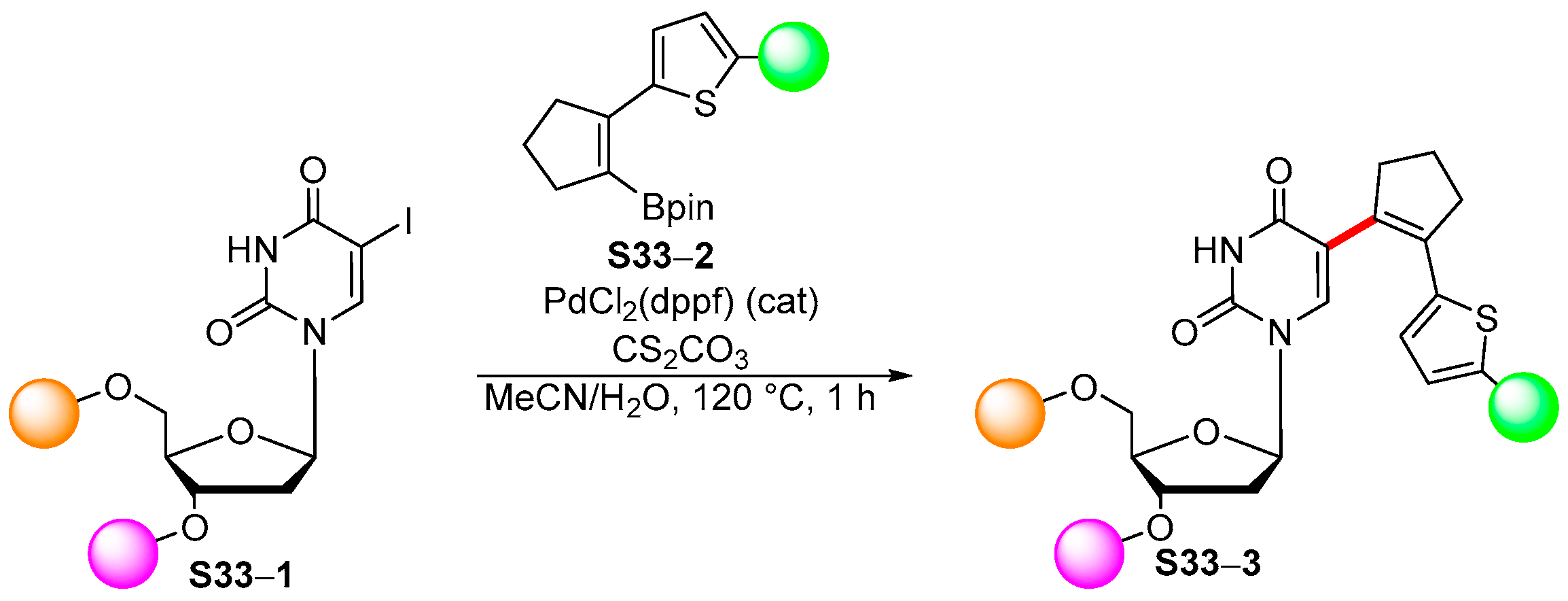
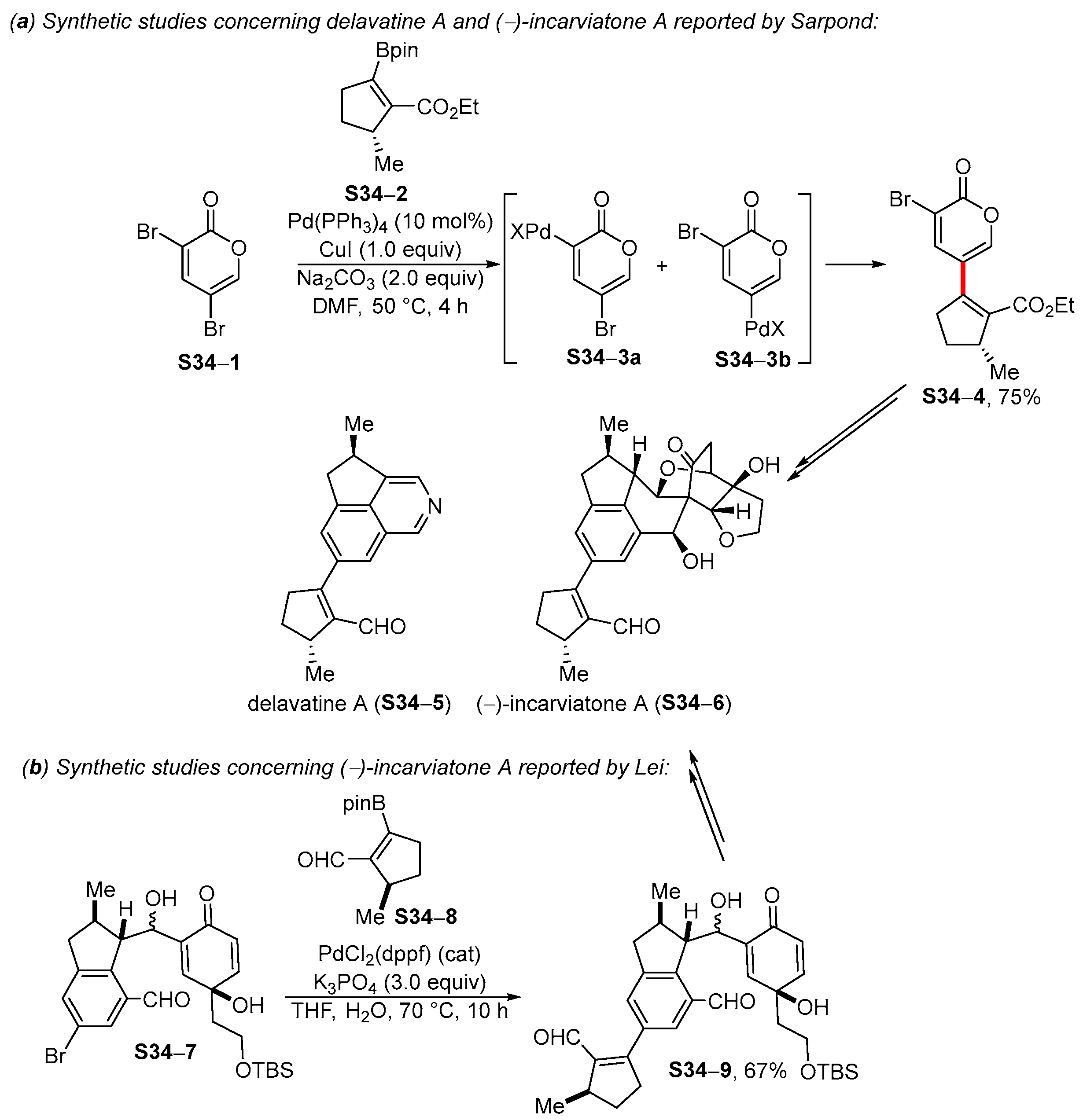
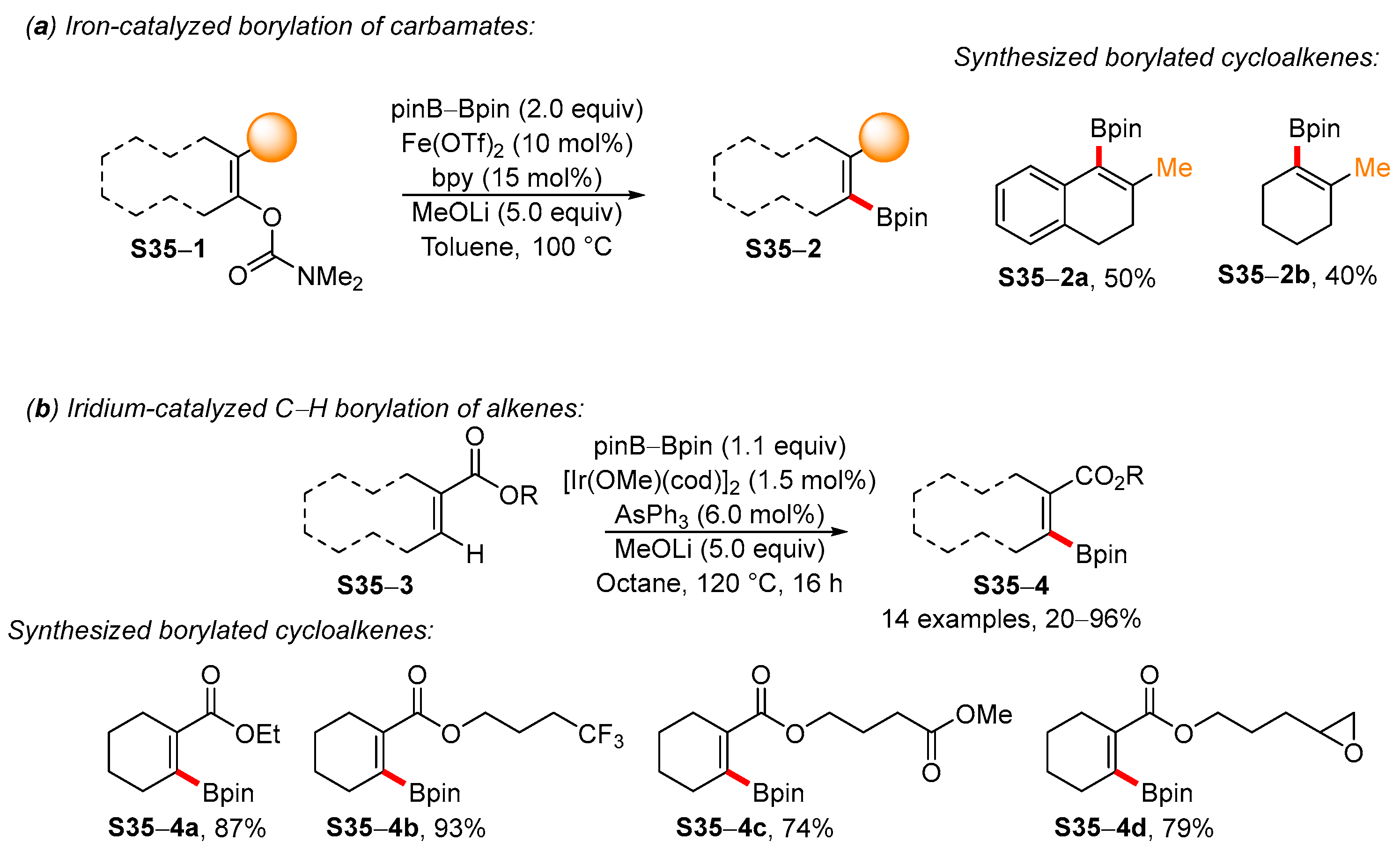
4. Synthetic Approaches to and Applications of Cycloalkenylboronates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albright, H.; Davis, A.J.; Gomez-Lopez, J.L.; Vonesh, H.L.; Quach, P.K.; Lambert, T.H.; Schindler, C.S. Carbonyl–Olefin Metathesis. Chem. Rev. 2021, 121, 9359–9406. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Du, H. Shi Epoxidation: A Great Shortcut to Complex Compounds. Chin. J. Chem. 2021, 39, 2016–2026. [Google Scholar] [CrossRef]
- Kraft, S.; Ryan, K.; Kargbo, R.B. Recent Advances in Asymmetric Hydrogenation of Tetrasubstituted Olefins. J. Am. Chem. Soc. 2017, 139, 11630–11641. [Google Scholar] [CrossRef] [PubMed]
- Wickham, L.M.; Giri, R. Transition Metal (Ni, Cu, Pd)-Catalyzed Alkene Dicarbofunctionalization Reactions. Acc. Chem. Res. 2021, 54, 3415–3437. [Google Scholar] [CrossRef]
- Yao, H.; Hu, W.; Zhang, W. Difunctionalization of Alkenes and Alkynes via Intermolecular Radical and Nucleophilic Additions. Molecules 2020, 26, 105. [Google Scholar] [CrossRef]
- Tandon, N.; Luxami, V.; Tandon, R.; Paul, K. Recent Advances in the Synthesis of Tamoxifen and Analogues in Medicinal Chemistry. Asian J. Org. Chem. 2020, 9, 1432–1465. [Google Scholar] [CrossRef]
- Rajendran, S.; Swaroop, S.S.; Roy, J.; Inemai, E.; Murugan, S.; Rayala, S.K.; Venkatraman, G. p21 activated kinase-1 and tamoxifen—A deadly nexus impacting breast cancer outcomes. BBA Rev. Cancer 2022, 1877, 188668. [Google Scholar] [CrossRef]
- Tsoi, H.; You, C.P.; Leung, M.H.; Man, E.P.S.; Khoo, U.S. Targeting Ribosome Biogenesis to Combat Tamoxifen Resistance in ER+ve Breast Cancer. Cancers 2022, 14, 1251. [Google Scholar] [CrossRef]
- Zewdie, K.A.; Hailu, H.G.; Ayza, M.A.; Tesfaye, B.A. Antileishmanial Activity of Tamoxifen by Targeting Sphingolipid Metabolism: A Review. Clin. Pharmacol. 2022, 14, 11–17. [Google Scholar] [CrossRef]
- Buttard, F.; Sharma, J.; Champagne, P.A. Recent advances in the stereoselective synthesis of acyclic all-carbon tetrasubstituted alkenes. Chem. Commun. 2021, 57, 4071–4088. [Google Scholar] [CrossRef]
- Flynn, A.B.; Ogilvie, W.W. Stereocontrolled Synthesis of Tetrasubstituted Olefins. Chem. Rev. 2007, 107, 4698–4745. [Google Scholar] [CrossRef]
- Fallis, A.G.; Forgione, P. Metal mediated carbometallation of alkynes and alkenes containing adjacent heteroatoms. Tetrahedron 2001, 57, 5899–5913. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakrabortty, R.; Ganesh, V. Dual Functionalization of Alkynes Utilizing the Redox Characteristics of Transition Metal Catalysts. ChemCatChem 2021, 13, 4262–4298. [Google Scholar] [CrossRef]
- Gillbard, S.M.; Lam, H.W. Nickel-Catalyzed Arylative Cyclizations of Alkyne- and Allene-Tethered Electrophiles using Arylboron Reagents. Chem. Eur. J. 2022, 28, e202104230. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, X.; Yu, X.; Wu, J. Double carbometallation of alkynes: An efficient strategy for the construction of polycycles. Chem. Soc. Rev. 2014, 43, 834–846. [Google Scholar] [CrossRef]
- Schitter, T.; Reding, A.; Werz, D.B. Cascades Involving anti-Carbopalladation Steps: From Our Initial Hypothesis to Natural Product Synthesis. Synlett 2019, 30, 1275–1288. [Google Scholar] [CrossRef]
- Edlová, T.; Čubiňák, M.; Tobrman, T. Cross-Coupling Reactions of Double or Triple Electrophilic Templates for Alkene Synthesis. Synthesis 2021, 53, 255–266. [Google Scholar]
