Design, Synthesis, and Photo-Responsive Properties of a Collagen Model Peptide Bearing an Azobenzene
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
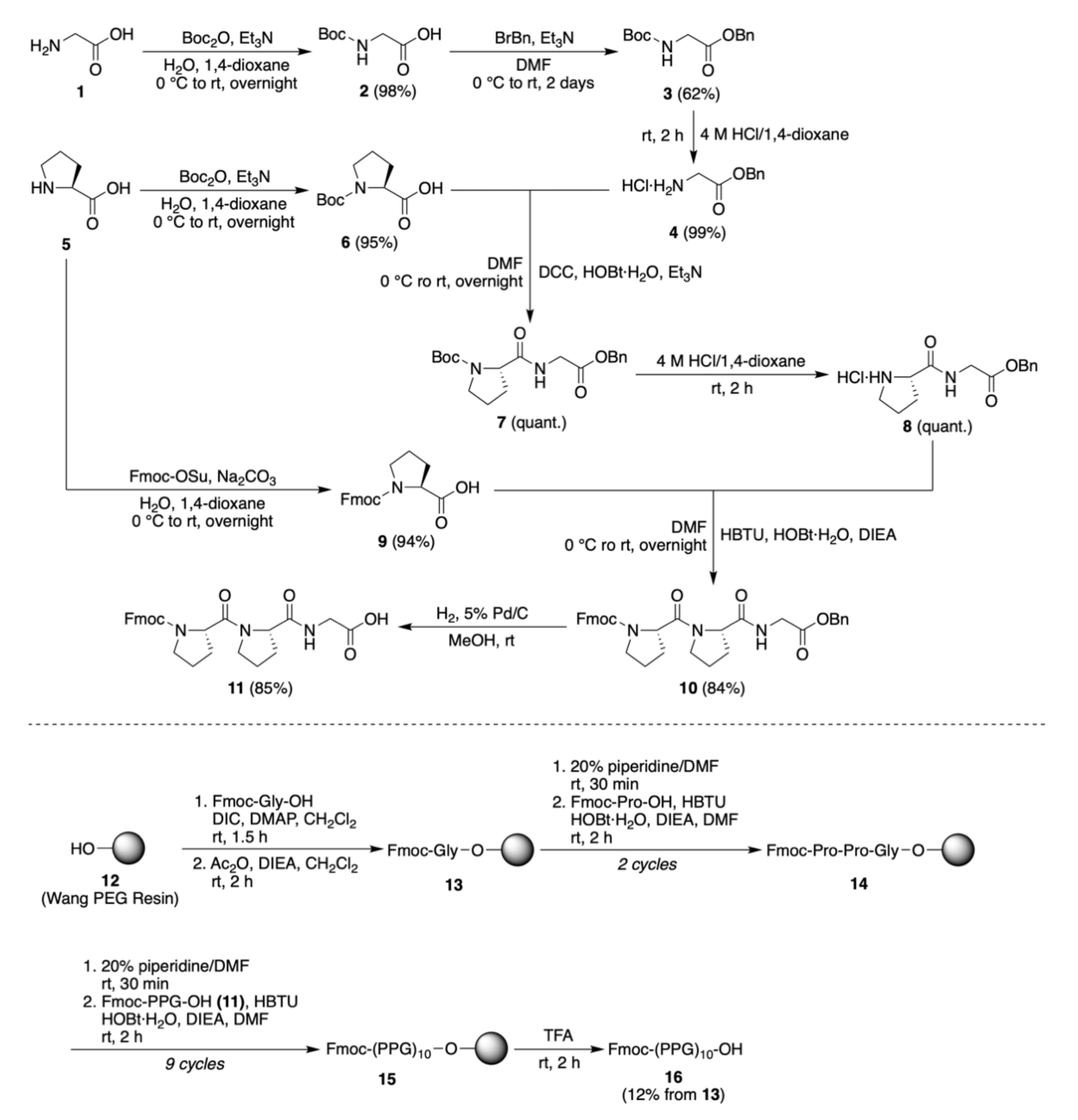
2.1.1. Synthesis of Fmoc-PPG-OH
2.1.2. Synthesis of Fmoc-(PPG)10-OH
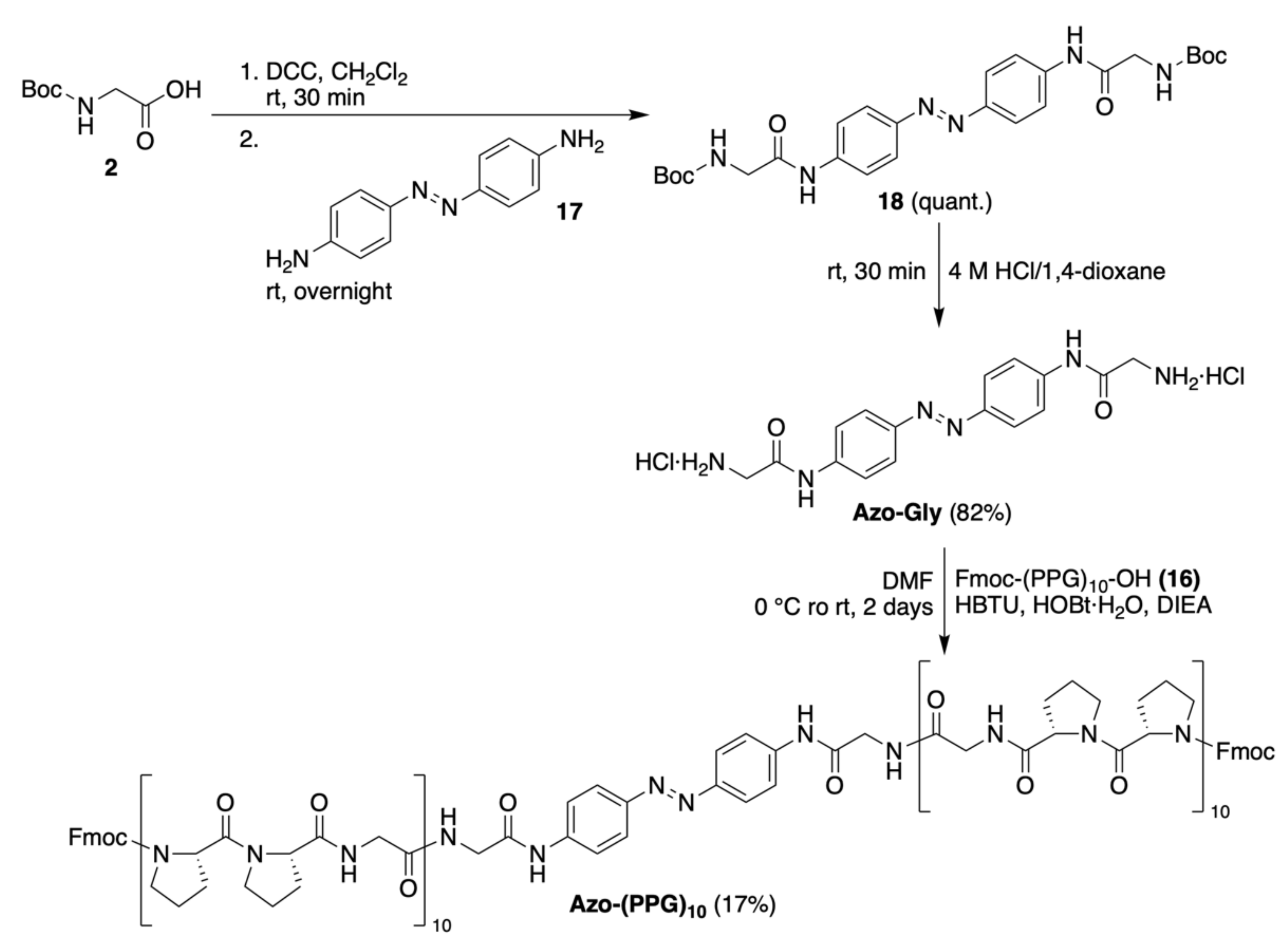
2.1.3. Synthesis of Azo-Gly and Azo-(PPG)10
2.2. Mesurements and Analysis
2.2.1. CD Measurements
2.2.2. Photo-Induced Denaturation of Collagen Triple Helix
2.2.3. Photo- and Thermal-Reversibility of Azobenzene Moiety
3. Results and Discussion
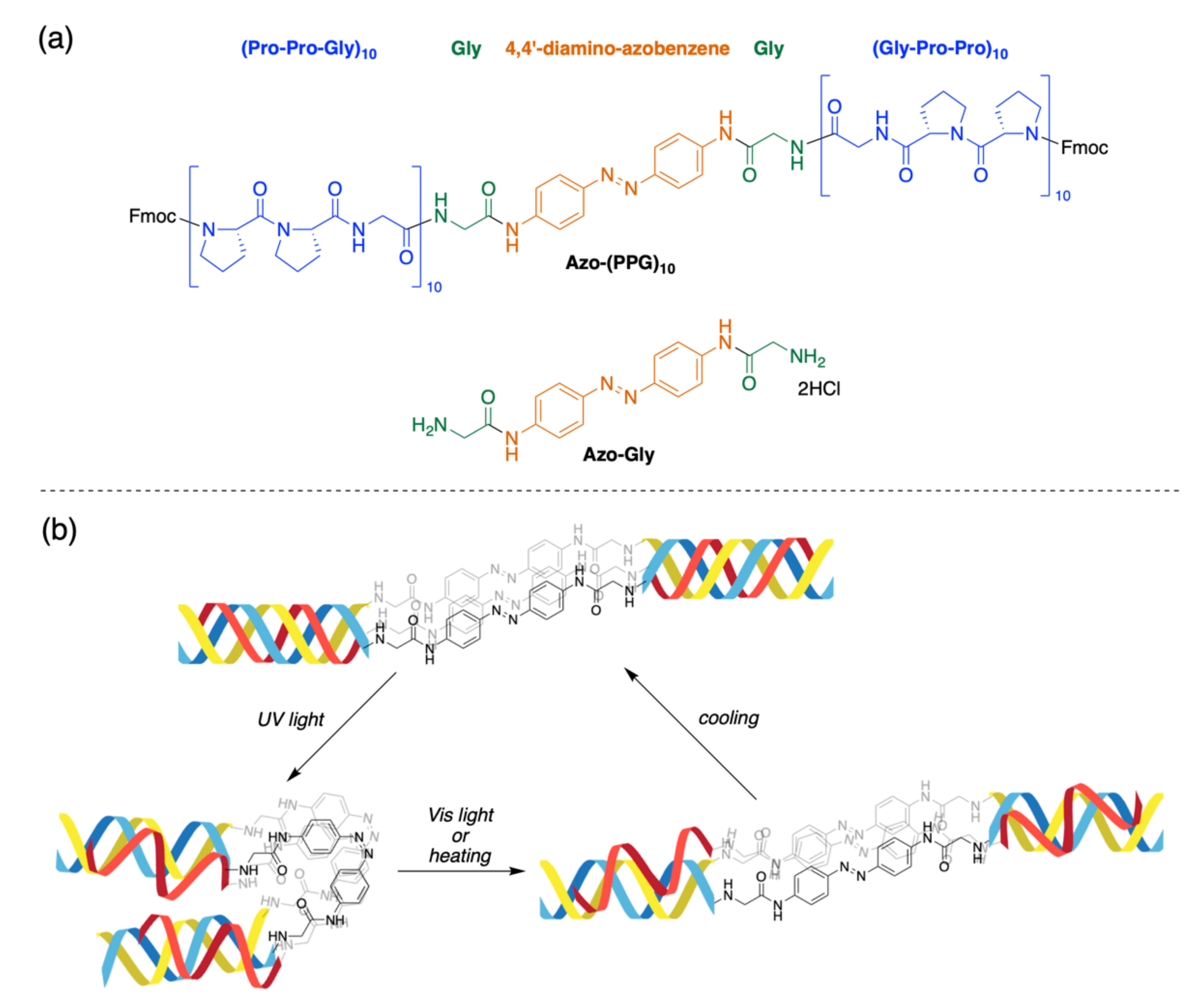
3.1. Synthesis of Photo-Responsive CMP
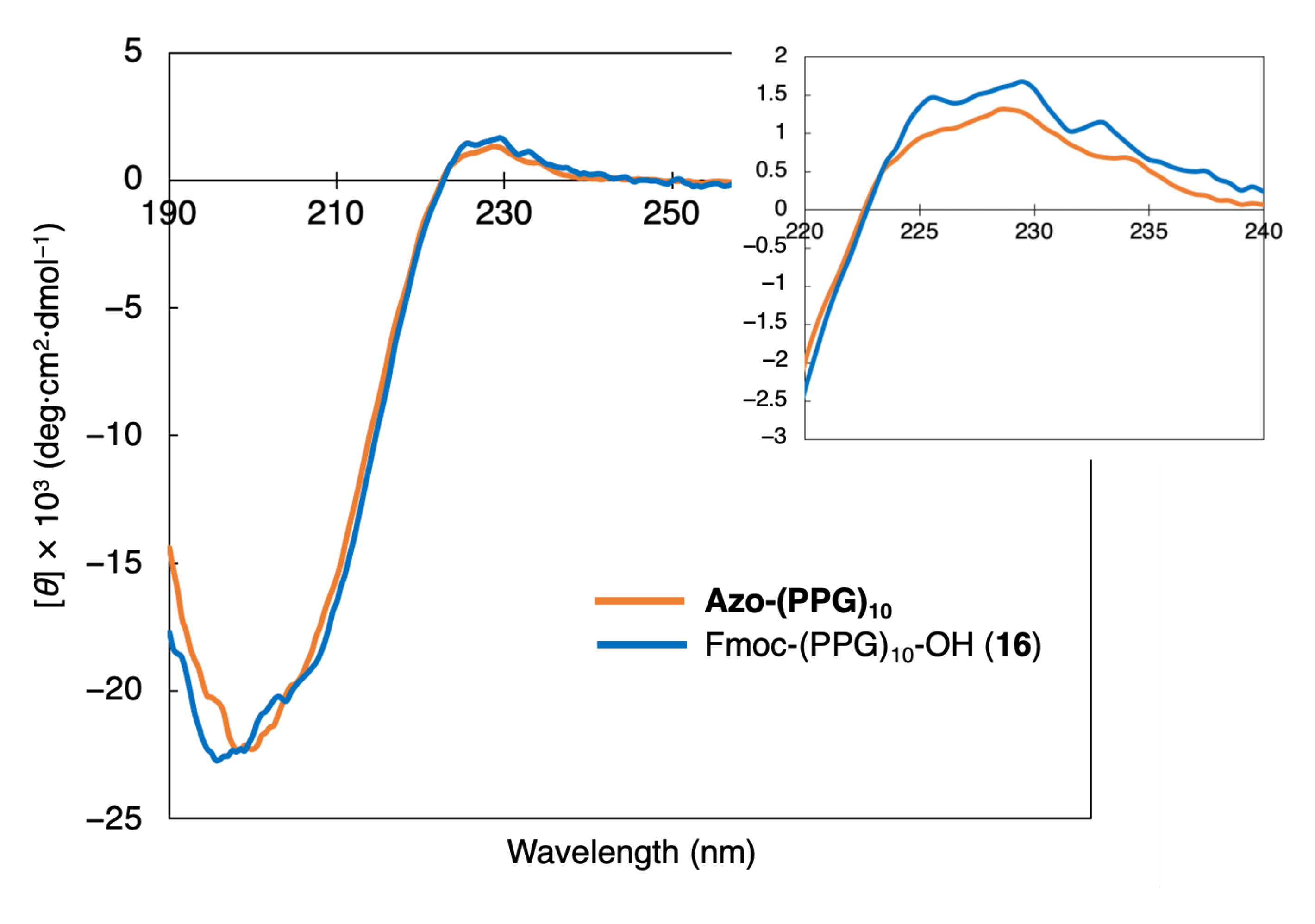
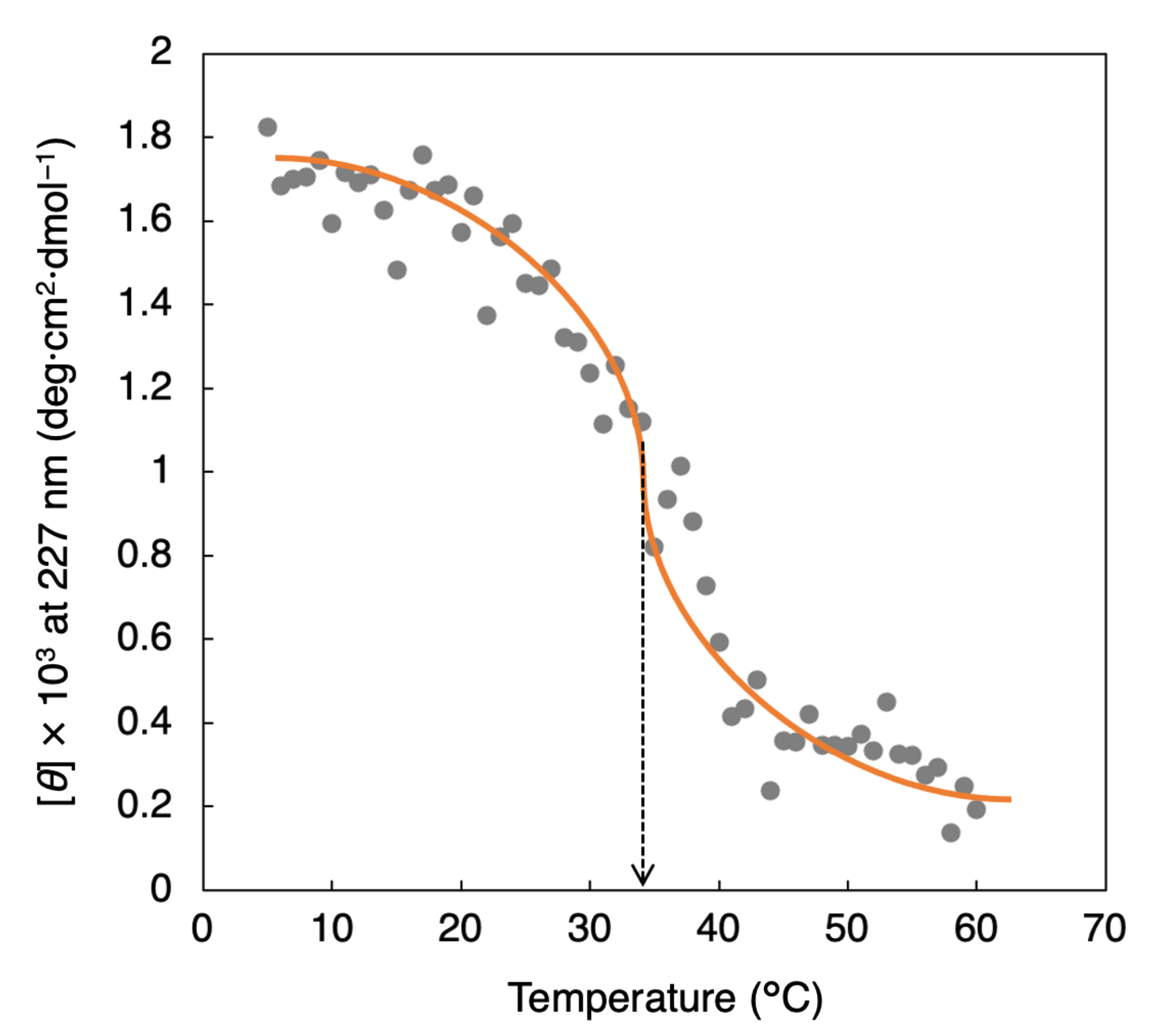
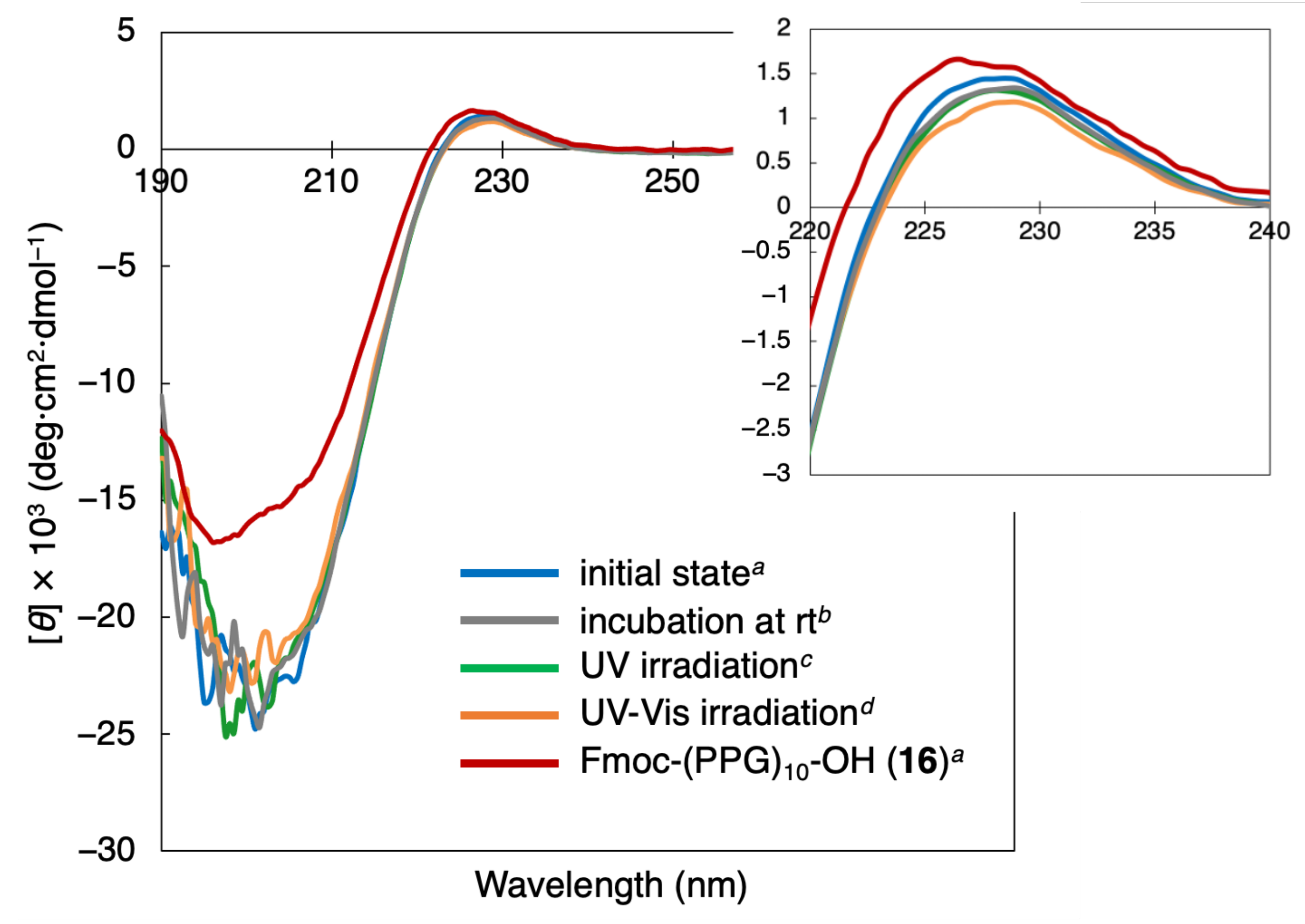
3.2. Investigation of Collagen Triple Helical Properties
3.3. Denaturation of the Collagen Triple Helix Structure by Light Irradiation
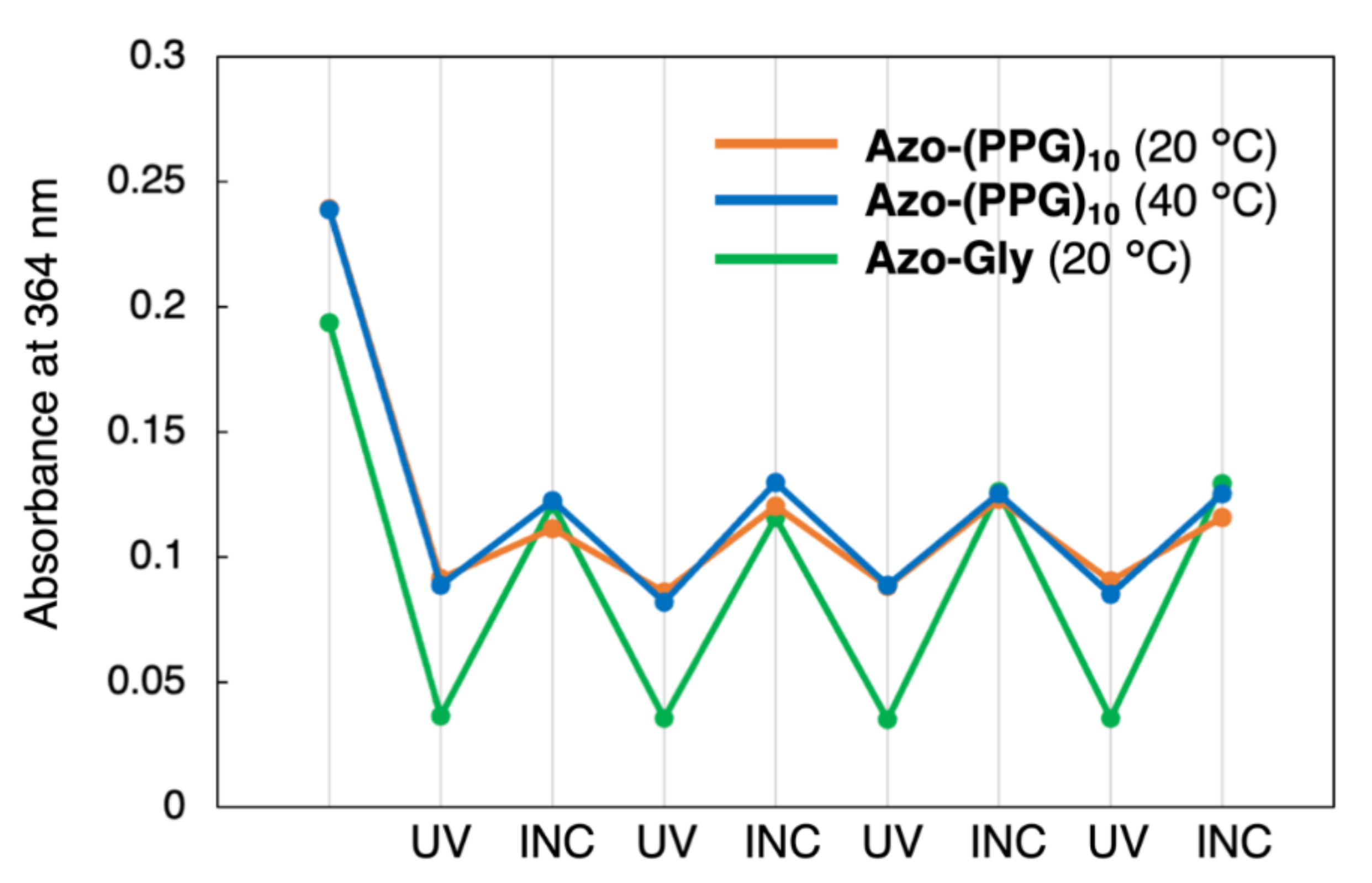
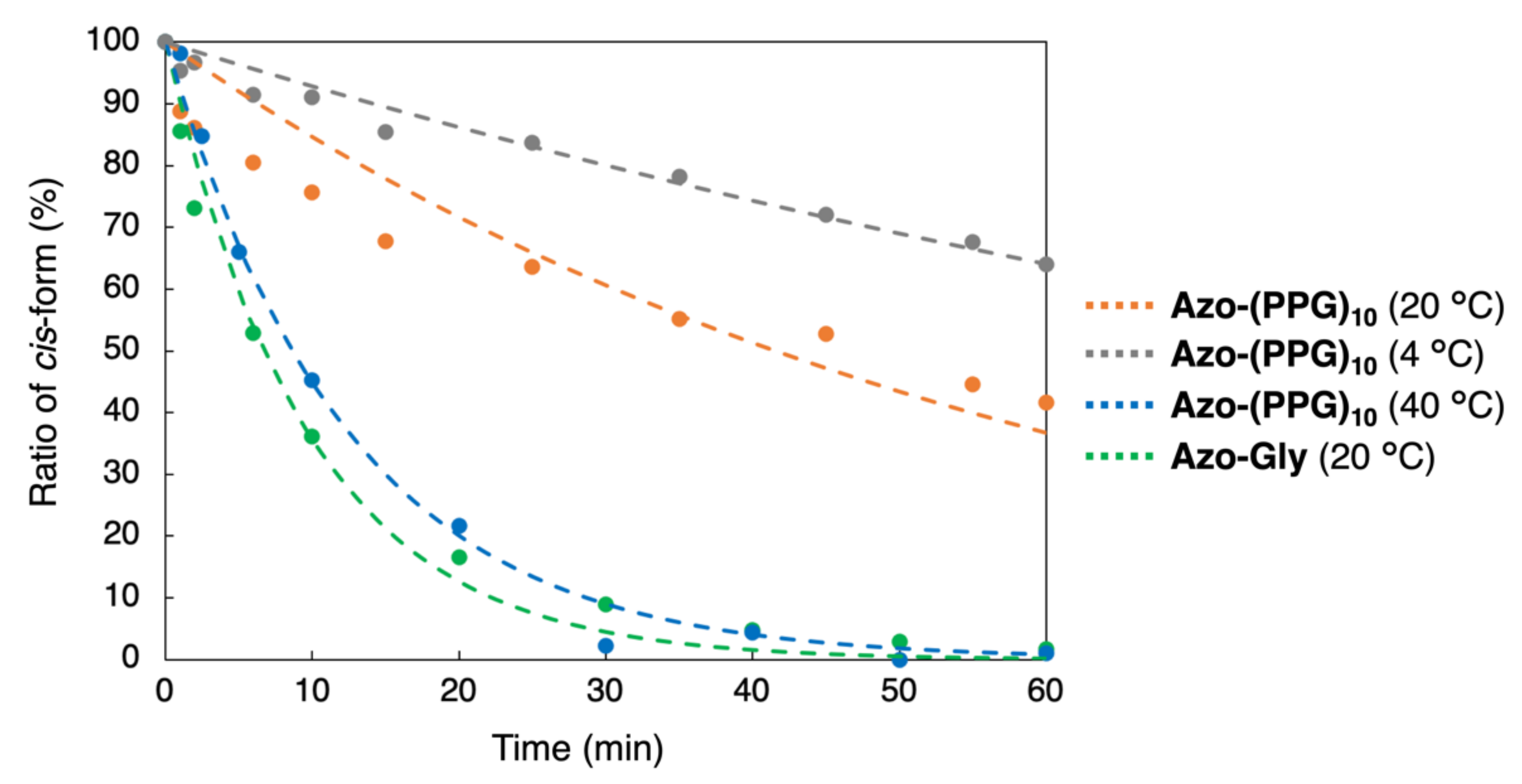
3.4. Isomerization of the Azobenzene Moiety by Light Irradiation or Thermal Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef] [PubMed]
- Owczarzy, A.; Kurasiński, R.; Kulig, K.; Rogóż, W.; Szkudlarek, A.; Maciążek-Jurczyk, M. Collagen—Structure, properties and application. Eng. Biomat. 2020, 156, 17–23. [Google Scholar] [CrossRef]
- Fields, G.B. Synthesis and biological applications of collagen-model triple-helical peptides. Org. Biomol. Chem. 2010, 8, 1237–1258. [Google Scholar] [CrossRef]
- O’Leary, L.E.R.; Fallas, J.A.; Bakota, E.L.; Kang, M.K.; Hartgerink, J.D. Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat. Chem. 2011, 3, 821–828. [Google Scholar] [CrossRef]
