Acid Catalyzed N-Alkylation of Pyrazoles with Trichloroacetimidates
Abstract
:1. Introduction
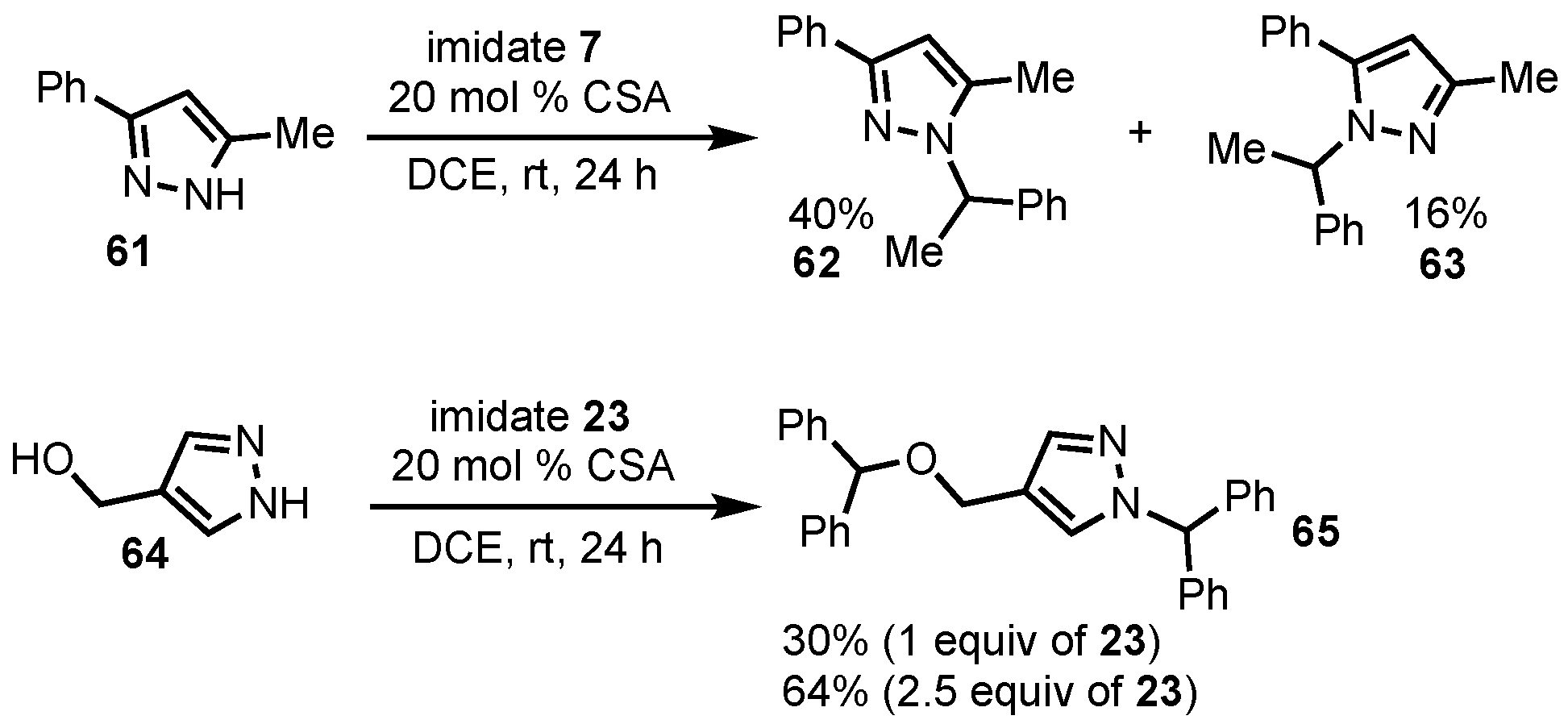
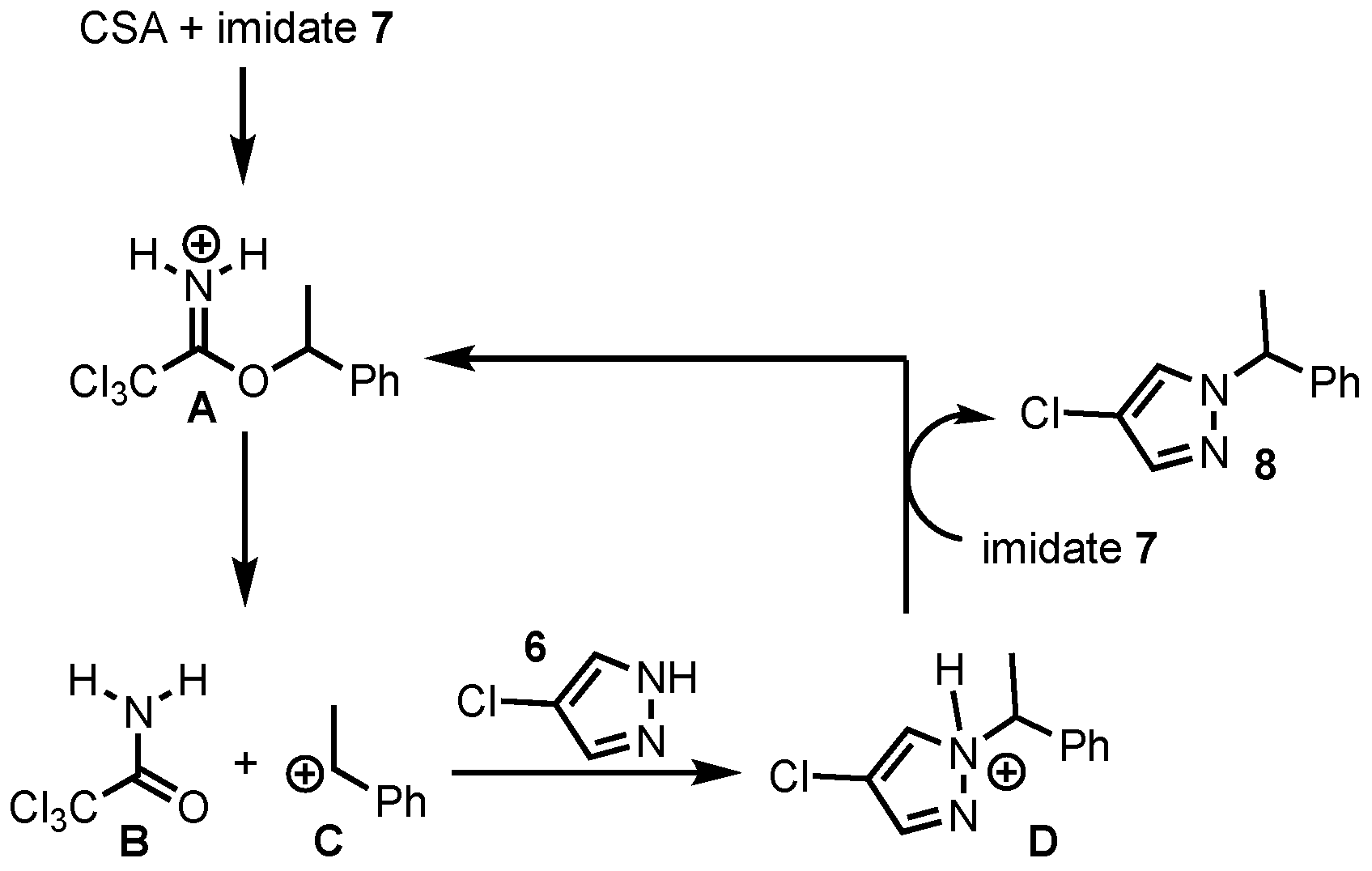
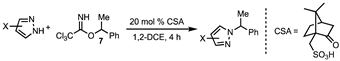
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Information
3.2. Preparation of Trichloroacetimidates
3.3. General Procedure for the Synthesis of N-Alkyl Pyrazoles
3.4. Tabulated Characterization Data for N-Alkyl Pyrazoles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gulia, N.; Daugulis, O. Palladium-Catalyzed Pyrazole-Directed sp3 C-H Bond Arylation for the Synthesis of β-Phenethylamines. Angew. Chem. Int. Ed. 2017, 56, 3630–3634. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Liu, C.; Wu, S.; Chen, C.; Zhu, B. Rhodium(III)-catalyzed unreactive C(sp3)-H alkenylation of N-alkyl-1H-pyrazoles with alkynes. Org. Biomol. Chem. 2019, 17, 7679–7683. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Liu, L.; Xu, S. Iridium-catalyzed regio- and enantioselective borylation of unbiased methylene C(sp3)-H bonds at the position β to a nitrogen center. Angew. Chem. Int. Ed. 2021, 60, 5843–5847. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, C.; Albert, R.; Floersheim, P.; Lemaire, M.; Bitch, F.; Weber, H.-P.; Andersen, E.; Hungerford, V.; Schreier, M.H. Pyrazole Bioisosteres of Leflunomide as B-Cell Immunosuppressants for Xenotransplantation and Chronic Rejection: Scope and Limitations. J. Med. Chem. 1998, 41, 3530–3538. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Carmona, A.V.; Tiwari, A.K.; Trippier, P.C. Amide Bond Bioisosteres: Strategies, Synthesis, and Successes. J. Med. Chem. 2020, 63, 12290–12358. [Google Scholar] [CrossRef]
- Graham, T.H.; Shu, M.; Verras, A.; Chen, Q.; Garcia-Calvo, M.; Li, X.; Lisnock, J.; Tong, X.; Tung, E.C.; Wiltsie, J.; et al. Pyrazoles as non-classical bioisosteres in prolylcarboxypeptidase (PrCP) inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1657–1660. [Google Scholar] [CrossRef]
- Wilkening, R.R.; Ratcliffe, R.W.; Fried, A.K.; Meng, D.; Sun, W.; Colwell, L.; Lambert, S.; Greenlee, M.; Nilsson, S.; Thorsell, A.; et al. Estrogen receptor β-subtype selective tetrahydrofluorenones: Use of a fused pyrazole as a phenol bioisostere. Bioorg. Med. Chem. Lett. 2006, 16, 3896–3901. [Google Scholar] [CrossRef]
