Palladium Catalyzed Ring-Opening of Diazabicylic Olefins with 4-Halo-1,3-Dicarbonyl Compounds: Accessing 3(2H)-Furanone-Appended Cyclopentenes
Abstract
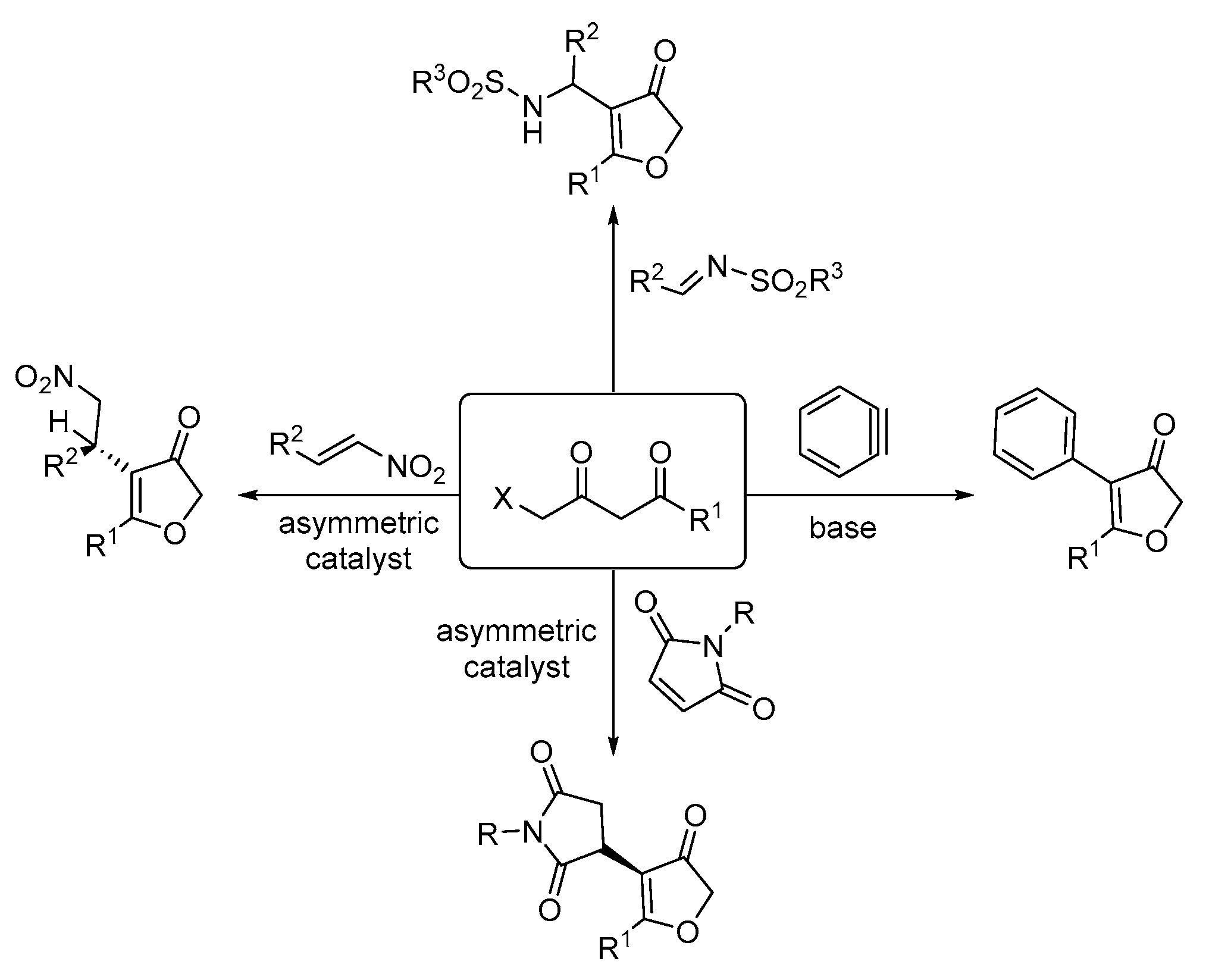
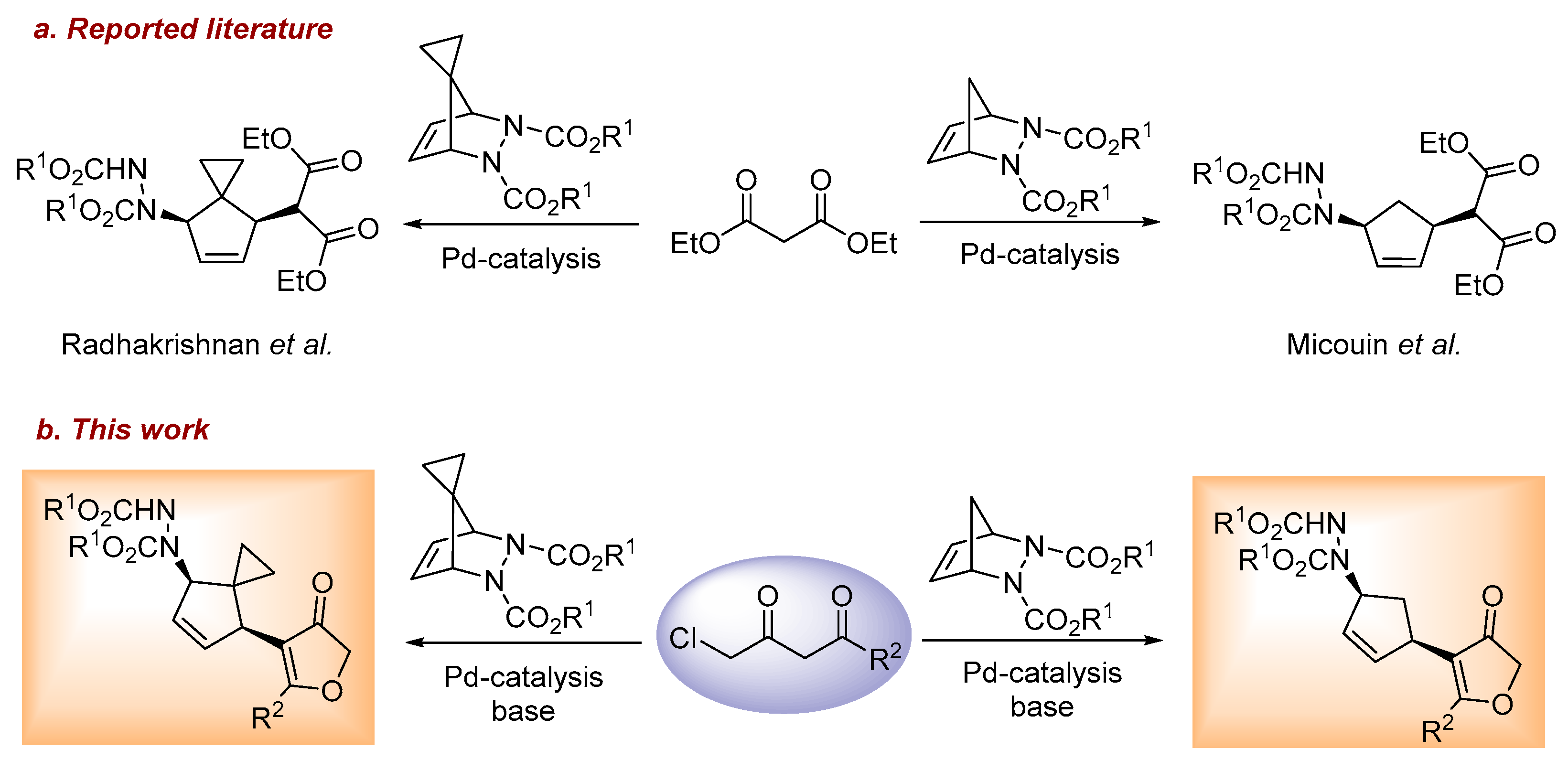
:1. Introduction
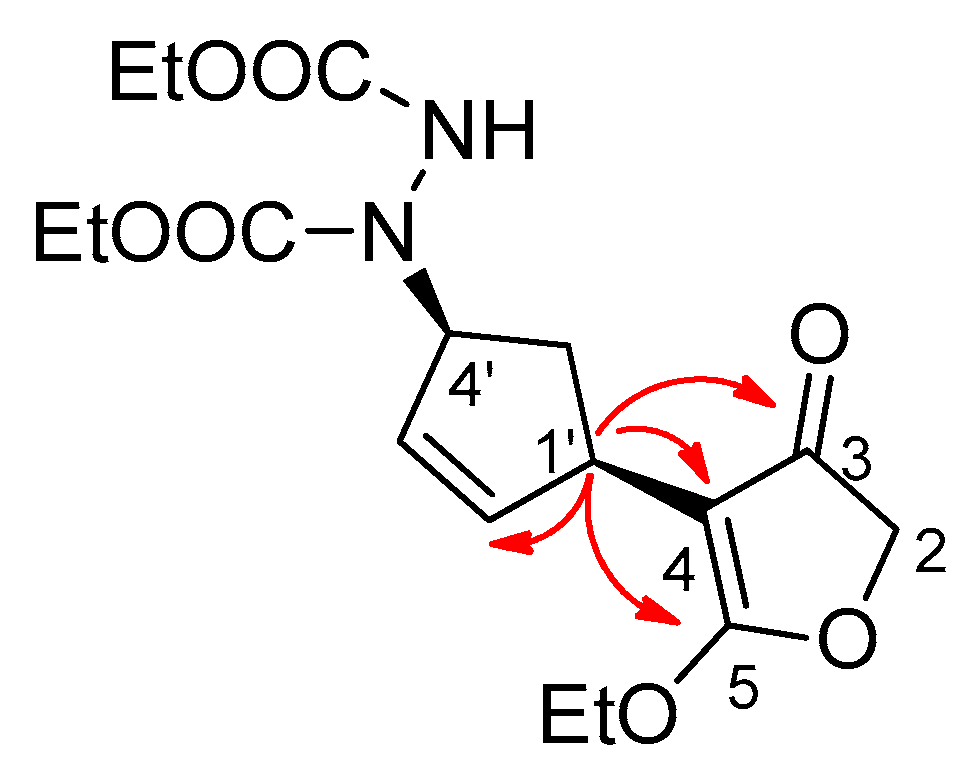
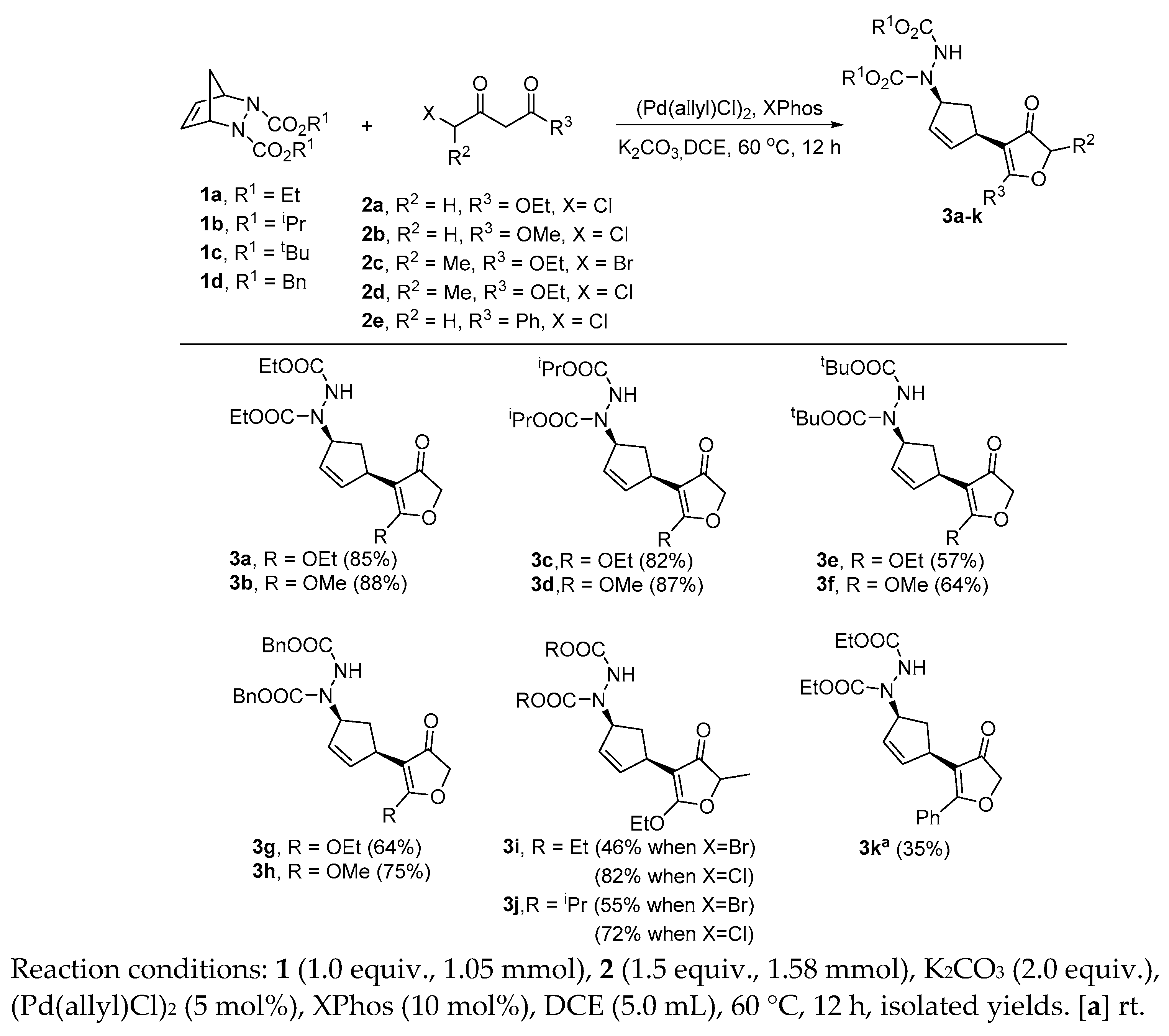
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colin Slaughter, J.C. The naturally occurring furanones: Formation and function from pheromone to food. Biol. Rev. 1999, 74, 259. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.B.; Nadanya, A.E.; Sweeney, J.B. Recent developments in the synthesis of furan-2(5H)-ones. J. Chem. Soc. Perkin Trans. 1. 2002, 2324. [Google Scholar] [CrossRef]
- de Nys, R.; Givskov, M.; Kumar, N.; Kjelleberg, S.; Steinberg, P.D. Marine Molecular Biotechnology; Fusetani, N., Clare, A.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 42. [Google Scholar]
- Schwab, W. Natural 4-Hydroxy-2,5-dimethyl-3(2H)-furanone. Molecules 2013, 18, 6936. [Google Scholar] [CrossRef]
- Husain, A.; Khan, S.A.; Iram, F.; Iqbal, M.A.; Asif, M. Insights into the chemistry and therapeutic potential of furanones: A versatile pharmacophore. Eur. J. Med. Chem. 2019, 171, 66. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B., III; Levenberg, P.A.; Jerris, P.J.; Scarborough, R.M., Jr.; Wovkulich, P.M. Synthesis and reactions of simple 3(2H)-furanones. J. Am. Chem. Soc. 1981, 103, 1501. [Google Scholar] [CrossRef]
- Jerris, P.J.; Smith, A.B., III. Synthesis and configurational assignment of geiparvarin: A novel antitumor agent. J. Org. Chem. 1981, 46, 577. [Google Scholar] [CrossRef]
