Abstract
The [2+2+2] cycloaddition (homo-Diels–Alder reaction) of N-substituted 1,2,4-triazoline-3,5-diones (TADs) with bicycloalkadienes produces strained heterocyclic compounds. A reaction with the unsubstituted dienes occurs readily to produce only the expected homo-Diels–Alder adducts. However, previous work in the literature showed that the attachment of a single electron-withdrawing group to the diene system results in the formation of not only the expected homo-Diels–Alder adducts, but also interesting “insertion” products. To probe the limits of reactivity of these diene systems, we investigated the reaction of N-methyl-1,2,4-triazoline-3,5-dione (MeTAD) with bicycloalkadienes substituted with two electron-withdrawing groups, i.e., two carbomethoxy or two cyano groups. We hoped to learn whether the reaction still proceeded, and if so, whether the homo-Diels–Alder adducts and/or other types of products were formed. We found that a reaction between MeTAD and the dienes takes place upon substitution with two carbomethoxy groups, albeit at a considerably slower rate than other reactions. The only products observed were the homo-Diels–Alder adducts. However, attachment of two CN groups completely inhibited reactivity.
1. Introduction
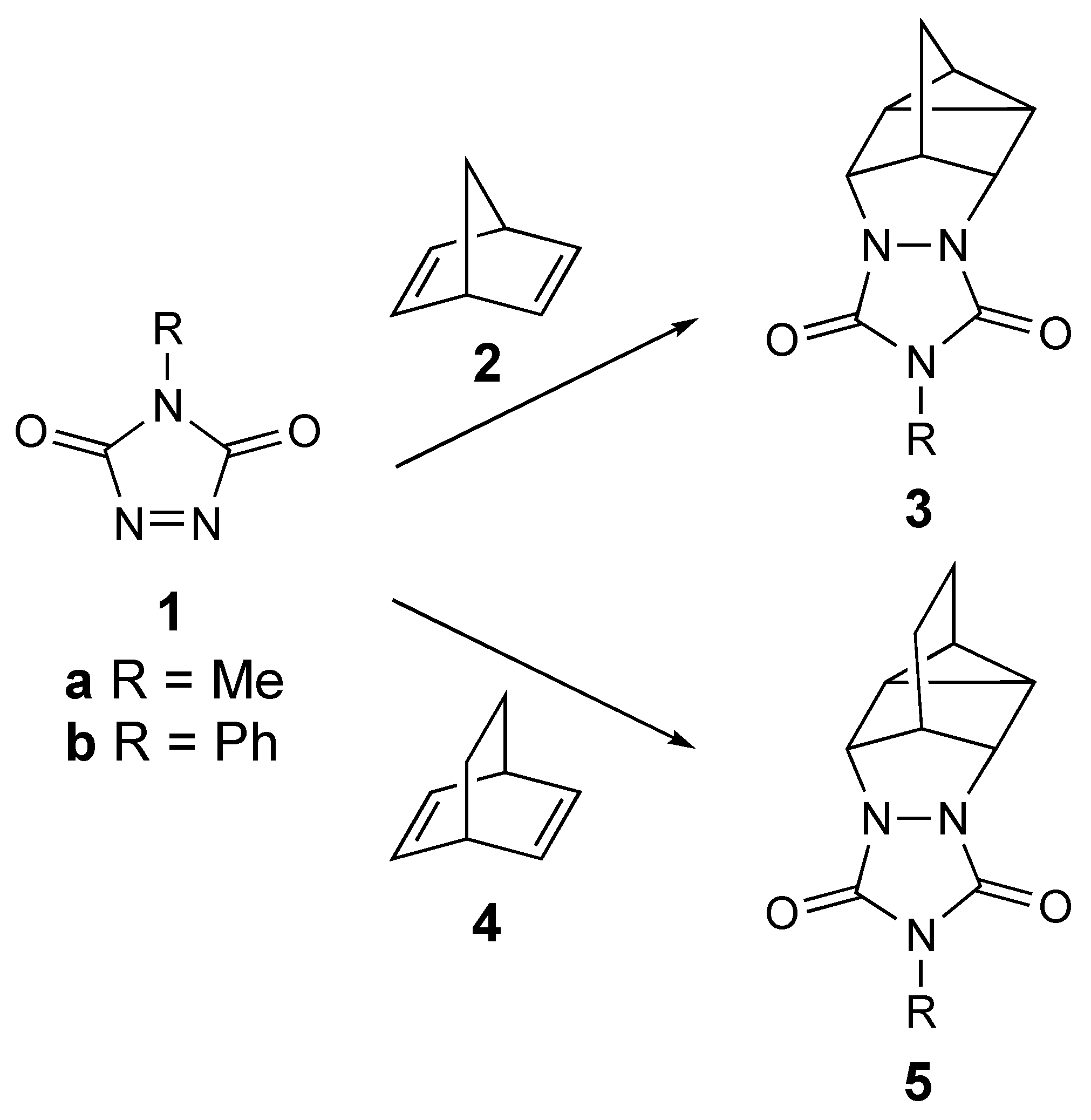
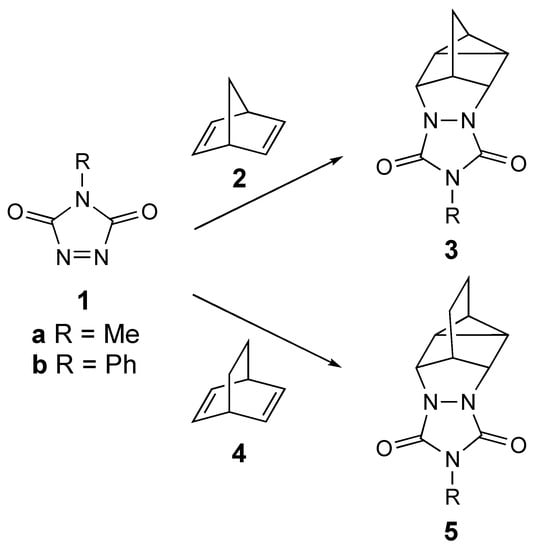
The [2+2+2] cycloaddition of N-substituted 1,2,4-triazoline-3,5-diones (TADs, 1) with bicyclodienes, also known as the homo-Diels–Alder (homo-DA) reaction allows for rapid access to structurally-interesting heterocyclic molecules (see Scheme 1) [1,2,3,4,5,6,7,8,9,10]. These adducts can be hydrolyzed to their corresponding hydrazine derivatives and then oxidized to provide strained azo compounds [11,12,13]. The reactivities of these azo compounds have provided important mechanistic insights into the reactivity of diradicals [11,12,13]. Both the N-methyl and N-phenyl TAD derivatives (MeTAD [1a] and PhTAD [1b], respectively) are known to add to bicyclo[2.2.1]hepta-2,5-diene (norbornadiene, 2) to produce the homo-Diels–Alder adducts 3 [1,2,3,10]. Similarly, 1b is known to react with bicyclo[2.2.2]octa-2,5-diene, 4, to give 5b [2].