- Negishi, E.-i.; Huang, Z.; Wang, G.; Mohan, S.; Wang, C.; Hattori, H. Recent Advances in Efficient and Selective Synthesis of Di-, Tri-, and Tetrasubstituted Alkenes via Pd-Catalyzed Alkenylation-Carbonyl Olefination Synergy. Acc. Chem. Res. 2008, 41, 1474–1485. [Google Scholar] [CrossRef]
- Polák, P.; Váňová, H.; Dvořák, D.; Tobrman, T. Recent progress in transition metal-catalyzed stereoselective synthesis of acyclic all-carbon tetrasubstituted alkenes. Tetrahedron Lett. 2016, 57, 3684–3693. [Google Scholar] [CrossRef]
- Reiser, O. Palladium-Catalyzed Coupling Reactions for the Stereoselective Synthesis of Tri- and Tetrasubstituted Alkenes. Angew. Chem. Int. Ed. 2006, 45, 2838–2840. [Google Scholar] [CrossRef]
- Yoshida, H. Borylation and Stannylation Reactions with Tuning of Lewis Acidity. Chem. Rec. 2021, 21, 3483–3497. [Google Scholar] [CrossRef]
- Yoshida, H. Borylation of Alkynes under Base/Coinage Metal Catalysis: Some Recent Developments. ACS Catal. 2016, 6, 1799–1811. [Google Scholar] [CrossRef]
- Velasco, R.; Feberero, C.; Sanz, R. α-Lithiated Aryl Benzyl Ethers: Inhibition of [1,2]-Wittig Rearrangement and Application to the Synthesis of Benzo[b]furan Derivatives. Org. Lett. 2015, 17, 4416–4419. [Google Scholar] [CrossRef]
- Eiji, S.; Tamejiro, H. Transition Metal-Catalyzed Carbostannylation of Alkynes and Dienes. Bull. Chem. Soc. Jpn. 2002, 75, 1435–1450. [Google Scholar] [CrossRef]
- Yoshida, H. Stannylation Reactions under Base Metal Catalysis: Some Recent Advances. Synthesis 2016, 48, 2540–2552. [Google Scholar] [CrossRef]
- Mukai, C.; Kozaka, T.; Suzuki, Y.; Kim, I.J. New entry to the Pauson–Khand reaction: Trimethylgermyl group at the triple bond terminus as a latent functional group. Tetrahedron 2004, 60, 2497–2507. [Google Scholar] [CrossRef]
- Mohamed, R.K.; Mondal, S.; Gold, B.; Evoniuk, C.J.; Banerjee, T.; Hanson, K.; Alabugin, I.V. Alkenes as Alkyne Equivalents in Radical Cascades Terminated by Fragmentations: Overcoming Stereoelectronic Restrictions on Ring Expansions for the Preparation of Expanded Polyaromatics. J. Am. Chem. Soc. 2015, 137, 6335–6349. [Google Scholar] [CrossRef]
- Dalkılıç, E.; Daştan, A. Synthesis of cyclopentadiene derivatives by retro-Diels–Alder reaction of norbornadiene derivatives. Tetrahedron 2015, 71, 1966–1970. [Google Scholar] [CrossRef]
- Shipe, W.D.; Sorensen, E.J. A Convergent Synthesis of the Tricyclic Architecture of the Guanacastepenes Featuring a Selective Ring Fragmentation. Org. Lett. 2002, 4, 2063–2066. [Google Scholar] [CrossRef]
- Shipe, W.D.; Sorensen, E.J. Convergent, Enantioselective Syntheses of Guanacastepenes A and E Featuring a Selective Cyclobutane Fragmentation. J. Am. Chem. Soc. 2006, 128, 7025–7035. [Google Scholar] [CrossRef]
- Masters, K.-S.; Flynn, B.L. An efficient synthesis of (±)-frondosin B using a Stille–Heck reaction sequence. Org. Biomol. Chem. 2010, 8, 1290–1292. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Qu, T.; Mulder, M.; Noetzel, M.J.; Lindsley, C.W.; Sulikowski, G.A. Synthesis and bioactivity of (±)-tetrahydrohaliclonacyclamine A. Tetrahedron 2010, 66, 4805–4810. [Google Scholar] [CrossRef]