- Marson, C.M.; Edaan, E.; Morrell, J.M.; Coles, S.J.; Hursthouse, M.B.; Davies, D.T. A catalytic asymmetric protocol for the enantioselective synthesis of 3(2H)-furanones. Chem. Commun. 2007, 2494. [Google Scholar] [CrossRef] [PubMed]
- Haug, T.T.; Kirsch, S.F. Synthesis and chemistry of 3(2H)-furanones. Targets Heterocycl. Syst. 2009, 13, 57. [Google Scholar]
- Omanakuttan, V.K.; John, J.; Hopf, H. Synthesis of 3(2H)-furanones-a review. Eur. J. Org. Chem. 2021, 2021, 163. [Google Scholar] [CrossRef]
- Dou, X.; Han, X.; Lu, Y. From the Feist–Bénary Reaction to Organocatalytic Domino Michael–Alkylation Reactions: Asymmetric Synthesis of 3(2H)-Furanones. Chem. Eur. J. 2012, 18, 85. [Google Scholar] [CrossRef]
- Yan, Y.-Y.; Lu, R.-J.; Wang, J.-J.; Xuan, Y.-N.; Yan, M. Synthesis of chiral tetronic acid derivatives via organocatalytic conjugate addition of ethyl 4-chloro-3-oxobutanoate to nitroalkenes. Tetrahedron 2012, 68, 6123. [Google Scholar] [CrossRef]
- Zhou, J.; Bai, L.; Liang, G.; Chen, Y.; Gan, Z.; Wang, W.; Zhou, H.; Yu, Y. Organocatalytic asymmetric domino Michael/O-alkylation reaction for the construction of succinimide substituted 3(2H)-furanones catalyzed by quinine. RSC Adv. 2017, 7, 39885. [Google Scholar] [CrossRef]
- John, J.; Hopf, H. Substituted 3(2H)-Furanones by a Tandem Michael Addition/Palladium-Catalyzed Ring-Closing Protocol. Eur. J. Org. Chem. 2013, 2013, 841. [Google Scholar] [CrossRef]
- John, J.; Târcoveanu, E.; Jones, P.G.; Hopf, H. A tandem Mannich addition–palladium catalyzed ring-closing route toward 4-substituted-3-(2H)-furanones. Beilstein J. Org. Chem. 2014, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Omanakuttan, V.K.; Aneeja, T.; Suresh, C.H.; Jones, P.G.; Hopf, H. Tandem α-Arylation/Cyclization of 4-Haloacetoacetates with Arynes: A Metal-Free Approach toward 4-Aryl-3-(2H)-furanones. J. Org. Chem. 2019, 84, 5957. [Google Scholar] [CrossRef]