Scheme 1.
The known reactions of triazolinediones (1a,b) with bicyclodienes (2,4) to give homo-Diels-Alder adducts 3 and 5.
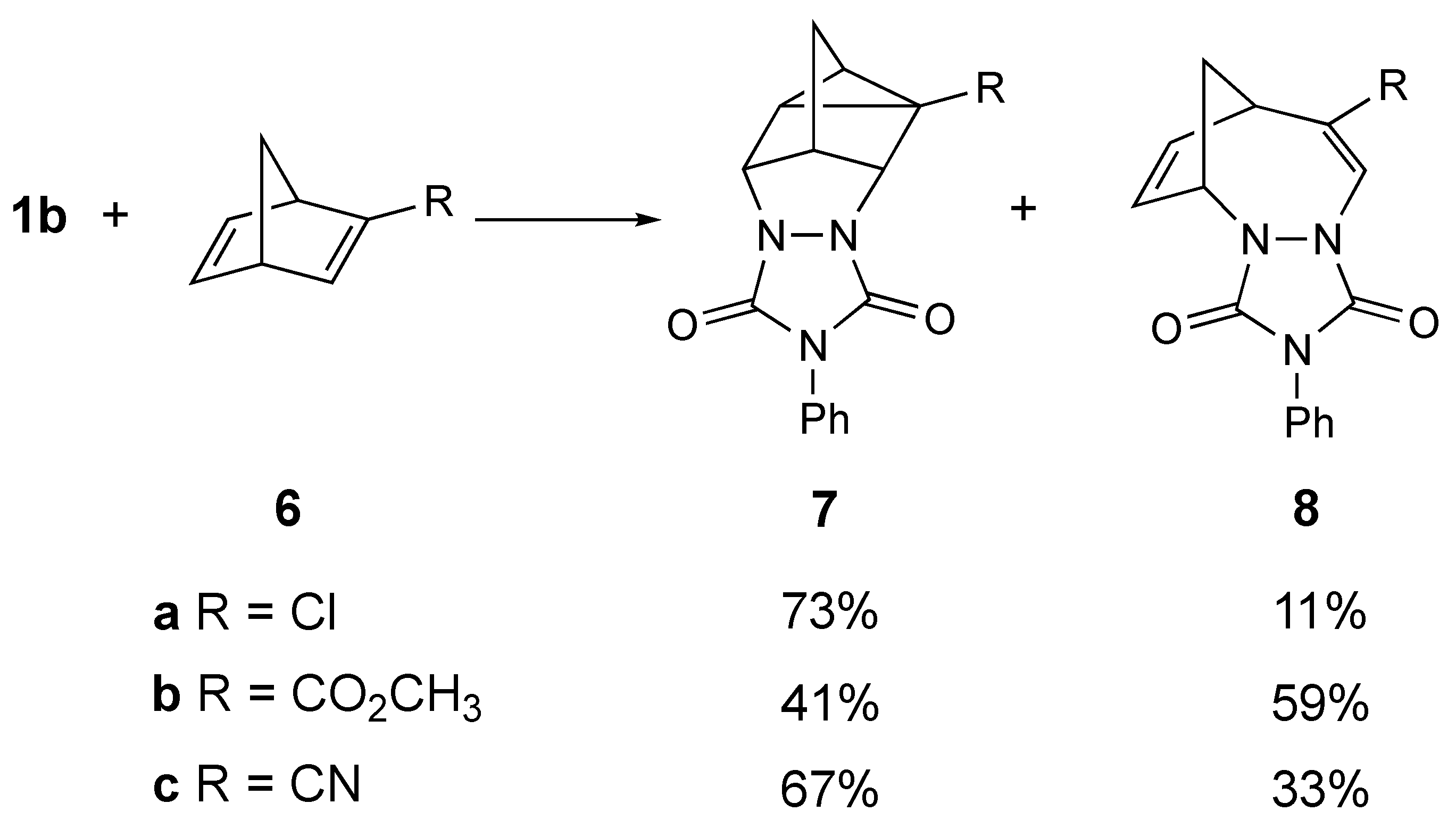
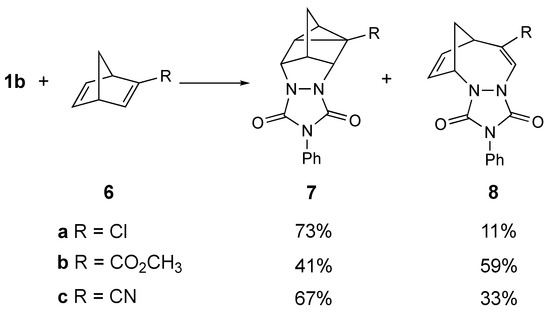
Adam et al. conducted studies several years ago that probed the effect of substituents on the course of the reaction of PhTAD with 2-substituted norbornadienes, 6 (Scheme 2) [8,9]. His group reported that the corresponding homo-DA adducts, 7, were still formed even when there were electron-withdrawing groups (i.e., –Cl, –CO2CH3, and –CN) attached. However, in each case, in addition to the expected homo-DA adduct, an interesting “insertion” product, 8, was also formed (note that the chloro derivative 6a also yielded other products, which are not shown). We found it interesting that this reaction was tolerant of the presence of strong electron-withdrawing groups, and even more interesting that these groups diverted at least some of the reactivity towards formation of the novel insertion product, 8.