- Schwärzer, K.; Rout, S.K.; Bessinger, D.; Lima, F.; Brocklehurst, C.E.; Karaghiosoff, K.; Bein, T.; Knochel, P. Selective functionalization of the 1H-imidazo[1,2-b]pyrazole scaffold. A new potential non-classical isostere of indole and a precursor of push-pull dyes. Chem. Sci. 2021, 12, 12993–13000. [Google Scholar] [CrossRef]
- Kucukguzel, S.G.; Senkardes, S. Recent advances in bioactive pyrazoles. Eur. J. Med. Chem. 2015, 97, 786–815. [Google Scholar] [CrossRef]
- Faria, J.V.; Vegi, P.F.; Miguita, A.G.C.; dos Santos, M.S.; Boechat, N.; Bernardino, A.M.R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 25, 5891–5903. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Tu, Z. Pyrazole scaffold synthesis, functionalization, and applications in Alzheimer’s disease and Parkinson’s disease treatment (2011–2020). Molecules 2021, 26, 1202. [Google Scholar] [CrossRef] [PubMed]
- Mykhailiuk, P.K. Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev. 2021, 121, 1670–1715. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.E.; Carreira, A.R.F.; Silva, V.L.M.; Braga, S.S. Natural and biomimetic antitumor pyrazoles, a perspective. Molecules 2020, 25, 1364. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.; Jean’ne, M.S. Nitrogen-rich azoles as high density energy materials: Reviewing the energetic footprints of heterocycles. Adv. Heterocycl. Chem. 2017, 121, 89–131. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, Z.; Lan, D.; Jia, Q.; Liu, N.; Zhang, J.; Kou, K. Recent advances in synthesis and properties of nitrated-pyrazoles based energetic compounds. Molecules 2020, 25, 3475. [Google Scholar] [CrossRef]
- Kashyap, S.; Singh, R.; Singh, U.P. Inorganic and organic anion sensing by azole family members. Coord. Chem. Rev. 2020, 417, 213369. [Google Scholar] [CrossRef]
- Tigreros, A.; Portilla, J. Recent progress in chemosensors based on pyrazole derivatives. RSC Adv. 2020, 10, 19693–19712. [Google Scholar] [CrossRef]
- El Boutaybi, M.; Taleb, A.; Touzani, R.; Bahari, Z. Metal-organic frameworks based on pyrazole subunit for batteries applications: A systematic review. Mater. Today Proc. 2020, 31, S96–S102. [Google Scholar] [CrossRef]