- Omanakuttan, V.K.; Santhini, P.V.; Shaludheen, S.; Varughese, S.; Hopf, H.; John, J. Tandem Reaction of 4-Halo-1,3-Dicarbonyl Compounds with Alkynes towards 4-Vinyl-3(2H)-Furanones and 3(2H)-Furanone fused 2-Pyridones. Asian J. Org. Chem. 2022, 11, e202200410. [Google Scholar] [CrossRef]
- Bournaud, C.; Chung, F.; Luna, A.; Pasco, M.; Errasti, G.; Lecourt, T.; Micouin, L. Stereoselective Transformations of meso Bicyclic Hydrazines: Versatile Access to Functionalized Aminocyclopentanes. Synthesis 2009, 2009, 869. [Google Scholar] [CrossRef]
- Sajisha, S.; Anas, S.; John, J.; Radhakrishnan, K.V. Desymmetrization of meso-Bicyclic Hydrazines: An Efficient Strategy towards the Synthesis of Functionalized Cyclopentenes. Synlett 2009, 2009, 2885. [Google Scholar] [CrossRef]
- Pineschi, M. The Binomial Copper-Catalysis and Asymmetric Ring Opening of Strained Heterocycles: Past and Future Challenges. Eur. J. Org. Chem. 2020, 2020, 2643. [Google Scholar] [CrossRef]
- Preethalayam, P.; Jijy, E.; Prakash, P.; Sarngadharan, S.C.; Vijayan, A.; Radhakrishnan, K.V.; John, J. Diazanorbornene: A Valuable Synthon towards Carbocycles and Heterocycles. Eur. J. Org. Chem. 2020, 2020, 6588. [Google Scholar] [CrossRef]
- Allred, E.L.; Anderson, C.L.; Smith, R.L. Hydroboration of 2,3-dicarbomethoxy-2,3-diazabicyclo [2.2.1]hept-5-ene. The elimination mechanism of the organoborane intermediate. Tetrahedron Lett. 1966, 9, 951. [Google Scholar] [CrossRef]