- Smith, B.J.; Sulikowski, G.A. Total Synthesis of (±)-Haliclonacyclamine C. Angew. Chem. Int. Ed. 2010, 49, 1599–1602. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Gladen, P.T.; Pinchman, J.R. Total Synthesis of Neodolastane Diterpenes Trichoaurantianolides C and D. J. Org. Chem. 2015, 80, 5474–5493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Li, J.; Yang, F.; Liu, G.; Tang, W.; He, W.; Fu, J.-J.; Shen, Y.-H.; Li, A.; et al. Total Synthesis and Stereochemical Assignment of Delavatine A: Rh-Catalyzed Asymmetric Hydrogenation of Indene-Type Tetrasubstituted Olefins and Kinetic Resolution through Pd-Catalyzed Triflamide-Directed C–H Olefination. J. Am. Chem. Soc. 2017, 139, 5558–5567. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, H.; Celik, M.A.; Dellermann, T.; Frenking, G.; Hammond, K.; Hupp, F.; Kelch, H.; Krummenacher, I.; Lindl, F.; Mailänder, L.; et al. Scope of the Thermal Ring-Expansion Reaction of Boroles with Organoazides. Chem. Eur. J. 2017, 23, 8006–8013. [Google Scholar] [CrossRef] [PubMed]
- Chaładaj, W.; Domański, S. Mild and Functional Group Tolerant Method for Tandem Palladium-Catalyzed Carbocyclization–Coupling of ε-Acetylenic β-Ketoesters with Aryl Bromides and Chlorides. Adv. Synth. Catal. 2016, 358, 1820–1825. [Google Scholar] [CrossRef]
- Iwasaki, M.; Araki, Y.; Iino, S.; Nishihara, Y. Synthesis of Multisubstituted Triphenylenes and Phenanthrenes by Cascade Reaction of o-Iodobiphenyls or (Z)-β-Halostyrenes with o-Bromobenzyl Alcohols through Two Sequential C–C Bond Formations Catalyzed by a Palladium Complex. J. Org. Chem. 2015, 80, 9247–9263. [Google Scholar] [CrossRef]
- Piers, E.; Romero, M.Á. Palladium-Catalyzed Intramolecular Decarboxylative Allylation of (2-Trimethylstannyl)allyl β-Oxocarboxylates. Synthesis 2011, 4017–4022. [Google Scholar] [CrossRef]
- Ruan, Y.; Wang, B.-Y.; Erb, J.M.; Chen, S.; Hadad, C.M.; Badjić, J.D. On the role of guests in enforcing the mechanism of action of gated baskets. Org. Biomol. Chem. 2013, 11, 7667–7675. [Google Scholar] [CrossRef]
- Tanino, K.; Yamada, T.; Yoshimura, F.; Suzuki, T. Cyanoazulene-based Multistage Redox Systems Prepared from Vinylcyclopropanecarbonitrile and Cyclopentenone via Divinylcyclopropane-rearrangement Approach. Chem. Lett. 2014, 43, 607–609. [Google Scholar] [CrossRef]
- Kats-Kagan, R.; Herzon, S.B. The Discovery of a Novel Route to Highly Substituted α-Tropolones Enables Expedient Entry to the Core of the Gukulenins. Org. Lett. 2015, 17, 2030–2033. [Google Scholar] [CrossRef]
- Dastan, A.; Uzundumlu, E.; Balci, M.; Fabris, F.; De Lucchi, O. An Investigation on the Synthesis of New Molecular Architectures from the Cyclotrimerisation of exo- and endo-Benzotricyclo[4.2.1.02,5]nonene. Eur. J. Org. Chem. 2004, 183–192. [Google Scholar] [CrossRef]
- Shintani, R.; Kurata, H.; Nozaki, K. Intermolecular Three-Component Arylsilylation of Alkynes under Palladium/Copper Cooperative Catalysis. J. Org. Chem. 2016, 81, 3065–3069. [Google Scholar] [CrossRef]
- Zhou, B.; Lu, A.; Shao, C.; Liang, X.; Zhang, Y. Palladium-catalyzed sequential three-component reactions to access vinylsilanes. Chem. Commun. 2018, 54, 10598–10601. [Google Scholar] [CrossRef]
- Wisthoff, M.F.; Pawley, S.B.; Cinderella, A.P.; Watson, D.A. Stereoselective Synthesis of Cis- and Trans-Tetrasubstituted Vinyl Silanes Using a Silyl-Heck Strategy and Hiyama Conditions for Their Cross-Coupling. J. Am. Chem. Soc. 2020, 142, 12051–12055. [Google Scholar] [CrossRef]
- Denmark, S.E.; Kallemeyn, J.M. Stereospecific Palladium-Catalyzed Cross-Coupling of (E)- and (Z)-Alkenylsilanolates with Aryl Chlorides. J. Am. Chem. Soc. 2006, 128, 15958–15959. [Google Scholar] [CrossRef]
- Kuang, Z.; Gao, G.; Song, Q. Base-catalyzed diborylation of alkynes: Synthesis and applications of cis-1,2-bis(boryl)alkenes. Sci. China Chem. 2019, 62, 62–66. [Google Scholar] [CrossRef]
- Ito, S.; Fukazawa, M.; Takahashi, F.; Nogi, K.; Yorimitsu, H. Sodium-Metal-Promoted Reductive 1,2-syn-Diboration of Alkynes with Reduction-Resistant Trimethoxyborane. Bull. Chem. Soc. Jpn. 2020, 93, 1171–1179. [Google Scholar] [CrossRef]