- Ganguly, H.K.; Basu, G. Conformational landscape of substituted prolines. Biophys. Rev. 2020, 12, 25–39. [Google Scholar] [CrossRef]
- Brodsky, B.; Thiagarajan, G.; Madhan, B.; Kar, K. Triple-helical peptides: An approach to collagen conformation, stability, and self-association. Biopolymers 2008, 89, 345–353. [Google Scholar] [CrossRef]
- Kubyshkin, V. Stabilization of the triple helix in collagen mimicking peptides. Org. Biomol. Chem. 2019, 17, 8031–8047. [Google Scholar] [CrossRef]
- Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering. Appl. Sci. 2021, 11, 11369. [Google Scholar] [CrossRef]
- Copes, F.; Pien, N.; Vlierberghe, S.V.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. BioMed. Eng. OnLine 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Luo, T.; Kiick, K.L. Collagen-like peptides and peptide–polymer conjugates in the design of assembled materials. Eur. Polym. J. 2013, 49, 2998–3009. [Google Scholar] [CrossRef] [PubMed]
- Brieke, C.; Rohrbach, F.; Gottschalk, A.; Mayer, G.; Heckel, A. Light-Controlled Tools. Angew. Chem. Int. Ed. 2012, 51, 8446–8476. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.; Li, Y.; Kim, D. Collagen mimetic peptides: Progress towards functional applications. Soft Matter. 2011, 7, 7927–7938. [Google Scholar] [CrossRef]
- Hamon, F.; Djedaini-Pilard, F.; Barbot, F.; Len, C. Azobenzenes—Synthesis and carbohydrate applications. Tetrahedron 2009, 65, 10105–10123. [Google Scholar] [CrossRef]