- Wilson, R.M.; Schnapp, K.A.; Merwin, R.K.; Ranganathan, R.; Moats, D.L.; Conrad, T.T. Synthesis of allylic alcohol single-chain PGH analogs. A synthetic application of the argon laser. J. Org. Chem. 1986, 51, 4028. [Google Scholar] [CrossRef]
- Bournaud, C.; Lecourt, T.; Micouin, L.; Méliet, C.; Agbossou-Niedercorn, F. Desymmetrization of meso-Bicyclic Hydrazines by Rhodium-Catalyzed Enantioselective Hydroformylation. Eur. J. Org. Chem. 2008, 2008, 2298. [Google Scholar] [CrossRef]
- Mellor, J.M.; Smith, N.M. Reductive cleavage of the nitrogen–nitrogen bond in hydrazine derivatives. J. Chem. Soc., Perkin Trans. 1 1984, 2927–2931. [Google Scholar] [CrossRef]
- Grabowski, S.; Armbruster, J.; Prinzbach, H. Biocatalysis in the chiral recognition of meso-diamides—An efficient route from cyclic olefinic hydrocarbons to optically pure diamino-polyols. Tetrahedron Lett. 1997, 38, 5485. [Google Scholar] [CrossRef]
- Storsberg, J.; Nandakumar, M.V.; Sankaranarayanan, S.; Kaufmann, D.E. Stereoselective Palladium-Catalyzed C-C Coupling Reactions with a Diazabicyclo [2.2.1]heptane. Adv. Synth. Catal. 2001, 343, 177. [Google Scholar] [CrossRef]
- Yao, M.-L.; Adiwidjaja, G.; Kaufmann, D.E. Two-Step, Stereoselective Hydrazidoarylation of 1,3-Cyclopentadiene. Angew. Chem. Int. Ed. 2002, 41, 3375. [Google Scholar] [CrossRef]
- Radhakrishnan, K.V.; Sajisha, V.S.; Anas, S.; Krishnan, K.S. Palladium-Catalyzed Reaction of Bicyclic Hydrazines with Allyl- and Arylstannanes in Ionic Liquid [bmim]PF6: A Facile Method for the Synthesis of Substituted Hydrazinocyclopentene Derivatives. Synlett 2005, 2005, 2273. [Google Scholar] [CrossRef]