- Nakagawa, N.; Hatakeyama, T.; Nakamura, M. Iron-Catalyzed Diboration and Carboboration of Alkynes. Chem. Eur. J. 2015, 21, 4257–4261. [Google Scholar] [CrossRef]
- Ferrand, L.; Lyu, Y.; Rivera-Hernández, A.; Fallon, B.J.; Amatore, M.; Aubert, C.; Petit, M. Hydroboration and Diboration of Internal Alkynes Catalyzed by a Well-Defined Low-Valent Cobalt Catalyst. Synthesis 2017, 49, 3895–3904. [Google Scholar] [CrossRef]
- Hyodo, K.; Suetsugu, M.; Nishihara, Y. Diborylation of Alkynyl MIDA Boronates and Sequential Chemoselective Suzuki–Miyaura Couplings: A Formal Carboborylation of Alkynes. Org. Lett. 2014, 16, 440–443. [Google Scholar] [CrossRef]
- Lai, Q.; Ozerov, O.V. Dehydrogenative Diboration of Alkynes Catalyzed by Ir/CO/tBuNC System. J. Organomet. Chem. 2021, 931, 121614. [Google Scholar] [CrossRef]
- Lee, C.-I.; Shih, W.-C.; Zhou, J.; Reibenspies, J.H.; Ozerov, O.V. Synthesis of Triborylalkenes from Terminal Alkynes by Iridium-Catalyzed Tandem C–H Borylation and Diboration. Angew. Chem. Int. Ed. 2015, 54, 14003–14007. [Google Scholar] [CrossRef]
- Liu, X.; Ming, W.; Friedrich, A.; Kerner, F.; Marder, T.B. Copper-Catalyzed Triboration of Terminal Alkynes Using B2pin2: Efficient Synthesis of 1,1,2-Triborylalkenes. Angew. Chem. Int. Ed. 2020, 59, 304–309. [Google Scholar] [CrossRef]
- Yoshii, D.; Jin, X.; Mizuno, N.; Yamaguchi, K. Selective Dehydrogenative Mono- or Diborylation of Styrenes by Supported Copper Catalysts. ACS Catal. 2019, 9, 3011–3016. [Google Scholar] [CrossRef]
- Semba, K.; Yoshizawa, M.; Ohtagaki, Y.; Nakao, Y. Arylboration of Internal Alkynes by Cooperative Palladium/Copper Catalysis. Bull. Chem. Soc. Jpn. 2017, 90, 1340–1343. [Google Scholar] [CrossRef]
- Huang, Y.; Bergmann, A.M.; Brown, M.K. (Hetero)arylboration of alkynes: A strategy for the synthesis of α,α-bis(hetero)arylketones. Org. Biomol. Chem. 2019, 17, 5913–5915. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Zou, J.-Y.; Zhong, Q.; Li, S.-S.; Zhao, J. Synergistic Pd/Cu-catalysed regio- and stereoselective borylation of allenylic carbonates. Chem. Commun. 2022, 58, 1037–1040. [Google Scholar] [CrossRef]
- Wigman, B.; Lee, W.; Wei, W.; Houk, K.N.; Nelson, H.M. Electrochemical Fluorination of Vinyl Boronates through Donor-Stabilized Vinyl Carbocation Intermediates. Angew. Chem. Int. Ed. 2022, 61, e202113972. [Google Scholar] [CrossRef]
- Nozawa, R.; Shinokubo, H. Synthesis and Properties of meso-Arylated Corrphycenes. Org. Lett. 2017, 19, 4928–4931. [Google Scholar] [CrossRef] [PubMed]
- La Cascia, E.; Cuenca, A.B.; Fernández, E. Opportune gem-Silylborylation of Carbonyl Compounds: A Modular and Stereocontrolled Entry to Tetrasubstituted Olefins. Chem. Eur. J. 2016, 22, 18737–18741. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Hartwig, J.F. Iridium-catalyzed diborylation of benzylic C–H bonds directed by a hydrosilyl group: Synthesis of 1,1-benzyldiboronate esters. Chem. Sci. 2014, 5, 694–698. [Google Scholar] [CrossRef]
- Zhou, Y.; You, W.; Smith, K.B.; Brown, M.K. Copper-Catalyzed Cross-Coupling of Boronic Esters with Aryl Iodides and Application to the Carboboration of Alkynes and Allenes. Angew. Chem. Int. Ed. 2014, 53, 3475–3479. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Xu, J.; Zhu, Q.; Liu, C. Synthesis of Allylboronates via Zweifel-type Deprotonative Olefination. Adv. Synth. Catal. 2021, 363, 2403–2407. [Google Scholar] [CrossRef]