- Pirone, D.; Bandeira, N.A.; Tylkowski, B.; Boswell, E.; Labeque, R.; Valls, R.G.; Giamberini, M. Contrasting Photo-Switching Rates in Azobenzene Derivatives: How the Nature of the Substituent Plays a Role. Polymers 2020, 12, 1019. [Google Scholar] [CrossRef]
- Hamon, F.; Blaszkiewicz, C.; Buchotte, M.; Banaszak-Léonard, E.; Bricout, H.; Tilloy, S.; Monflier, E.; Cézard, C.; Bouteiller, L.; Len, C.; et al. Synthesis and characterization of a new photoinduced switchable β-cyclodextrin dimer. Beilstein J. Org. Chem. 2014, 10, 2874–2885. [Google Scholar] [CrossRef]
- Beharry, A.A.; Woolley, G.A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011, 40, 4422–4437. [Google Scholar] [CrossRef]
- Szymański, W.; Beierle, J.M.; Kistemaker, H.A.V.; Velema, W.A.; Feringa, B.L. Reversible Photocontrol of Biological Systems by the Incorporation of Molecular Photoswitches. Chem. Rev. 2013, 113, 6114–6178. [Google Scholar] [CrossRef]
- Wang, H.; Bisoyi, H.K.; Zhang, X.; Hassan, F.; Li, Q. Visible Light-Driven Molecular Switches and Motors: Recent Developments and Applications. Chem. Eur. J. 2022, 28, e202103906. [Google Scholar] [CrossRef]
- Lee, I.N.; Dobre, O.; Richards, D.; Ballestrem, C.; Curran, J.M.; Hunt, J.A.; Richardson, S.M.; Swift, J.; Wong, L.S. Photoresponsive Hydrogels with Photoswitchable Mechanical Properties Allow Time-Resolved Analysis of Cellular Responses to Matrix Stiffening. ACS Appl. Mater. Interfaces 2018, 10, 7765–7776. [Google Scholar] [CrossRef] [PubMed]
- Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Nather, C.; Renth, F.; Temps, F.J. Highly Efficient Reversible Z−E Photoisomerization of a Bridged Azobenzene with Visible Light through Resolved S1(nπ*) Absorption Bands. Am. Chem. Soc. 2009, 131, 15594–15595. [Google Scholar] [CrossRef] [PubMed]
- Beharry, A.A.; Sadovski, O.; Woolley, G.A.J. Azobenzene Photoswitching without Ultraviolet Light. Am. Chem. Soc. 2011, 133, 19684–119687. [Google Scholar] [CrossRef]
- Yang, Y.; Hughes, R.P.; Aprahamian, I.J. Near-Infrared Light Activated Azo-BF2 Switches. Am. Chem. Soc. 2014, 136, 13190–13193. [Google Scholar] [CrossRef] [PubMed]
- Weis, P.; Wu, S. Light-Switchable Azobenzene-Containing Macromolecules: From UV to Near Infrared. Macromol. Rapid Commun. 2018, 39, 1700220. [Google Scholar] [CrossRef]
- Kumita, J.R.; Smart, O.S.; Woolley, G.A. Photo-control of helix content in a short peptide. Proc. Natl. Acad. Sci. USA 2000, 97, 3803–3808. [Google Scholar] [CrossRef] [PubMed]
- Flint, D.G.; Kumita, J.R.; Smart, O.S.; Woolley, G.A. Using an Azobenzene Cross-Linker to Either Increase or Decrease Peptide Helix Content upon Trans-to-Cis Photoisomerization. Chem. Biol. 2002, 9, 391–397. [Google Scholar] [CrossRef]
- Bredenbeck, J.; Helbing, J.; Kumita, J.R.; Woolley, G.A.; Hamm, P. α-Helix formation in a photoswitchable peptide tracked from picoseconds to microseconds by time-resolved IR spectroscopy. Proc. Natl. Acad. Sci. USA 2005, 102, 2379–2384. [Google Scholar] [CrossRef]
- Zhang, F.; Sadovski, O.; Woolley, G.A. Synthesis and Characterization of a Long, Rigid Photoswitchable Cross-Linker for Promoting Peptide and Protein Conformational Change. ChemBioChem 2008, 9, 2147–2154. [Google Scholar] [CrossRef]