- Matos, I.; Perez-Mayoral, E.; Soriano, E.; Zukal, A.; Martin-Aranda, R.M.; Lopez-Peinado, A.J.; Fonseca, I.; Cejka, J. Experimental and theoretical study of pyrazole N-alkylation catalyzed by basic modified molecular sieves. Chem. Eng. J. 2010, 161, 377–383. [Google Scholar] [CrossRef]
- Almena, I.; Diez-Barra, E.; De La Hoz, A.; Ruiz, J.; Sanchez-Migallon, A.; Elguero, J. Alkylation and arylation of pyrazoles under solvent-free conditions: Conventional heating versus microwave irradiation. J. Heterocycl. Chem. 1998, 35, 1263–1268. [Google Scholar] [CrossRef]
- Diez-Barra, E.; De la Hoz, A.; Sanchez-Migallon, A.; Tejeda, J. Synthesis of N-alkylpyrazoles by phase transfer catalysis without solvent. Synth. Commun. 1990, 20, 2849–2853. [Google Scholar] [CrossRef]
- Wang, R.; Chen, Y.; Zhao, X.; Yu, S.; Yang, B.; Wu, T.; Guo, J.; Hao, C.; Zhao, D.; Cheng, M. Design, synthesis and biological evaluation of novel 7H-pyrrolo[2,3-d]pyrimidine derivatives as potential FAK inhibitors and anticancer agents. Eur. J. Med. Chem. 2019, 183, 111716. [Google Scholar] [CrossRef] [PubMed]
- Mosallanejad, A.; Lorthioir, O. Application of Tsunoda reagent to the convenient synthesis of drug-like pyrazoles. Tetrahedron Lett. 2018, 59, 1708–1710. [Google Scholar] [CrossRef]
- Gill, A.; Werz, U.R.; Maas, G. N-vinylation and N-allylation of 3,5-disubstituted pyrazoles by N-H insertion of vinylcarbenoids. Z. Naturforsch. B J. Chem. Sci. 2015, 70, 747–756. [Google Scholar] [CrossRef]
- Beattie, D.; Brearley, A.; Brown, Z.; Charlton, S.J.; Cox, B.; Fairhurst, R.A.; Fozard, J.R.; Gedeck, P.; Kirkham, P.; Meja, K.; et al. Synthesis and evaluation of two series of 4′-aza-carbocyclic nucleosides as adenosine A2A receptor agonists. Bioorg. Med. Chem. Lett. 2010, 20, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Haydl, A.M.; Xu, K.; Breit, B. Regio- and Enantioselective Synthesis of N-Substituted Pyrazoles by Rhodium-Catalyzed Asymmetric Addition to Allenes. Angew. Chem. Int. Ed. 2015, 54, 7149–7153. [Google Scholar] [CrossRef]
- Arredondo, V.; Hiew, S.C.; Gutman, E.S.; Premachandra, I.D.U.A.; Van Vranken, D.L. Enantioselective Palladium-Catalyzed Carbene Insertion into the N-H Bonds of Aromatic Heterocycles. Angew. Chem. Int. Ed. 2017, 56, 4156–4159. [Google Scholar] [CrossRef]
- Wang, H.; Guo, C. Enantioselective γ-Addition of Pyrazole and Imidazole Heterocycles to Allenoates Catalyzed by Chiral Phosphine. Angew. Chem. Int. Ed. 2019, 58, 2854–2858. [Google Scholar] [CrossRef] [PubMed]