- Sajisha, V.S.; Mohanlal, S.; Anas, S.; Radhakrishnan, K.V. A facile synthesis of 3-allyl-4-hydrazinocyclopentenes by the palladium/Lewis acid mediated ring opening of bicyclic hydrazines with allyltributyltin and allyltrimethylsilane. Tetrahedron 2006, 62, 3997. [Google Scholar] [CrossRef]
- Sajisha, V.S.; Radhakrishnan, K.V. Palladium/Lewis Acid-Catalyzed Reactions of Bicyclic Hydrazines with Organostannanes: A General Methodology for the Stereoselective Synthesis of 3,4-Disubstituted Cyclopentenes. Adv. Synth. Catal. 2006, 348, 924. [Google Scholar] [CrossRef]
- John, J.; Sajisha, V.S.; Mohanlal, S.; Radhakrishnan, K.V. Iodine assisted modified Suzuki type reaction of bicyclic hydrazines: Stereoselective synthesis of functionalized cyclopentene. Chem. Commun. 2006, 3510–3512. [Google Scholar] [CrossRef]
- Bournaud, C.; Falciola, C.; Lecourt, T.; Rosset, S.; Alexakis, A.; Micouin, L. On the Use of Phosphoramidite Ligands in Copper-Catalyzed Asymmetric Transformations with Trialkylaluminum Reagents. Org. Lett. 2006, 8, 3581. [Google Scholar] [CrossRef] [PubMed]
- Anas, S.; John, J.; Sajisha, V.S.; Rajan, R.; Suresh, E.; Radhakrishnan, K.V. Iodine assisted palladium catalyzed ring opening of bicyclic hydrazines with organoboronic acids: Stereoselective synthesis of functionalized cyclopentenes and alkylidene cyclopentenes. Org. Biomol. Chem. 2007, 5, 4010. [Google Scholar] [CrossRef]
- John, J.; Anas, S.; Sajisha, V.S.; Viji, S.; Radhakrishnan, K.V. Palladium-catalyzed ring opening of azabicyclic olefins with organoindium reagents: A simple, clean, and efficient synthesis of functionalized cyclopentenes. Tetrahedron Lett. 2007, 48, 7225. [Google Scholar] [CrossRef]