Scheme 2.
Partial results of the reaction of PhTAD (1b) with monosubstituted norbornenes (6a,b,c) to give homo-Diels–Alder adducts (7) and insertion products (8) as reported by Adam [8,9]. The chloro derivative (6a) also yielded other products, which are not shown.
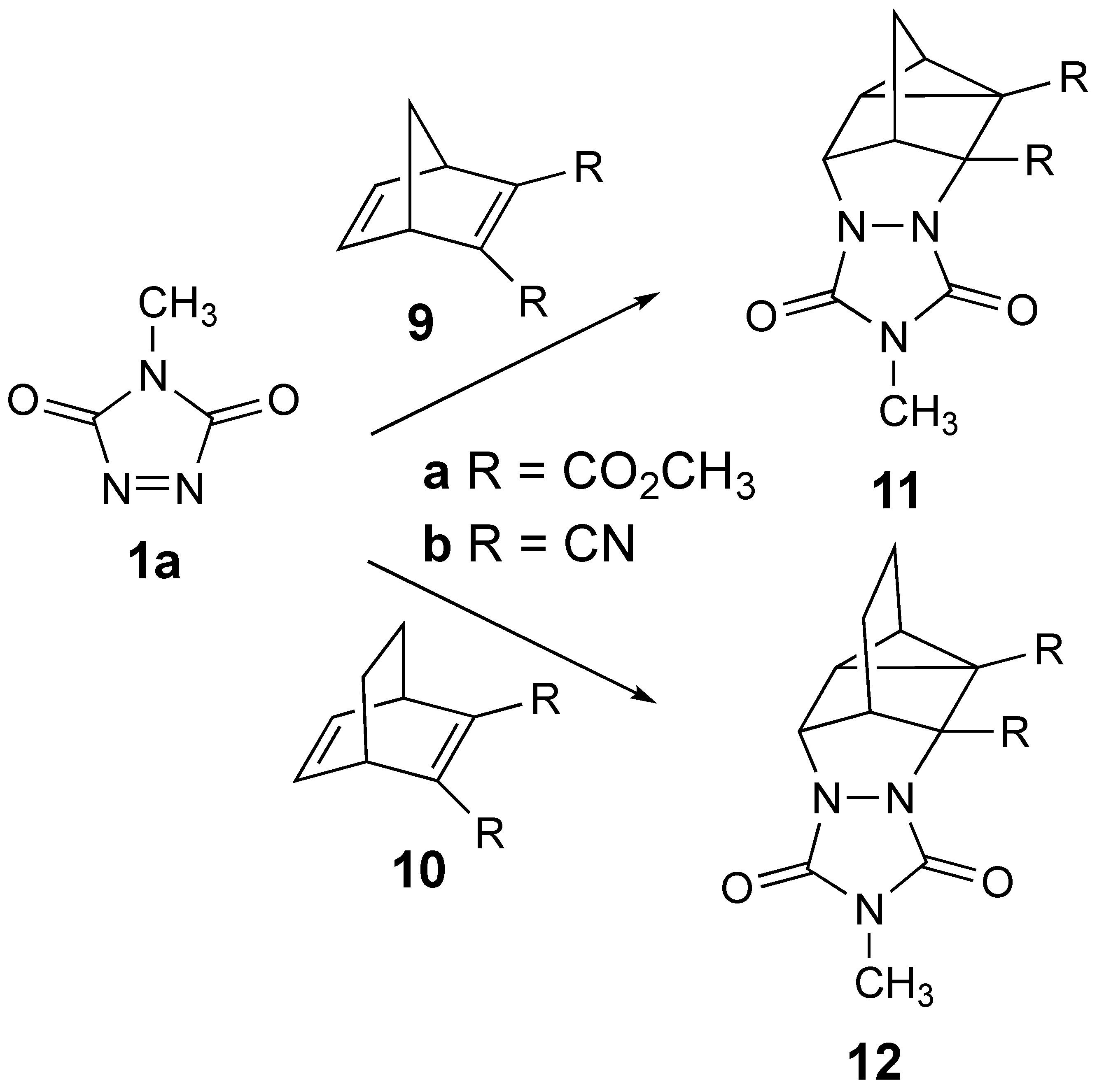
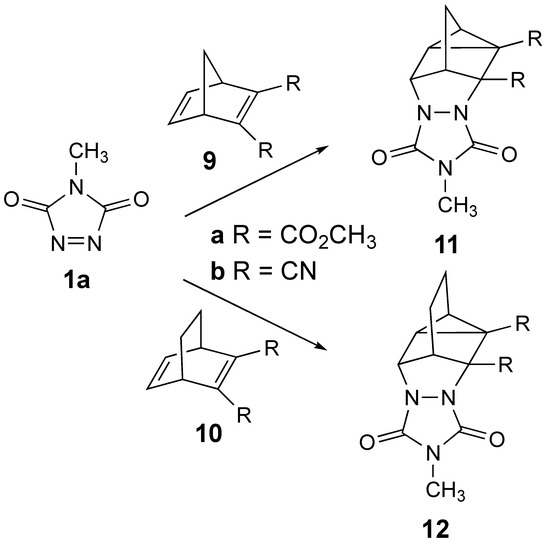
Given the recently renewed interest in the homo-DA reaction [14,15], we decided to investigate the reaction of MeTAD with the series of 2,3-disubstituted bicyclo-2,5-dienes 9a,b and 10a,b shown in Scheme 3. We wondered whether disubstitution on the bicyclo-2,5-diene frameworks with these electron-withdrawing groups would still allow for homo-DA reactivity and/or whether the reactivity would be diverted towards an insertion or other type of product. Herein, we report our findings on these reactions.