- Bengel, L.L.; Aberle, B.; Egler-Kemmerer, A.-N.; Kienzle, S.; Hauer, B.; Hammer, S.C. Engineered Enzymes Enable Selective N-Alkylation of Pyrazoles With Simple Haloalkanes. Angew. Chem. Int. Ed. 2021, 60, 5554–5560. [Google Scholar] [CrossRef]
- Arachchi, M.K.; Nguyen, H.M. Iridium-Catalyzed Enantioselective Allylic Substitutions of Racemic, Branched Trichloroacetimidates with Heteroatom Nucleophiles: Formation of Allylic C-O, C-N, and C-S Bonds. Adv. Synth. Catal. 2021, 363, 4239–4246. [Google Scholar] [CrossRef]
- Arnold, J.S.; Nguyen, H.M. Rhodium-catalyzed asymmetric amination of allylic trichloroacetimidates. Synthesis 2013, 45, 2101–2108. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.H.L.; Hor, T.S.; Hii, K.K. Silver-catalysed intramolecular hydroamination of alkynes with trichloroacetimidates. Chem. Commun. 2013, 49, 9272–9274. [Google Scholar] [CrossRef] [PubMed]
- Maleckis, A.; Klimovica, K.; Jirgensons, A. Catalytic Enantioselective Synthesis of 4-Vinyl-2-trichloromethyloxazoline: An Access to Enantioenriched Vinylglycinol Surrogate. J. Org. Chem. 2010, 75, 7897–7900. [Google Scholar] [CrossRef]
- Anderson, C.E.; Donde, Y.; Douglas, C.J.; Overman, L.E. Catalytic Asymmetric Synthesis of Chiral Allylic Amines. Evaluation of Ferrocenyloxazoline Palladacycle Catalysts and Imidate Motifs. J. Org. Chem. 2005, 70, 648–657. [Google Scholar] [CrossRef]
- Wallach, D.R.; Stege, P.C.; Shah, J.P.; Chisholm, J.D. Brønsted Acid Catalyzed Monoalkylation of Anilines with Trichloroacetimidates. J. Org. Chem. 2015, 80, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Wallach, D.R.; Chisholm, J.D. Alkylation of Sulfonamides with Trichloroacetimidates under Thermal Conditions. J. Org. Chem. 2016, 81, 8035–8042. [Google Scholar] [CrossRef]
- Mate, N.A.; Meador, R.I.L.; Joshi, B.D.; Chisholm, J.D. Alkylation of isatins with trichloroacetimidates. Org. Biomol. Chem. 2022, 20, 2131–2136. [Google Scholar] [CrossRef]
- McHardy, S.F.; Vetelino, M.G. Preparation of 1-diphenylmethylpyrazoles as Opioid Receptor Ligands. U.S. Patent 6960609B2, 1 November 2005. [Google Scholar]
- Overman, L.E.; Carpenter, N.E. The allylic trihaloacetimidate rearrangement. Org. React. 2005, 66, 1–107. [Google Scholar]