- John, J.; Adarsh, B.; Radhakrishnan, K.V. Palladium catalyzed ring opening of azabicyclic olefins with organoindium and gallium reagents: A facile access towards benzylated cyclopentanoids. Tetrahedron 2010, 66, 1383. [Google Scholar] [CrossRef]
- Joseph, N.; Rajan, R.; John, J.; Devika, N.V.; Chand, S.S.; Suresh, E.; Pihko, P.M.; Radhakrishnan, K.V. An exclusive approach to 3,4-disubstituted cyclopentenes and alkylidene cyclopentenes via the palladium catalyzed ring opening of azabicyclic olefins with aryl halides. RSC Adv. 2013, 3, 7751. [Google Scholar] [CrossRef]
- John, J.; Indu, U.; Suresh, E.; Radhakrishnan, K.V. Palladium Catalyzed Tandem Ring Opening−Ring Closing Reaction of Diazabicyclic Alkenes: A Facile One Pot Strategy for Cyclopentannulation of Heterocycles. J. Am. Chem. Soc. 2009, 131, 5042. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Rajan, R.; Chand, S.S.; Prakash, P.; Joseph, N.; Suresh, E.; Radhakrishnan, K.V. Palladium catalyzed reaction of ortho-functionalized aryl iodides with bicyclic hydrazines: Facile route toward heteroannulated cyclopentenes and azabicycles. Tetrahedron 2013, 69, 152. [Google Scholar] [CrossRef]
- Jijy, E.; Prakash, P.; Shimi, M.; Pihko, P.M.; Joseph, N.; Radhakrishnan, K.V. Rhodium catalyzed oxidative coupling of salicylaldehydes with diazabicyclic olefins: A one pot strategy involving aldehyde C–H cleavage and π-allyl chemistry towards the synthesis of fused ring chromanones. Chem. Commun. 2013, 49, 7349. [Google Scholar] [CrossRef]
- Santhini, P.V.; Nimisha, G.; John, J.; Suresh, E.; Varma, R.L.; Radhakrishnan, K.V. Pd-Catalyzed oxidative annulation of enamides with diazabicyclic olefins: Rapid access to cyclopentene fused 2-pyrrolines. Chem. Commun. 2017, 53, 1848. [Google Scholar] [CrossRef]