Scheme 3.
The reactions of 1a with disubstituted bicyclo-2,5-dienes 9a,b and 10a,b to be investigated to possibly provide the resulting homo-DA adducts 11a,b and 12a,b.
2. Materials and Methods
2.1. General Methods
Column chromatography was conducted on silica gel (234–400 mesh). Thin-layer chromatography was performed on pre-coated silica gel plates (250 mm) and visualized using ultraviolet light. 1H and 13C NMR spectra were obtained on a 400 MHz NMR spectrometer. Chemical shifts are reported in units of parts per million downfield from TMS. Structural assignments were made using additional information obtained from COSY experiments. High-resolution mass spectra (HRMS) were acquired via electron spray ionization on an LTQ-FTMS hybrid mass spectrometer. N-Methyl-1,3,5-triazoline-3,5-dione (MeTAD) was synthesized via oxidation of N-methylurazole with DABCO-Br2, as described in the literature [16,17]. Compounds 9a,b and 10a,b were synthesized according to the methods described in the literature [18,19].
2.2. Experimental Procedures
2.2.1. Reaction of MeTAD with 9a
(A) Reaction at room temperature. To begin, 100 mg (2 equiv) of MeTAD as a solid was added to a solution of 95 mg (0.46 mmol) of diene 9a in 3 mL of anhydrous 1,2-dichloroethane, and the two components were stirred together. The resulting deep red solution was sealed with parafilm, wrapped in foil to prevent exposure to light, and stirred for 3 weeks during which time the red color of the MeTAD was discharged. The resulting pale orange solution was concentrated in vacuo and subjected to column chromatography (100% EtOAc) to produce 22 mg (15% yield) of homo-DA adduct 11a as a white solid: m.p. 132–133 °C; IR cm−1 2962, 2927, 1774, 1712, 1441, 128, 1111, 784; 1H NMR (400 MHz, CDCl3) δ 4.57 (t, J = 2.0 Hz, 1H), 3.93 (s, 3H), 3.67 (s, 3H), 3.05 (s, 3H), 2.96–2.92 (m, 1H), 2.41 (d, J = 5.2 Hz, 1H), 2.37 (dd, J = 1.4, 5.2 Hz, 1H), 2.24 (d, J = 12.5 Hz, 1H), 1.91 (d, J = 12.5 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) 168.5, 166.0, 156.7, 154.9, 75.6, 64.3, 53.5, 52.4, 51.8, 31.9, 27.6, 27.4, 25.9, 24.6; HRMS (ESI) m/z [M+H]+ Calcd for C14H16N3O6 322.10336; Found 322.10289. (B) Reaction at 80 °C. To begin, 50 mg (1 equiv) of MeTAD as a solid was added to a solution of 95 mg (0.46 mmol) of diene 9a in 2 mL of anhydrous 1,2-dichloroethane, and the two components were stirred together. A water condenser with a drying tube was attached to the top of the round-bottomed flask and the solution was heated to reflux until the red color of the MeTAD was discharged (6 h). The resulting orange solution was concentrated in vacuo and subjected to column chromatography (100% EtOAc) to produce 21 mg (14% yield) of homo-DA adduct 11a as a white solid: characterization as above.
2.2.2. Attempted Reaction of MeTAD with 9b
To begin, 35 mg (1 equiv) of MeTAD as a solid was added to a solution of 45 mg (0.32 mmol) of diene 9b in 2 mL of anhydrous 1,2-dichloroethane was added, and the two components were stirred together. A water condenser with a drying tube was attached to the top of the round-bottomed flask and the solution was heated to reflux for 24 h. The red color of the MeTAD persisted. The reaction mixture was cooled to room temperature and concentrated in vacuo. Analysis by 1H NMR spectroscopy provided no evidence of a reaction.
2.2.3. Reaction of MeTAD with 10a
To begin, 50 mg (1 equiv) of MeTAD as a solid was added to a solution of 111 mg (0.5 mmol) of diene 10a in 2 mL of anhydrous 1,2-dichloroethane was added, and the two components were stirred together. A water condenser with a drying tube was attached to the top of the round-bottomed flask and the solution was heated to reflux until the red color of the MeTAD was discharged (24 h). The reaction mixture was concentrated in vacuo and subjected to column chromatography (100% EtOAc) to produce 82 mg (55% yield) of homo-DA adduct 12a as a white crystalline solid: m.p. 194–195 °C; IR cm−1 2954, 1761, 1715, 1458, 1441, 1230, 1119, 782. 1H NMR (400 MHz, CDCl3) δ 4.48 (br s, 1H), 3.97 (s, 3H), 3.67 (s, 3H), 3.00 (s, 3H), 2.67 (br s, 1H), 2.35 (d, J = 7.9 Hz, 1H), 2.13–2.02 (m, 1H), 1.9–1.75 (m, 4H); 13C{1H} NMR (100 MHz, CDCl3) 169.8, 166.5, 156.0, 154.5, 73.4, 59.5, 53.2, 52.4, 47.1, 32.3, 25.9, 25.7, 23.7, 16.5, 13.0; HRMS (ESI) m/z [M+H]+ Calcd for C15H18N3O6 336.11901; Found 336.11850.
2.2.4. Attempted Reaction of MeTAD with 10b
To begin, 18 mg (1 equiv) of MeTAD as a solid was added to a solution of 25 mg (0.16 mmol) of diene 10b in 1 mL of anhydrous 1,2-dichloroethane was added, and the two components were stirred together. A water condenser with a drying tube was attached to the top of the round-bottomed flask and the solution was heated to reflux for 24 h. The red color of the MeTAD persisted. The reaction mixture was cooled to room temperature and concentrated in vacuo. Analysis by 1H NMR spectroscopy provided no evidence of a reaction.