- Samanta, S.; Woolley, A. Bis-Azobenzene Crosslinkers for Photocontrol of Peptide Structure. ChemBioChem 2011, 12, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Kumita, J.R.; Flint, D.G.; Woolley, G.A.; Smart, O.S. Achieving photo-control of protein conformation and activity: Producing a photo-controlled leucine zipper. Faraday Discuss. 2002, 122, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Schrader, T.E.; Cordes, T.; Schreier, W.J.; Koller, F.O.; Dong, S.L.; Moroder, L.; Zinth, W.J. Folding and Unfolding of Light-Triggered β-Hairpin Model Peptides. Phys. Chem. B 2011, 115, 5219–5226. [Google Scholar] [CrossRef] [PubMed]
- Spekowius, J.; Pfister, R.; Helbing, J.J. Folding and Unfolding of the Tryptophan Zipper in the Presence of Two Thioamide Substitutions. Phys. Chem. B 2021, 125, 7662–7670. [Google Scholar] [CrossRef]
- Tochitsky, I.; Banghart, M.R.; Mourot, A.; Yao, J.Z.; Gaub, B.; Kramer, R.H.; Trauner, D. Optochemical control of genetically engineered neuronal nicotinic acetylcholine receptors. Nat. Chem. 2012, 4, 105–111. [Google Scholar] [CrossRef]
- Schierling, B.; Noël, A.J.; Wende, W.; Hien, L.T.; Volkov, E.; Kubareva, E.; Oretskaya, T.; Kokkinidis, M.; Römpp, A.; Spengler, B.; et al. Controlling the enzymatic activity of a restriction enzyme by light. Proc. Natl. Acad. Sci. USA 2010, 107, 1361–1366. [Google Scholar] [CrossRef]
- Fortin, D.L.; Banghart, M.R.; Dunn, T.W.; Borges, K.; Wagenaar, D.A.; Gaudry, Q.; Karakossian, M.H.; Otis, T.S.; Kristan, W.B.; Trauner, D.; et al. Photochemical control of endogenous ion channels and cellular excitability. Nat. Methods 2008, 5, 331–338. [Google Scholar] [CrossRef]