- Bin, H.-Y.; Wei, X.; Zi, J.; Zuo, Y.-J.; Wang, T.-C.; Zhong, C.-M. Substrate-Controlled Regio- and Stereoselective Synthesis of Boron-Substituted 1,4-Dienes via Copper-Catalyzed Boryl–Allylation of Alkynes with Allyl Phosphates and Bis(pinacolato)diboron. ACS Catal. 2015, 5, 6670–6679. [Google Scholar] [CrossRef]
- Itoh, T.; Shimizu, Y.; Kanai, M. Ligand-Enabled, Copper-Catalyzed Regio- and Stereoselective Synthesis of Trialkylsubstituted Alkenylboronates from Unactivated Internal Alkynes. J. Am. Chem. Soc. 2016, 138, 7528–7531. [Google Scholar] [CrossRef]
- Sanderson, M.D.; Kamplain, J.W.; Bielawski, C.W. Quinone-Annulated N-Heterocyclic Carbene-Transition-Metal Complexes: Observation of π-Backbonding Using FT-IR Spectroscopy and Cyclic Voltammetry. J. Am. Chem. Soc. 2006, 128, 16514–16515. [Google Scholar] [CrossRef]
- Kubota, K.; Iwamoto, H.; Yamamoto, E.; Ito, H. Silicon-Tethered Strategy for Copper(I)-Catalyzed Stereo- and Regioselective Alkylboration of Alkynes. Org. Lett. 2015, 17, 620–623. [Google Scholar] [CrossRef]
- Muchnij, J.A.; Kwaramba, F.B.; Rahaim, R.J. Sterically Directed Iridium-Catalyzed Hydrosilylation of Alkenes in the Presence of Alkynes. Org. Lett. 2014, 16, 1330–1333. [Google Scholar] [CrossRef]
- Tamao, K.; Ishida, N.; Kumada, M. (Diisopropoxymethylsilyl)methyl Grignard reagent: A new, practically useful nucleophilic hydroxymethylating agent. J. Org. Chem. 1983, 48, 2120–2122. [Google Scholar] [CrossRef]
- Iwamoto, H.; Ozawa, Y.; Kubota, K.; Ito, H. Copper(I)-Catalyzed Regio- and Stereoselective Intramolecular Alkylboration of Propargyl Ethers and Amines. J. Org. Chem. 2017, 82, 10563–10573. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, B.; Qiu, Y.; Bäckvall, J.-E. Olefin-Directed Palladium-Catalyzed Regio- and Stereoselective Hydroboration of Allenes. Chem. Eur. J. 2016, 22, 2939–2943. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, B.; Zhu, C.; Bäckvall, J.-E. Palladium-Catalyzed Oxidative Carbocyclization–Borylation of Enallenes to Cyclobutenes. Angew. Chem. Int. Ed. 2016, 55, 6520–6524. [Google Scholar] [CrossRef]
- Jin, H.; Fürstner, A. Regioselective trans-Carboboration of Propargyl Alcohols. Org. Lett. 2019, 21, 3446–3450. [Google Scholar] [CrossRef]
- Takahashi, F.; Nogi, K.; Sasamori, T.; Yorimitsu, H. Diborative Reduction of Alkynes to 1,2-Diboryl-1,2-Dimetalloalkanes: Its Application for the Synthesis of Diverse 1,2-Bis(boronate)s. Org. Lett. 2019, 21, 4739–4744. [Google Scholar] [CrossRef]
- Tani, T.; Takahashi, N.; Sawatsugawa, Y.; Osano, M.; Tsuchimoto, T. Stepwise Suzuki–Miyaura Cross-Coupling of Triborylalkenes Derived from Alkynyl–B(dan)s: Regioselective and Flexible Synthesis of Tetrasubstituted Alkenes. Adv. Synth. Catal. 2021, 363, 2427–2442. [Google Scholar] [CrossRef]
- Tani, T.; Sawatsugawa, Y.; Sano, Y.; Hirataka, Y.; Takahashi, N.; Hashimoto, S.; Sugiura, T.; Tsuchimoto, T. Alkynyl–B(dan)s in Various Palladium-Catalyzed Carbon–Carbon Bond-Forming Reactions Leading to Internal Alkynes, 1,4-Enynes, Ynones, and Multiply Substituted Alkenes. Adv. Synth. Catal. 2019, 361, 1815–1834. [Google Scholar] [CrossRef]
- Tsuchimoto, T.; Utsugi, H.; Sugiura, T.; Horio, S. Alkynylboranes: A Practical Approach by Zinc-Catalyzed Dehydrogenative Coupling of Terminal Alkynes with 1,8-Naphthalenediaminatoborane. Adv. Synth. Catal. 2015, 357, 77–82. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, Y.; Stang, P.J.; Zhao, W. Divergent and Stereoselective Synthesis of Tetraarylethylenes from Vinylboronates. Angew. Chem. Int. Ed. 2020, 59, 20090–20098. [Google Scholar] [CrossRef]