2.3. X-ray Crystallographic Analysis
Colorless single crystals suitable for X-ray diffraction were obtained from a methanol solution.
The diffraction data were collected on a Rigaku XtaLAB Synergy-S Dualflex HyPix diffractometer with monochromated Cu-Kα radiation. The structure was solved by direct methods (OLEX2.solve [20,21]) and refined by full-matrix least-squares on F2 values (SHELXL [22]). All the heavy atoms were refined anisotropically. The hydrogen atoms were localized from the difference electron density maps, after which they were refined isotropically (Uiso with a factor of 1.2 for CH and CH2 groups and a factor of 1.5 for CH3 groups) with riding coordinates or as rotation CH3 groups. Mercury [23] was used for the structure presentation in Figure 1.
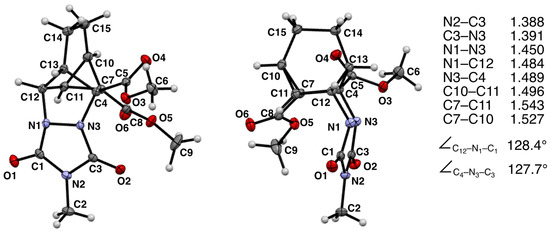
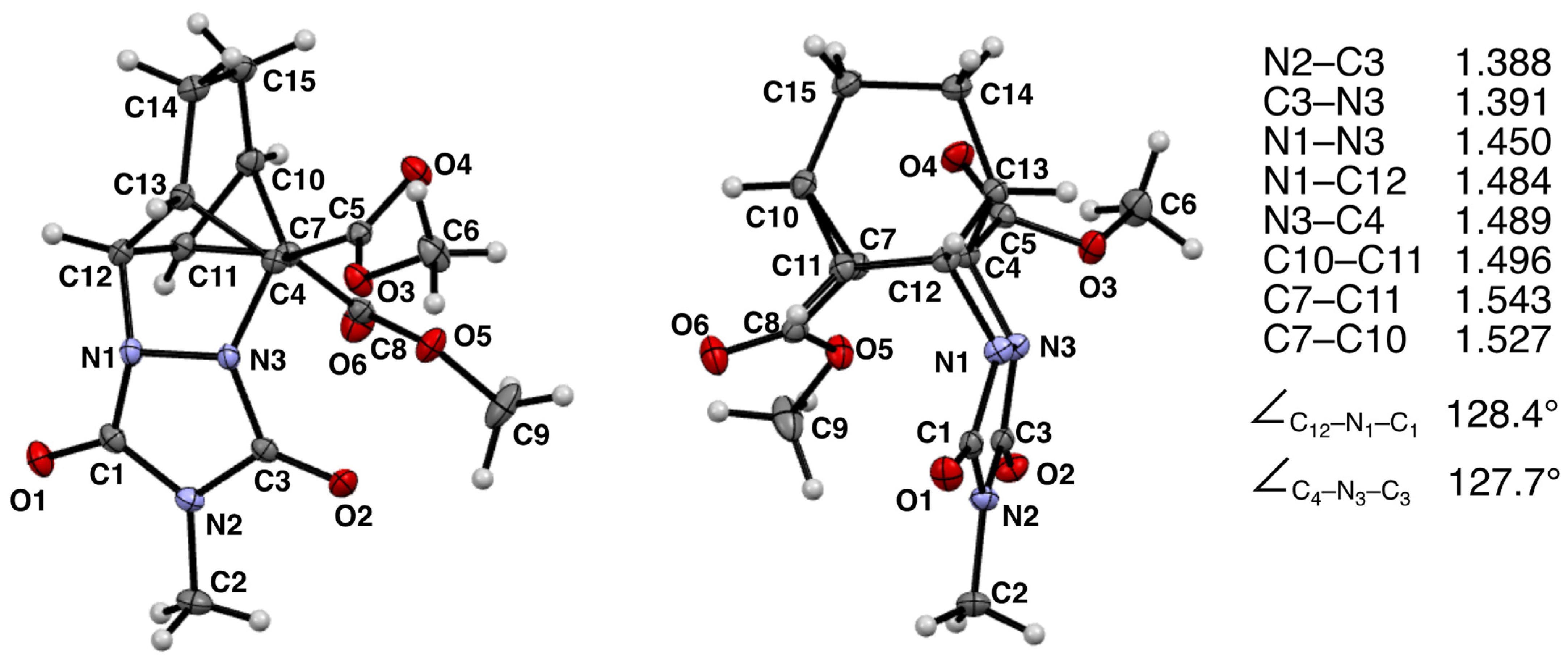
Figure 1.
Ortep diagram for compound 12a as provided by X-ray crystallographic analysis. Non-hydrogen atom thermal elipsoids are at the 50% probability limit. Hydrogen atoms are represented as spheres of arbitrarily small radii. Some selected bond distances (in Å) and bond angles are provided from the X-ray structure.
The X-ray structure of the homo-Diels–Alder adduct 12a is as follows: C15H17N3O6, M = 335.32 g/mol, monoclinic system, space group P21/a, a = 7.6971(1) Å, b = 20.5045(2) Å, c = 9.7683(1) Å, β = 108.129(2)°, Z = 4, V = 1465.15(3) Å3, Dc = 1.52 g cm−3, μ(Cu Kα) = 1.011 mm−1, T = 100 K, and crystal dimensions of 0.152 × 0.389 × 0.423 mm. The final model converged to final values of R = 0.0362 and Rw = 0.0932 using 2954 independent reflections (θmax = 77.03°).