- Umeki, N.; Yoshizawa, T.; Sugimoto, Y.; Mitsui, T.; Wakabayashi, K.; Maruta, S. Incorporation of an Azobenzene Derivative into the Energy Transducing Site of Skeletal Muscle Myosin Results in Photo-Induced Conformational Changes. J. Biochem. 2004, 136, 839–846. [Google Scholar] [CrossRef]
- Asanuma, H.; Liang, X.; Nishioka, H.; Matsunaga, D.; Liu, M.; Komiyama, M. Synthesis of azobenzene-tethered DNA for reversible photo-regulation of DNA functions: Hybridization and transcription. Nat. Prot. 2007, 2, 203–212. [Google Scholar] [CrossRef]
- Kusebauch, U.; Cadamuro, S.A.; Musiol, H.J.; Lenz, M.O.; Wachtveitl, J.; Moroder, L.; Renner, C. Photocontrolled Folding and Unfolding of a Collagen Triple Helix. Angew. Chem. Int. Ed. 2006, 45, 7015–7018. [Google Scholar] [CrossRef]
- Kusebauch, U.; Cadamuro, S.A.; Musiol, H.J.; Moroder, L.; Renner, C. Photocontrol of the Collagen Triple Helix: Synthesis and Conformational Characterization of Bis-cysteinyl Collagenous Peptides with an Azobenzene Clamp. Chem. Eur. J. 2007, 13, 2966–2973. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, L.; Kusebauch, U.; Moroder, L.; Wachtveitl, J. Temperature- and Photocontrolled Unfolding/Folding of a Triple-Helical Azobenzene-Stapled Collagen Peptide Monitored by Infrared Spectroscopy. ChemPhysChem 2016, 17, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Higashi, N.; Yoshikawa, R.; Koga, T. Photo-responsive azobenzene interactions promote hierarchical self-assembly of collagen triple-helical peptides to various higher-order structures. RSC Adv. 2020, 10, 15947–15954. [Google Scholar] [CrossRef]