- Biswas, S.; Jana, D.; Kumar, G.S.; Maji, S.; Kundu, P.; Ghorai, U.K.; Giri, R.P.; Das, B.; Chattopadhyay, N.; Ghorai, B.K.; et al. Supramolecular Aggregates of Tetraphenylethene-Cored AIEgen toward Mechanoluminescent and Electroluminescent Devices. ACS Appl. Mater. Interfaces 2018, 10, 17409–17418. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Wang, Z.; Luo, Q. Triptycene-scaffolded tetraphenylethylenes with irregular temperature-dependence AIE. Tetrahedron Lett. 2019, 60, 439–443. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, Q.; Li, J.; Zhang, S.; Zhang, C.; Lu, F.; Yang, B.; Lu, P. Efficient pyrene-imidazole derivatives for organic light-emitting diodes. RSC Adv. 2016, 6, 17239–17245. [Google Scholar] [CrossRef]
- Dong, Y.; Shen, J.; Li, W.; Zhao, R.; Pan, Y.; Song, Q.; Zhang, C. Opposite ESIPT characteristic of two AIE-active isomers with different linkage sites. Tetrahedron 2019, 75, 2670–2675. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, H.; Ma, J.; Chen, M.; Zhu, H.; Wang, W. Ladder-type conjugated oligomers prepared by the Scholl oxidative cyclodehydrogenation reaction: Synthesis, characterization and application in field effect transistors. J. Mater. Chem. C 2015, 3, 6200–6208. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, W.; Yang, J.; Ma, J.; Chen, M.; Zhu, H.; Wang, W. The synthesis, characterization and flexible OFET application of three (Z)-1,2-bis(4-(tert-butyl)phenyl)ethane based copolymers. Polym. Chem. 2016, 7, 538–545. [Google Scholar] [CrossRef]
- Yang, J.; Huang, W.; Lin, T.; Pan, X.; Zhu, H.; Huang, Y.; Wang, W. Intramolecular oxidative cyclodehydrogenation route for the synthesis of strap-like conjugated polymers. RSC Adv. 2017, 7, 10763–10773. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Zhang, X.; Li, N.; Quan, Y.; Cheng, Y. Doping-free circularly polarized electroluminescence of AIE-active chiral binaphthyl-based polymers. Chem. Commun. 2018, 54, 9663–9666. [Google Scholar] [CrossRef]
- Bijesh, S.; Misra, R. Triphenylamine Functionalized Unsymmetrical Quinoxalines. Asian J. Org. Chem. 2018, 7, 1882–1892. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Li, X.; Quan, Y.; Cheng, Y. The amplified circularly polarized luminescence regulated from D–A type AIE-active chiral emitters via liquid crystals system. Chem. Commun. 2020, 56, 1117–1120. [Google Scholar] [CrossRef]
- Maragani, R.; Sharma, R.; Misra, R. Donor-Acceptor Triphenylvinyl and Tetraphenyl Conjugates: Synthesis, Aggregation and Computational Studies. ChemistrySelect 2017, 2, 10033–10037. [Google Scholar] [CrossRef]
- Qi, J.; Duan, X.; Cai, Y.; Jia, S.; Chen, C.; Zhao, Z.; Li, Y.; Peng, H.-Q.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Simultaneously boosting the conjugation, brightness and solubility of organic fluorophores by using AIEgens. Chem. Sci. 2020, 11, 8438–8447. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, A.T.; Liu, L.H. Many-membered Carbon Rings. VII. Cycloöctyne. J. Am. Chem. Soc. 1953, 75, 2153–2154. [Google Scholar] [CrossRef]
- Green, J.R. Cycloheptyne–cobalt complexes via allylation of stabilized γ-carbonyl cations. Chem. Commun. 1998, 1751–1752. [Google Scholar] [CrossRef]
- Farmer, M.E.; Ehehalt, L.E.; Pabst, T.P.; Tudge, M.T.; Chirik, P.J. Well-Defined Cationic Cobalt(I) Precatalyst for Olefin-Alkyne [2 + 2] Cycloaddition and Olefin-Diene Hydrovinylation Reactions: Experimental Evidence for Metallacycle Intermediates. Organometallics 2021, 40, 3599–3607. [Google Scholar] [CrossRef]