The crystallographic data for the structure reported in this paper has been deposited with the Cambridge Crystallographic Data Centre as a supplementary publication. Copies of the data (CCDC registration number 2215026) can be obtained from the CCDC free of charge by sending an application to the following e-mail address: deposit@ccdc.cam.ac.uk.
3. Results
The disubstituted bicyclo-2,5-dienes 9a,b and 10a,b were synthesized according to the procedures described in the literature [18,19]. The reaction of equimolar amounts of MeTAD with 9a in 1,2-dichloroethane as a solvent was allowed to proceed for three weeks at room temperature until the deep red color of the MeTAD was discharged. Analysis of the crude reaction mixture by TLC and 1H NMR spectroscopy suggested a complete consumption of the starting materials and the formation of a single major product. Careful column chromatography resulted in a 15% yield of the homo-DA adduct 11a. The structural assignment was consistent with the results from the 1H, 13C, and COSY NMR spectral analyses (spectra are provided in the Supplementary Materials). Although refluxing the reaction mixture in 1,2-dichloroethane (~80 °C) drove the reaction to completion within six hours, the yield was not improved (14% yield). For both reactions, in addition to 11a, only intractable, undefined materials that often accompany triazolinedione reactions were formed, as Adam has also reported [8,9].
Dicyano-substituted diene 9b failed to show any signs of reaction with MeTAD, even upon refluxing the reaction mixture in 1,2-dichloroethane for 24 h.
Heating a solution of MeTAD and 10a in 1,2-dichloroethane for 24 h resulted in a single isolable product that was identified as a homo-DA adduct 12a (55% yield). The 1H NMR spectrum was similar to that collected for 11a, and the structural assignment was supported by the expected proton connectivities revealed in the COSY spectrum (see Supplementary Materials). Fortunately, this homo-DA adduct was particularly crystalline in nature which enabled us to confirm the structures of these strained heterocycles. This was a notable accomplishment given that we were unable to find an instance in the literature in which the fundamental structures of these diaza homo-DA adducts had been definitively determined via X-ray crystallography. Slow cooling of a saturated boiling solution of 12a in methanol produced colorless plates suitable for X-ray analysis. The observed structure is provided in Figure 1 from two different vantage points. Therefore, both the presence of the strained cyclopropyl ring and the connectivity of the urazole ring system to the carbon framework were confirmed. Some selected bond lengths and bond angles are provided (see Figure 1).
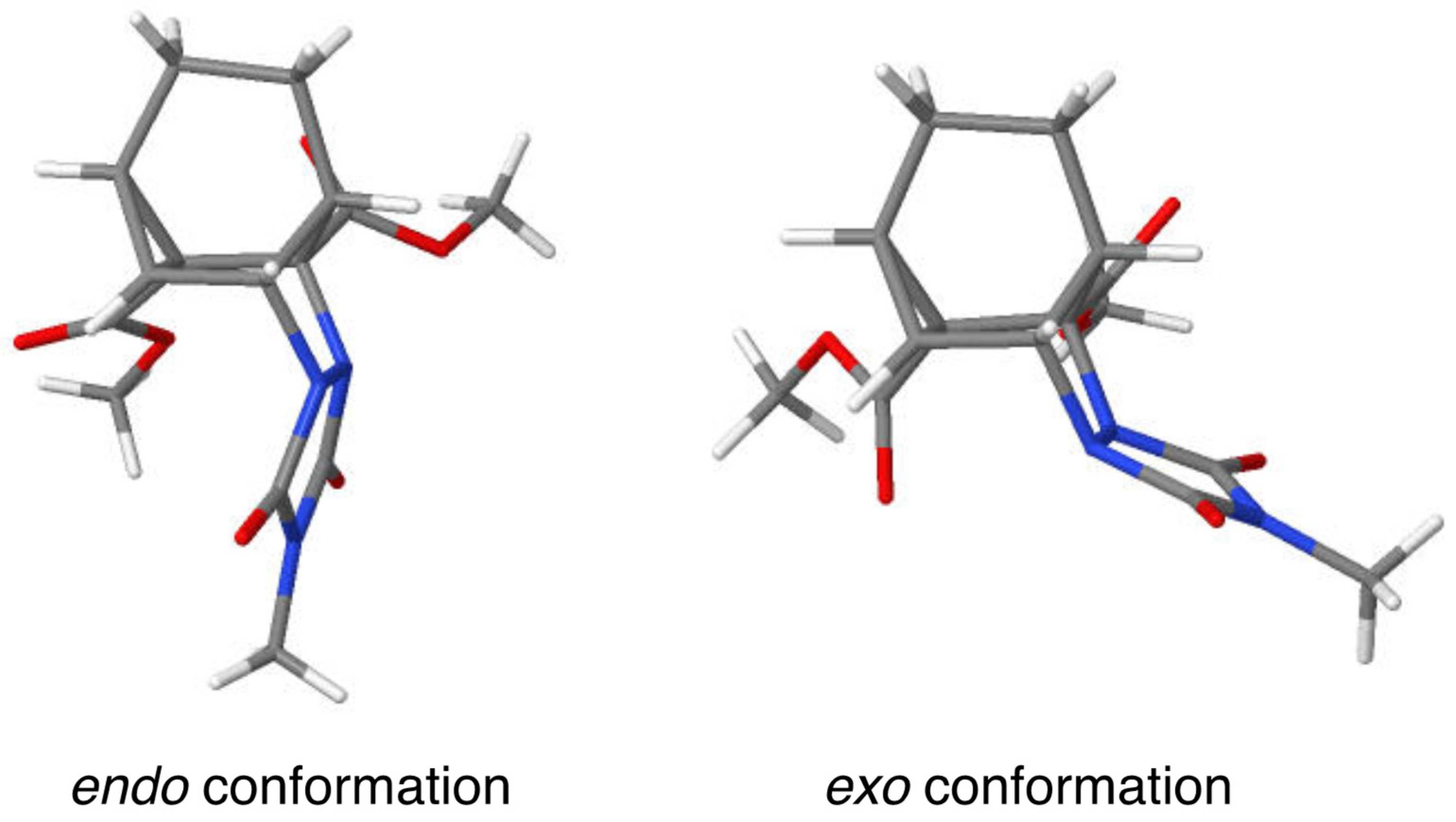
The newly formed C-C bond of the cyclopropyl ring system appears to experience the most strain as its bond length (1.543 Å) is longer than that of the other two bonds (1.496 and 1.527 Å). The two C-N bonds joining the nitrogen atoms of the urazole ring to the carbon backbone are similar in length (1.484 and 1.489 Å). Note that the urazole nitrogen atoms adopt pyramidalization such that the urazole ring is tucked underneath the bicyclic system in an endo fashion rather than being extended outward (i.e., exo) in what would appear to be a less sterically congested arrangement (see Figure 2). It is likely that crystal packing forces may favor the endo conformation because of its greater compactness.