- Cran, J.; Vidhani, D.; Krafft, M. Copper-Catalyzed N-tert-Butylation of Aromatic Amines under Mild Conditions Using tert-Butyl 2,2,2-Trichloroacetimidate. Synlett 2014, 25, 1550–1554. [Google Scholar] [CrossRef]
- Iversen, T.; Bundle, D.R. Benzyl trichloroacetimidate, a versatile reagent for acid-catalyzed benzylation of hydroxy-groups. J. Chem. Soc. Chem. Commun. 1981, 1240–1241. [Google Scholar] [CrossRef]
- Nakajima, N.; Horita, K.; Abe, R.; Yonemitsu, O. MPM (4-methoxybenzyl)-protection of hydroxy functions under mild acidic conditions. Tetrahedron Lett. 1988, 29, 4139–4142. [Google Scholar] [CrossRef]
- Ali, I.A.I.; El Ashry, E.S.H.; Schmidt, R.R. Protection of Hydroxy Groups with Diphenylmethyl and 9-Fluorenyl Trichloroacetimidates—Effect on Anomeric Stereocontrol. Eur. J. Org. Chem. 2003, 2003, 4121–4131. [Google Scholar] [CrossRef]
- Howard, K.T.; Duffy, B.C.; Linaburg, M.R.; Chisholm, J.D. Formation of DPM ethers using O-diphenylmethyl trichloroacetimidate under thermal conditions. Org. Biomol. Chem. 2016, 14, 1623–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pangborn, A.B.; Giardello, M.A.; Grubbs, R.H.; Rosen, R.K.; Timmers, F.J. Safe and Convenient Procedure for Solvent Purification. Organometallics 1996, 15, 1518–1520. [Google Scholar] [CrossRef]
- Zhao, C.; Toste, F.D.; Raymond, K.N.; Bergman, R.G. Nucleophilic Substitution Catalyzed by a Supramolecular Cavity Proceeds with Retention of Absolute Stereochemistry. J. Am. Chem. Soc. 2014, 136, 14409–14412. [Google Scholar] [CrossRef]
- Adhikari, A.A.; Suzuki, T.; Gilbert, R.T.; Linaburg, M.R.; Chisholm, J.D. Rearrangement of Benzylic Trichloroacetimidates to Benzylic Trichloroacetamides. J. Org. Chem. 2017, 82, 3982–3989. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Mixdorft, J.C.; Reynders, G.J.; Nguyen, H.M. Rhodium-catalyzed benzylic fluorination of trichloroacetimidates. Tetrahedron 2015, 71, 5932–5938. [Google Scholar] [CrossRef]
- Mahajani, N.S.; Chisholm, J.D. Synthesis of 1,1′-Diarylethanes and Related Systems by Displacement of Trichloroacetimidates with Trimethylaluminum. J. Org. Chem. 2018, 83, 4131–4139. [Google Scholar] [CrossRef]
- Li, C.K.; Li, W.B.; Wang, J.B. Gold(I)-catalyzed arylmethylation of terminal alkynes. Tetrahedron Lett. 2009, 50, 2533–2535. [Google Scholar] [CrossRef]