- Polák, P.; Tobrman, T. Novel Selective Approach to Terminally Substituted [n]Dendralenes. Eur. J. Org. Chem. 2019, 2019, 957–968. [Google Scholar] [CrossRef]
- Nowikow, C.; Fuerst, R.; Kauderer, M.; Dank, C.; Schmid, W.; Hajduch, M.; Rehulka, J.; Gurska, S.; Mokshyna, O.; Polishchuk, P.; et al. Synthesis and biological evaluation of cis-restrained carbocyclic combretastatin A-4 analogs: Influence of the ring size and saturation on cytotoxic properties. Bioorg. Med. Chem. 2019, 27, 115032. [Google Scholar] [CrossRef]
- León, T.; Fernández, E. The Pauson–Khand reaction using alkynylboronic esters: Solving a long-standing regioselectivity issue. Chem. Commun. 2016, 52, 9363–9366. [Google Scholar] [CrossRef][Green Version]
- Albarghouti, G.; Rayyan, S. General Method for the Synthesis of Substituted Cyclopentenones via α-Borylzirconacyclopentene Intermediates. Org. Prep. Proced. Int. 2020, 52, 1–8. [Google Scholar] [CrossRef]
- Sarter, C.; Dey, S.; Jäschke, A. Photoswitchable Oligonucleotides Containing Different Diarylethene-Modified Nucleotides. ACS Omega 2019, 4, 12125–12129. [Google Scholar] [CrossRef]
- Buckup, T.; Sarter, C.; Volpp, H.-R.; Jäschke, A.; Motzkus, M. Ultrafast Time-Resolved Spectroscopy of Diarylethene-Based Photoswitchable Deoxyuridine Nucleosides. J. Phys. Chem. Lett. 2015, 6, 4717–4721. [Google Scholar] [CrossRef]
- Kellis, D.L.; Sarter, C.; Cannon, B.L.; Davis, P.H.; Graugnard, E.; Lee, J.; Pensack, R.D.; Kolmar, T.; Jäschke, A.; Yurke, B.; et al. An All-Optical Excitonic Switch Operated in the Liquid and Solid Phases. ACS Nano 2019, 13, 2986–2994. [Google Scholar] [CrossRef]
- Altenhofer, E.; Harmata, M. Radical isomerization of borylated allylic sulfones. Tetrahedron Lett. 2015, 56, 3176–3178. [Google Scholar] [CrossRef]
- Altenhofer, E.F.; Harmata, M. Suzuki–Miyaura Coupling Reactions of Conjunctive Reagents: 2-Borylated Allylic Sulfones. J. Org. Chem. 2015, 80, 8168–8174. [Google Scholar] [CrossRef]
- Palani, V.; Hugelshofer, C.L.; Kevlishvili, I.; Liu, P.; Sarpong, R. A Short Synthesis of Delavatine A Unveils New Insights into Site-Selective Cross-Coupling of 3,5-Dibromo-2-pyrone. J. Am. Chem. Soc. 2019, 141, 2652–2660. [Google Scholar] [CrossRef]
- Palani, V.; Hugelshofer, C.L.; Sarpong, R. A Unified Strategy for the Enantiospecific Total Synthesis of Delavatine A and Formal Synthesis of Incarviatone A. J. Am. Chem. Soc. 2019, 141, 14421–14432. [Google Scholar] [CrossRef]
- Hong, B.; Li, C.; Wang, Z.; Chen, J.; Li, H.; Lei, X. Enantioselective Total Synthesis of (−)-Incarviatone A. J. Am. Chem. Soc. 2015, 137, 11946–11949. [Google Scholar] [CrossRef]
- Geng, S.; Zhang, J.; Chen, S.; Liu, Z.; Zeng, X.; He, Y.; Feng, Z. Development and Mechanistic Studies of Iron-Catalyzed Construction of Csp2–B Bonds via C–O Bond Activation. Org. Lett. 2020, 22, 5582–5588. [Google Scholar] [CrossRef]
- Sasaki, I.; Taguchi, J.; Doi, H.; Ito, H.; Ishiyama, T. Iridium(I)-catalyzed C–H Borylation of α,β-Unsaturated Esters with Bis(pinacolato)diboron. Chem. Asian J. 2016, 11, 1400–1405. [Google Scholar] [CrossRef]
- Heinrich, C.F.; Durand, D.; Starck, J.; Michelet, V. Ruthenium Metathesis: A Key Step To Access a New Cyclic Tetrasubstituted Olefin Platform. Org. Lett. 2020, 22, 7064–7067. [Google Scholar] [CrossRef]
- Warner, A.J.; Lawson, J.R.; Fasano, V.; Ingleson, M.J. Formation of C(sp2)–Boronate Esters by Borylative Cyclization of Alkynes Using BCl3. Angew. Chem. Int. Ed. 2015, 54, 11245–11249. [Google Scholar] [CrossRef] [PubMed]