- Koga, T.; Ikejiri, A.; Higashi, N. Narcissistic Self-Sorting of Amphiphilic Collagen-Inspired Peptides in Supramolecular Vesicular Assembly. Langmuir 2022, 38, 2294–2300. [Google Scholar] [CrossRef]
- Koga, T.; Kingetsu, S.; Higashi, N. Supramolecular Nanofibers from Collagen-Mimetic Peptides Bearing Various Aromatic Groups at N-Termini via Hierarchical Self-Assembly. Int. J. Mol. Sci. 2021, 22, 4533. [Google Scholar] [CrossRef]
- Ottl, J.; Musiol, H.J.; Moroder, L.J. Heterotrimeric collagen peptides containing functional epitopes. Synthesis of single-stranded collagen type I peptides related to the collagenase cleavage site. Pept. Sci. 1999, 5, 103–110. [Google Scholar] [CrossRef]
- Xu, Y.; Kirchner, M. Collagen Mimetic Peptides. Bioengineering 2021, 8, 5. [Google Scholar] [CrossRef]
- Holmgren, S.K.; Bretscher, L.E.; Taylor, K.M.; Raines, R.T. A hyperstable collagen mimic. Chem. Biol. 1999, 6, 63–70. [Google Scholar] [CrossRef]
- Zitnay, J.L.; Li, Y.; Qin, Z.; San, B.H.; Depalle, B.; Reese, S.P.; Buehler, M.J.; Yu, S.M.; Weiss, J.A. Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nat. Commun. 2017, 8, 14913. [Google Scholar] [CrossRef]
- Zitnay, J.L.; Jung, G.S.; Lin, A.H.; Qin, Z.; Li, Y.; Yu, S.M.; Buehler, M.J.; Weiss, J.A. Accumulation of collagen molecular unfolding is the mechanism of cyclic fatigue damage and failure in collagenous tissues. Sci. Adv. 2020, 6, eaba2795. [Google Scholar] [CrossRef]