- Ali, I.A.I.; El Ashry, E.S.H.; Schmidt, R.R. Imidomethylation of C-nucleophiles using O-phthalimidomethyl trichloroacetimidate and catalytic amounts of TMSOTf. Tetrahedron 2004, 60, 4773–4780. [Google Scholar] [CrossRef]
- Song, C.; Dong, X.; Yi, H.; Chiang, C.-W.; Lei, A. DDQ-Catalyzed Direct C(sp3)-H Amination of Alkylheteroarenes: Synthesis of Biheteroarenes under Aerobic and Metal-Free Conditions. ACS Catal. 2018, 8, 2195–2199. [Google Scholar] [CrossRef]
- Olsen, K.L.; Jensen, M.R.; MacKay, J.A. A mild halogenation of pyrazoles using sodium halide salts and Oxone. Tetrahedron Lett. 2017, 58, 4111–4114. [Google Scholar] [CrossRef]
- Shi, J.; Yuan, T.; Wang, R.; Zheng, M.; Wang, X. Boron carbonitride photocatalysts for direct decarboxylation: The construction of C(sp3)-N or C(sp3)-C(sp2) bonds with visible light. Green Chem. 2021, 23, 3945–3949. [Google Scholar] [CrossRef]

| Entry | Catalyst | Solvent | Temp. (°C) | Time | Yield |
|---|---|---|---|---|---|
| 1 | none | 1,2-DCE | rt | 24 | 0 |
| 2 | none | 1,2-DCE | reflux | 24 | 3 |
| 3 | 20 mol% TMSOTf | 1,2-DCE | 23 | 24 | 61 |
| 4 | 20 mol% BF3•OEt2 | 1,2-DCE | 23 | 24 | 68 |
| 5 | 20 mol% CSA 1 | 1,2-DCE | 23 | 24 | 76 |
| 6 | 20 mol% CSA | toluene | 23 | 24 | 69 |
| 7 | 20 mol% CSA | DCM | 23 | 24 | 70 |
| 8 | 20 mol% CSA | MeCN | 23 | 24 | 29 |
| 9 | 10 mol% CSA | 1,2-DCE | 23 | 24 | 62 |
| 10 | 20 mol% CSA | 1,2-DCE | 23 | 18 | 77 |
| 11 | 20 mol% CSA | 1,2-DCE | 23 | 8 | 75 |
| 12 | 20 mol% CSA | 1,2-DCE | 23 | 6 | 70 |
| 13 | 20 mol% CSA | 1,2-DCE | 23 | 4 | 71 |
| 14 | 20 mol% CSA | 1,2-DCE | 23 | 2 | 45 |
| 15 | 20 mol% CSA | 1,2-DCE | reflux | 4 | 71 |
| 16 | 50 mol% CSA | 1,2-DCE | 23 | 24 | 54 |

| Entry | Imidate | Product | Yield (%) |
|---|---|---|---|
| 1 |  |  | 71 |
| 2 |  | 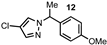 | 97 |
| 3 | 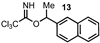 | 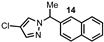 | 67 |
| 4 |  | 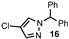 | 59 |
| 5 |  | 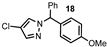 | 71 |
| 6 |  | 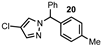 | 98 |
| 7 | 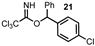 |  | 76 |
| 8 | 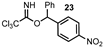 | 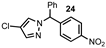 | 0 |
| 9 |  | 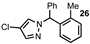 | 85 |