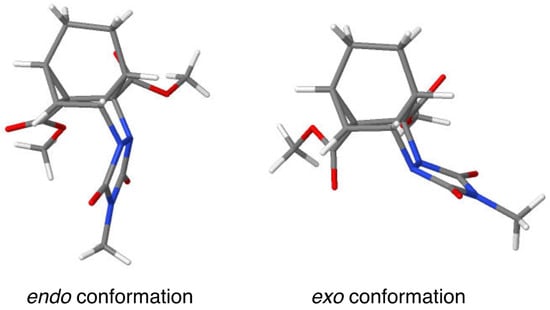
Figure 2.
Models representing the endo and exo conformers of cycloadduct 12a.
Unfortunately, and as was previously observed for diene 9b, refluxing a solution of MeTAD and dicyano-substituted diene 10b in 1,2-dichloroethane failed to produce any indication of cycloadduct formation.
4. Discussion
Attaching two substituents onto the bicycloalkadiene frameworks might be expected to inhibit reactivity with the MeTAD dienophile for two reasons. First, both the carbomethoxy and cyano groups are sterically larger than the hydrogen atoms present on the unsubstituted diene analogues 2 and 4. The MeTAD will therefore experience steric hindrance as it approaches the diene to form the homo-DA product. The effect of the larger, and conformationally mobile carbomethoxy group would be expected to result in a greater steric rate retardation compared to the linear cyano group. However, since homo-DA adduct formation was still observed for the dienes substituted with the carbomethoxy groups, but not upon substitution with the cyano groups, apparently steric hindrance is not the primary factor dictating reactivity. In addition to steric effects, the substituents will also exert electronic effects. Substitution on the C=C bond by electron-withdrawing groups will deplete electron density in the diene system, thereby rendering the diene less attractive as a cycloaddition partner with the strongly electrophilic MeTAD. Hammett parameters (σp) provide a measure of the electron-withdrawing character of various substituents [24]. The σp-value for the cyano group (0.66) is significantly greater than that for the carbomethoxy group (0.45). While Adam demonstrated that the homo-DA reaction can still proceed with a single cyano group attached to the diene system [8,9], apparently two cyano groups leads to depletion of the electron density of the diene system to the extent that reaction with MeTAD is prohibited. Surprisingly however, sufficient reactivity is maintained even in the presence of the two fairly strong electron-withdrawing carbomethoxy groups. Remarkably, in both cases, the only isolable product was the homo-DA adduct and no diversion of reactivity to form any characterizable insertion or other products was observed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org4010002/s1, Figure S1: 1H NMR (400 MHz, CDCl3) of homo-Diels–Alder adduct 11a; Figure S2: 13C{1H} NMR (100 MHz, CDCl3) of homo-Diels–Alder adduct 11a; Figure S3: COSY spectrum (400 MHz, CDCl3) of homo-Diels–Alder adduct 11a; Figure S4: 1H NMR (400 MHz, CDCl3) of homo-Diels–Alder adduct 12a; Figure S5: 13C{1H} NMR (100 MHz, CDCl3) of homo-Diels–Alder adduct 12a; Figure S6: COSY spectrum (400 MHz, CDCl3) of homo-Diels–Alder adduct 12a.
Author Contributions
Data curation, G.W.B.; Formal analysis, G.W.B. and K.L.M.; Funding acquisition, G.W.B.; Investigation, G.W.B. and K.L.M.; Methodology, G.W.B. and K.L.M.; Writing—original draft, G.W.B.; Writing—review & editing, G.W.B. and K.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Berry College.
Data Availability Statement
Crystallographic data have been deposited with the Cambridge Crystallographic Data Center (Deposition Number 2215026) and can be obtained free of charge. NMR data for novel compounds are available in the Supplementary Information.