- Kamio, S.; Yoshida, H. Synthetic Chemistry with Lewis Acidity-Diminished B(aam) and B(dan) Groups: Borylation Reactions and Direct Cross-Couplings. Adv. Synth. Catal. 2021, 363, 2310–2324. [Google Scholar] [CrossRef]
- Kubota, K.; Iwamoto, H.; Ito, H. Formal nucleophilic borylation and borylative cyclization of organic halides. Org. Biomol. Chem. 2017, 15, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Steven, A. Micelle-Mediated Chemistry in Water for the Synthesis of Drug Candidates. Synthesis 2019, 51, 2632–2647. [Google Scholar] [CrossRef]
- Yamamoto, E.; Ukigai, S.; Ito, H. Boryl substitution of functionalized aryl-, heteroaryl- and alkenyl halides with silylborane and an alkoxy base: Expanded scope and mechanistic studies. Chem. Sci. 2015, 6, 2943–2951. [Google Scholar] [CrossRef]
- Gunther, M.J.; Pavlović, R.Z.; Finnegan, T.J.; Wang, X.; Badjić, J.D. Enantioselective Construction of Modular and Asymmetric Baskets. Angew. Chem. Int. Ed. 2021, 60, 25075–25081. [Google Scholar] [CrossRef]
- Zhao, X.; Song, C.; Rainier, J.D. Stereodivergent Photoelectrocyclization Reactions of Bis-aryl Cycloalkenones: Intercepting Photoelectrocyclization Intermediates with Acid. Org. Lett. 2019, 21, 8611–8614. [Google Scholar] [CrossRef]
- Zhao, X.; Song, C.; Rainier, J.D. Photoelectrocyclization Reactions of Conjugated Cycloalkenones: Scope and Reactivity. J. Org. Chem. 2020, 85, 5449–5463. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, C.; Zhang, Z.; Han, C.; Wang, X.; Li, Y.; Chen, K.; Zhao, J. Cut and sew: Benzofuran-ring-opening enabled cyclopentenone ring formation. Chem. Commun. 2020, 56, 12817–12820. [Google Scholar] [CrossRef]
- Deng, T.; Mazumdar, W.; Ford, R.L.; Jana, N.; Izar, R.; Wink, D.J.; Driver, T.G. Oxidation of Nonactivated Anilines to Generate N-Aryl Nitrenoids. J. Am. Chem. Soc. 2020, 142, 4456–4463. [Google Scholar] [CrossRef]
- Deng, T.; Shi, E.; Thomas, E.; Driver, T.G. I(III)-Catalyzed Oxidative Cyclization–Migration Tandem Reactions of Unactivated Anilines. Org. Lett. 2020, 22, 9102–9106. [Google Scholar] [CrossRef]
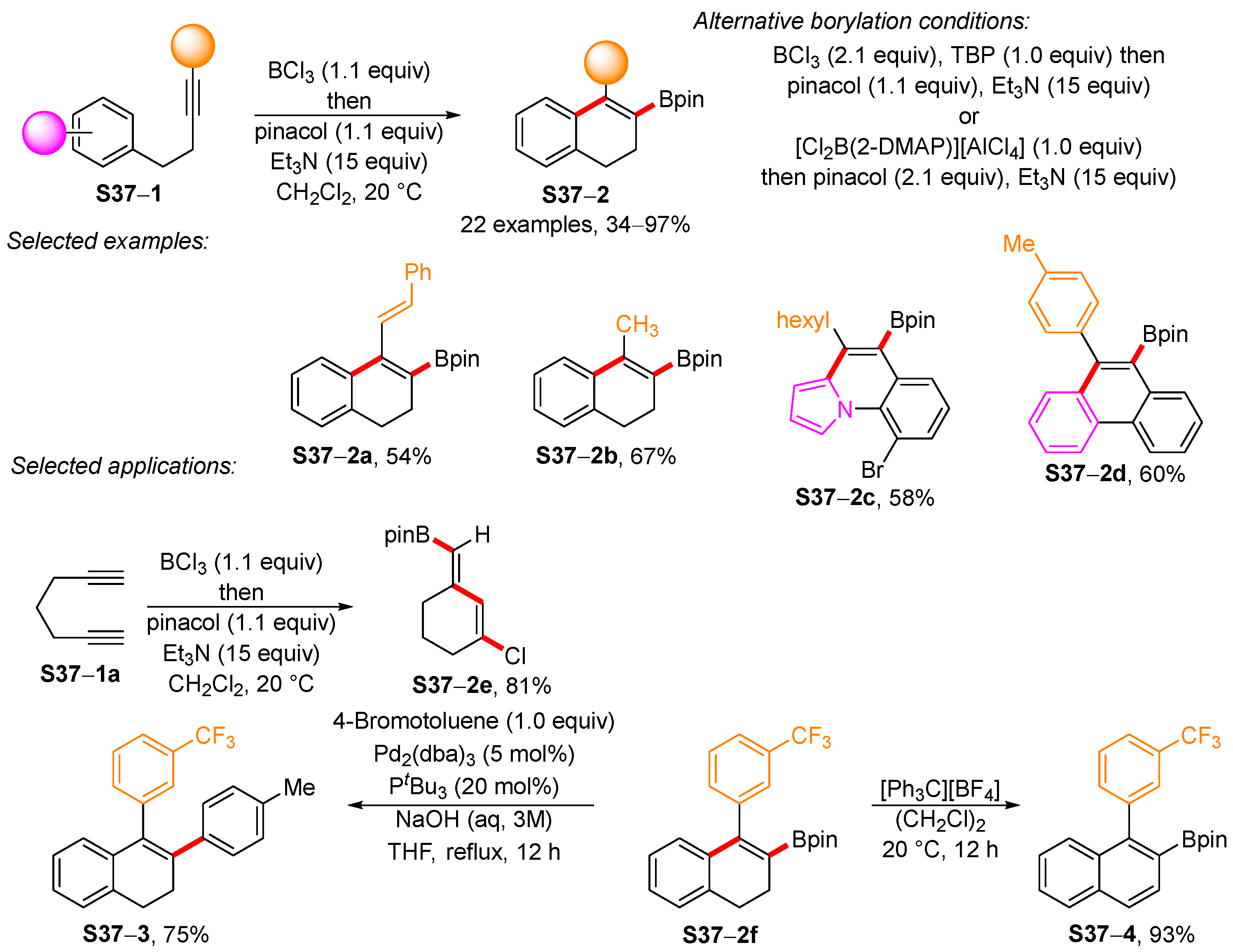
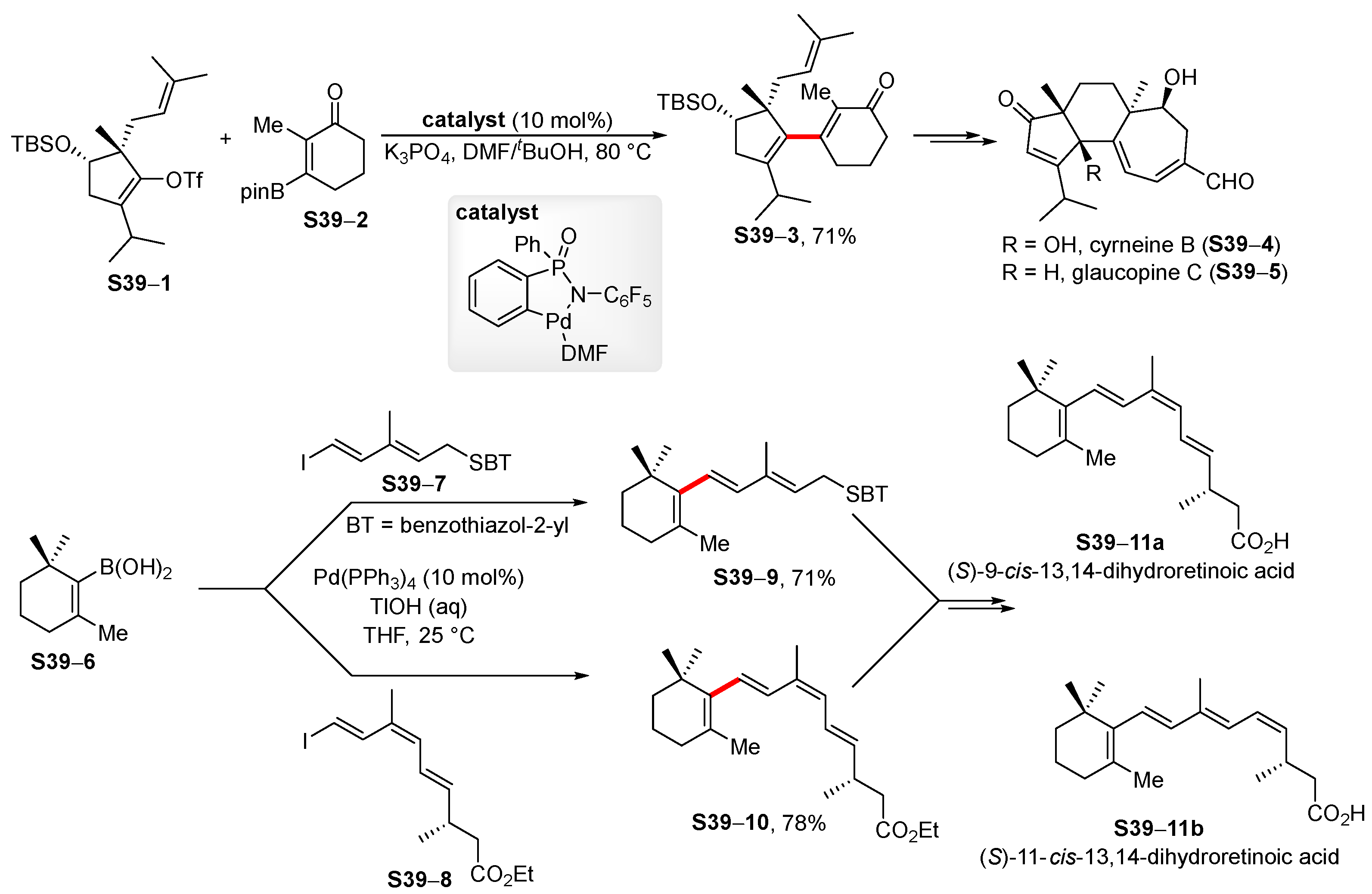
- Wu, G.-J.; Han, X. An improved synthesis of the [5.6.7]-tricyclic core of cyrneine B and glaucopine C. Org. Chem. Front. 2020, 7, 2960–2964. [Google Scholar] [CrossRef]
- Vaz, B.; Alvarez, R.; de Lera, A.R. Stereocontrolled synthesis of (S)-9-cis- and (S)-11-cis-13,14-dihydroretinoic acid. Tetrahedron 2016, 72, 3898–3904. [Google Scholar] [CrossRef]


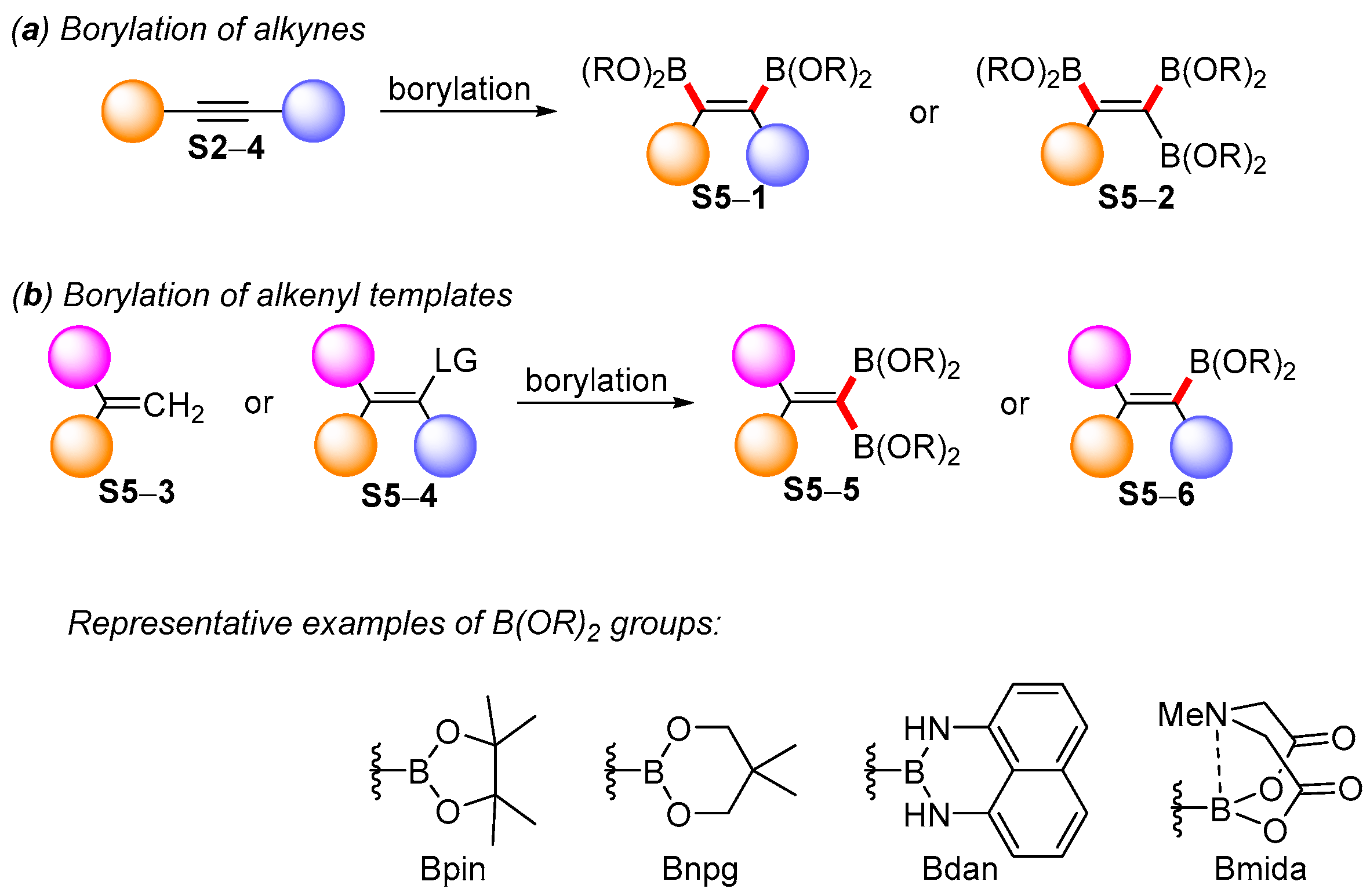






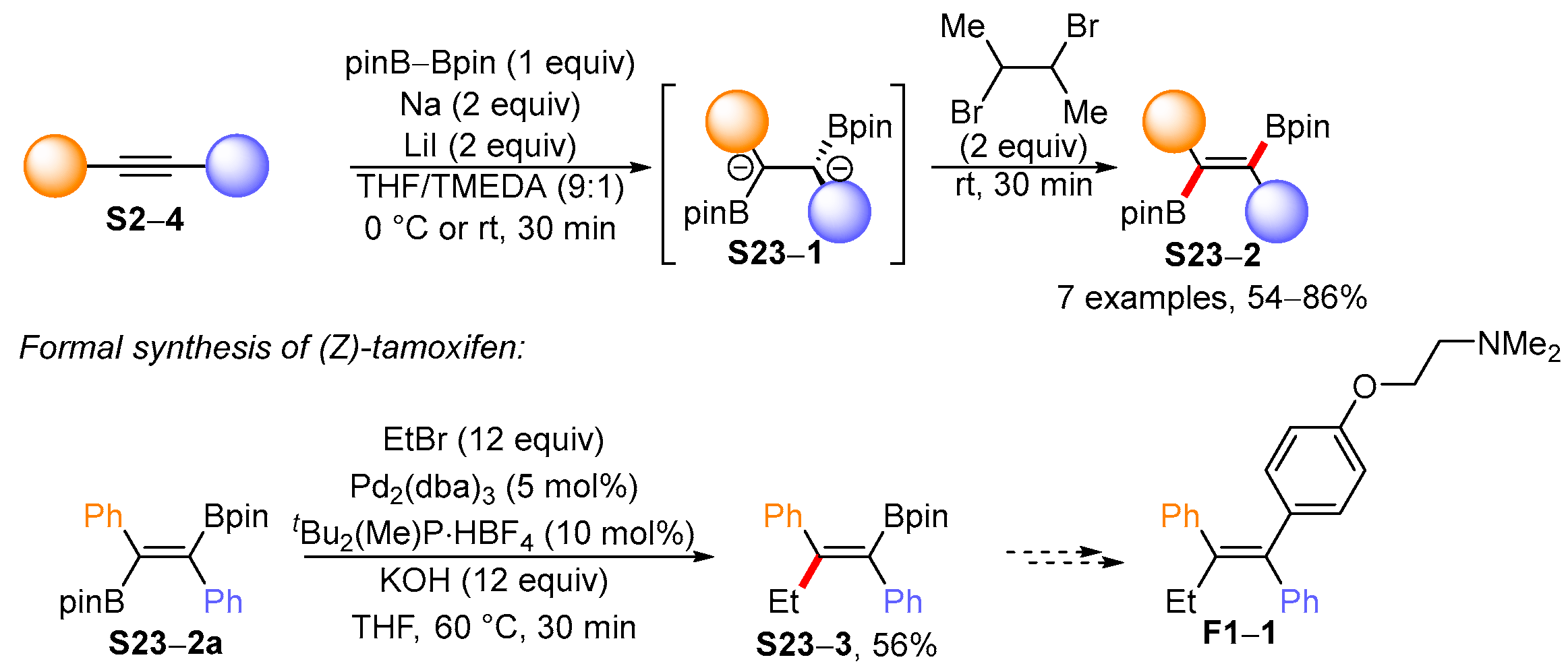
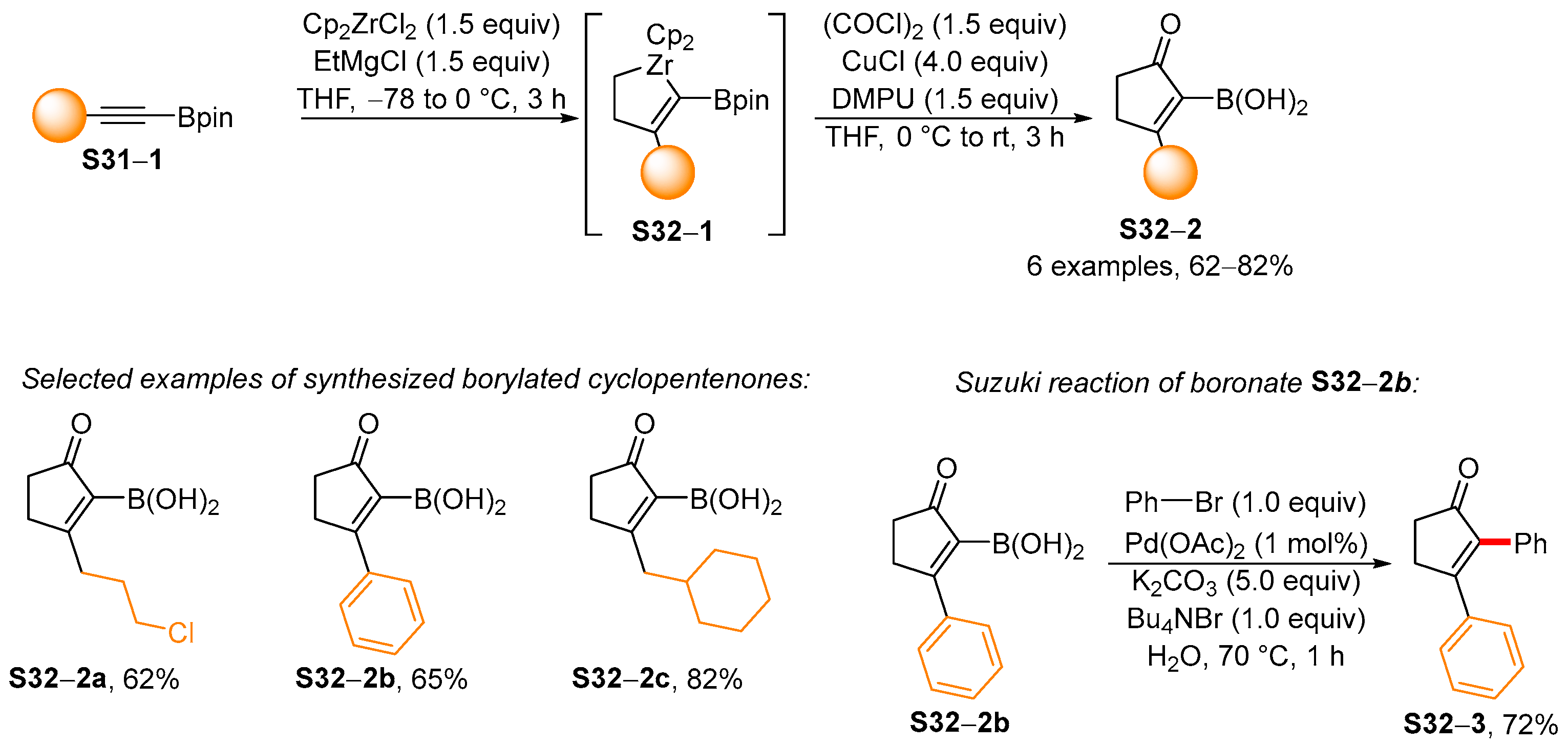


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobrman, T.; Mrkobrada, S. Palladium-Catalyzed Cross-Coupling Reactions of Borylated Alkenes for the Stereoselective Synthesis of Tetrasubstituted Double Bond. Organics 2022, 3, 210-239. https://doi.org/10.3390/org3030017
Tobrman T, Mrkobrada S. Palladium-Catalyzed Cross-Coupling Reactions of Borylated Alkenes for the Stereoselective Synthesis of Tetrasubstituted Double Bond. Organics. 2022; 3(3):210-239. https://doi.org/10.3390/org3030017
Chicago/Turabian StyleTobrman, Tomáš, and Sergej Mrkobrada. 2022. "Palladium-Catalyzed Cross-Coupling Reactions of Borylated Alkenes for the Stereoselective Synthesis of Tetrasubstituted Double Bond" Organics 3, no. 3: 210-239. https://doi.org/10.3390/org3030017
APA StyleTobrman, T., & Mrkobrada, S. (2022). Palladium-Catalyzed Cross-Coupling Reactions of Borylated Alkenes for the Stereoselective Synthesis of Tetrasubstituted Double Bond. Organics, 3(3), 210-239. https://doi.org/10.3390/org3030017