- Hartmann, J.; Zacharias, M. Mechanism of collagen folding propagation studied by Molecular Dynamics simulations. PLoS Comput. Biol. 2021, 17, e1009079. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.; Brodsky, B. Real-time NMR investigations of triple-helix folding and collagen folding diseases. Fold. Des. 1997, 2, 53–60. [Google Scholar] [CrossRef]
| Sample | k (s−1) | t1/2 (min) |
|---|---|---|
| Azo-(PPG)10 (20 °C) | 1.00 | 41.6 |
| Azo-(PPG)10 (4 °C) | 0.446 | 93.3 |
| Azo-(PPG)10 (40 °C) | 4.82 | 8.63 |
| Azo-Gly (20 °C) | 6.19 | 6.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, D.; Goto, H.; Ishizaki, Y.; Narimatsu, T.; Kato, T. Design, Synthesis, and Photo-Responsive Properties of a Collagen Model Peptide Bearing an Azobenzene. Organics 2022, 3, 415-429. https://doi.org/10.3390/org3040027
Sato D, Goto H, Ishizaki Y, Narimatsu T, Kato T. Design, Synthesis, and Photo-Responsive Properties of a Collagen Model Peptide Bearing an Azobenzene. Organics. 2022; 3(4):415-429. https://doi.org/10.3390/org3040027
Chicago/Turabian StyleSato, Daisuke, Hitomi Goto, Yui Ishizaki, Tetsuya Narimatsu, and Tamaki Kato. 2022. "Design, Synthesis, and Photo-Responsive Properties of a Collagen Model Peptide Bearing an Azobenzene" Organics 3, no. 4: 415-429. https://doi.org/10.3390/org3040027
APA StyleSato, D., Goto, H., Ishizaki, Y., Narimatsu, T., & Kato, T. (2022). Design, Synthesis, and Photo-Responsive Properties of a Collagen Model Peptide Bearing an Azobenzene. Organics, 3(4), 415-429. https://doi.org/10.3390/org3040027