Acknowledgments
G.W.B. and K.L.M. thank John Bacsa, Facilities Director of Emory University’s X-ray Crystallography Center (supported by the National Science Foundation under CHE-1626172) for collecting the X-ray crystal data on compound 12a.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cookson, R.C.; Gilani, S.S.H.; Stevens, I.D.R. Diels-Alder Reactions of 4-Phenyl-1,2,4-triazoline-3,5-dione. J. Chem. Soc. C. 1967, 1905–1909. [Google Scholar] [CrossRef]
- Allred, E.L.; Johnson, A.L. The Influence of Geometry on Cyclopropyl Participation in the Thermolysis of Azo Compounds. J. Am. Chem. Soc. 1971, 93, 1300–1301. [Google Scholar] [CrossRef]
- Burrage, M.E.; Cookson, R.C.; Gupte, S.S.; Stevens, I.D.R. Substituent and Solvent Effects on the Diels-Alder Reactions of Triazolinediones. J. Chem. Soc. Perkin Trans. 2 1975, 1325–1334. [Google Scholar] [CrossRef]
- Erden, I.; de Meijere, A. Convenient New Synthesis of Snoutene Utilizing a Dipolar Cycloaddition of 4-Phenyl-1,2,4-triazolin-3,5-dione. Tetrahedron Lett. 1980, 21, 1837–1840. [Google Scholar] [CrossRef]
- Gassman, P.G.; Hoye, R.C. Anomalous Cycloaddition Reactions of Distorted Cyclohexa-1,4-dienes. Cycloaddition of N-phenyltriazolinedione to (i,o)-Bicyclo[n.2.2]alkadienes. J. Am. Chem. Soc. 1981, 103, 2496–2498. [Google Scholar] [CrossRef]
- Adam, W.; De Lucchi, O. Reaction of Bicyclo[3.2.1]octadiene with 4-Methyl-1,2,4-triazoline-3,5-dione: Competitive Dipolar and Homo-Cycloaddition. Tetrahedron Lett. 1981, 22, 3501–3504. [Google Scholar] [CrossRef]
- Adam, W.; De Lucchi, O.; Peters, K.; Peters, E.-M.; von Schnering, H.G. Reaction of Bicyclo[3.2.1]octadiene with 1,2,4-triazoline-3,5-diones: Competitive Dipolar and HomoCycloaddition. J. Am. Chem. Soc. 1982, 104, 161–166. [Google Scholar] [CrossRef]
- Adam, W.; Arias, L.A.; De Lucchi, O. Unusual Cycloaddition Behavior of 2-Substituted Norbornadienes with Triazolinediones. Tetrahedron Lett. 1982, 23, 399–402. [Google Scholar] [CrossRef]
- Adam, W.; de Lucchi, O.; Pasquato, L.; Will, B. Cycloaddition Behavior of 2-Substituted Norbornadienes towards 4-Phenyl-4H-1,2,4-triazole-3,5-dione (PTAD): Homo Diels-Alder Reactivity versus Insertion, Rearrangement, and [2+2] Cycloaddition. Chem. Ber. 1987, 120, 531–535. [Google Scholar] [CrossRef]
- Kiselev, V.D.; Shakirova, I.I.; Kornilov, D.A.; Kashaeva, H.A.; Potapova, L.N.; Konovalov, A.I. Homo-Diels-Alder Reaction of a Very Inactive Diene, Bicyclo[2,2,1]hepta-2,5-diene, with the Most Active Dienophile, 4-Phenyl-1,2,4-triazolin-3,5-dione. Solvent, Temperature, and High Pressure Influence on the Reaction Rate. J. Phys. Org. Chem. 2013, 26, 47–53. [Google Scholar] [CrossRef]
- Adam, W.; De Lucchi, O.; Erden, I. Bicyclic Azoalkanes via Urazoles Derived from Cycloaddition of N-Phenyl-1,2,4-triazoline-3,5-dione with Strained Bicycloalkenes. J. Am. Chem. Soc. 1980, 102, 4806–4809. [Google Scholar] [CrossRef]
- Adam, W.; Dorr, M.; Kron, J.; Rosenthal, R.J. On the Question of 1,4-Diradical Intermediates in the Di-p-methane Rearrangement of Benzobicyclo[3.2.1]octadienes: Azoalkanes as Mechanistic Probes. J. Am. Chem. Soc. 1987, 109, 7074–7081. [Google Scholar] [CrossRef]
- Zimmerman, H.W.; Boettcher, R.J.; Buehler, N.E.; Keck, G.E.; Steinmetz, M.G. Independent generation of cyclopropyldicarbinyl diradical species of the di-.pi.-methane rearrangement. Excited singlet, triplet, and ground-state hypersurfaces of barrelene photochemistry. Mechanistic and exploratory organic photochemistry. J. Am. Chem. Soc. 1976, 98, 7680–7689. [Google Scholar] [CrossRef]
- Li, Y.; Fang, D.-C. DFT Calculations on Kinetic Data for Some [4+2] Reactions in Solution. Phys. Chem. Chem. Phys. 2014, 16, 15224–15230. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-P.; Seeman, J.I.; Houk, K.N. Rolf Huisgen’s Classic Studies of Cyclic Triene Diels-Alder Reactions Elaborated by Modern Computational Analysis. Angew. Chem. Int. Ed. 2020, 59, 12506–12519. [Google Scholar] [CrossRef] [PubMed]
- Breton, G.W.; Turlington, M. Alternative Synthetic Routes to N-Methyl-1,2,4-Triazoline-3,5-Dione (MeTAD) and Other Triazolinedione Derivatives. Tetrahedron Lett. 2014, 55, 4661–4663. [Google Scholar] [CrossRef]
- Billiet, S.; De Bruycker, K.; Driessen, F.; Goossens, H.; Van Speybroeck, V.; Winne, J.M.; Du Prez, F.E. Triazolinediones Enable Ultrafast and Reversible Click Chemistry for the Design of Dynamic Polymer Systems. Nat. Chem. 2014, 6, 815–821. [Google Scholar] [CrossRef]
- Vialulin, R.A.; Arisco, T.M.; Kutateladze, A.G. Photoinduced Intramolecular Cyclopentanation vs. Photoprotolytic Oxametathesis in Polycyclic Alkenes Outfitted with Conformationally Constrained Aroylmethyl Chromophores. J. Org. Chem. 2013, 78, 2012–2025. [Google Scholar] [CrossRef]
- Ono, N.; Hirao, A.; Takaisa, K.; Aramaki, S.; Okushima, T.; Hashimo, Y.; Akiyama, S. Manufacture of Heat-Stable Organic Pigments and Organic Electric Elements, Organic Pigment Precursors, and Phtalocyanie Analogs. JP2009215547, September 2009. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The Anatomy of a Comprehensive Constrained, Restrained Refinement Program for the Modern Computing Environment—Olex2 Dissected. Acta Cryst. 2015, A71, 59–75. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry, Part A: Structure and Mechanisms, 5th ed.; Springer: New York, NY, USA, 2007; pp. 335–344. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).