| 10 | 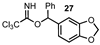 | 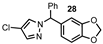 | 98 |
| 11 | 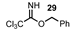 | 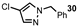 | 73 |
| 12 | 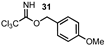 | 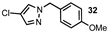 | 92 |
| 13 | 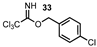 | 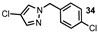 | 37 |
| 14 | 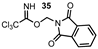 | 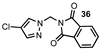 | 62 |
| 15 | 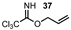 | 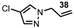 | 0 |
| 16 |  | 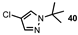 | 0 |
| 17 |  | 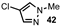 | 0 |
| Entry | Pyrazole | Product | Yield (%) |
|---|---|---|---|
| 1 | 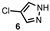 | 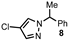 | 71 |
| 2 | 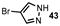 |  | 70 |
| 3 |  | 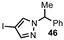 | 59 |
| 4 | 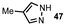 | 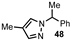 | 62 |
| 5 | 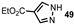 | 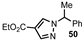 | 50 |
| 6 |  | 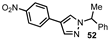 | 43 |
| 7 |  | 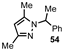 | 50 |
| 8 | 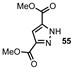 |  | 44 |
| 9 | 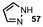 | 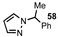 | 45 |
| 10 | 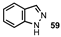 |  | 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meador, R.I.L.; Mate, N.A.; Chisholm, J.D. Acid Catalyzed N-Alkylation of Pyrazoles with Trichloroacetimidates. Organics 2022, 3, 111-121. https://doi.org/10.3390/org3020009
Meador RIL, Mate NA, Chisholm JD. Acid Catalyzed N-Alkylation of Pyrazoles with Trichloroacetimidates. Organics. 2022; 3(2):111-121. https://doi.org/10.3390/org3020009
Chicago/Turabian StyleMeador, Rowan I. L., Nilamber A. Mate, and John D. Chisholm. 2022. "Acid Catalyzed N-Alkylation of Pyrazoles with Trichloroacetimidates" Organics 3, no. 2: 111-121. https://doi.org/10.3390/org3020009
APA StyleMeador, R. I. L., Mate, N. A., & Chisholm, J. D. (2022). Acid Catalyzed N-Alkylation of Pyrazoles with Trichloroacetimidates. Organics, 3(2), 111-121. https://doi.org/10.3390/org3020009