- Santhini, P.V.; Smrithy, A.S.; Jesin, C.P.I.; Varughese, S.; John, J.; Radhakrishnan, K.V. Accessing highly functionalized cyclopentanoids via a cascade palladation approach: Unprecedented benzylic C–H activation towards cyclopentenoindanes. Chem. Commun. 2018, 54, 2982. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.P.; Cesario, M.; Bonin, M.; Micouin, L. Stereoselective Ring Opening of meso Bicyclic Hydrazines: A Straightforward Approach to Hydrazino Cyclopentenic Cores. Org. Lett. 2003, 5, 4771. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; John, J.; Thulasi, S.; Joseph, N.; Radhakrishnan, K.V.; Sawant, R.C. Trapping the π-Allylpalladium Intermediate from Fulvene-Derived Azabicyclic Olefin with Soft Nucleophiles. Synthesis 2010, 2010, 3649. [Google Scholar] [CrossRef]
- Jijy, E.; Prakash, P.; Baiju, V.; Shimi, M.; Yamamoto, Y.; Suresh, E.; Radhakrishnan, K.V. Palladium-Catalyzed Ring Opening of Cyclopropane-Appended Spirotricyclic Olefins with Soft Nucleophiles and Organoboronic Acids: Facile Synthesis of Functionalized Spiro [2.4]heptenes. Synthesis 2014, 46, 2629. [Google Scholar] [CrossRef]
- Negishi, E. Hand Book of Organopalladium Chemistry for Organic Synthesis; Wiley-Interscience: New York, NY, USA, 2002; Volume 2. [Google Scholar]
- Pashkovskii, F.S.; Shchukina, E.M.; Gribovskii, M.G.; Lakhvich, F.A. Heterocyclic Analogs of Prostaglandins: III. Synthesis of 10-Oxa-13-Aza, 11-Oxa-13-Aza, and 9-Oxa-7-Aza Prostanoids from 3-Acyl-and 3-(3-Arylprop-2-enoyl)Furan-2,4-Diones. Russ. J. Org. Chem. 2006, 42, 527. [Google Scholar] [CrossRef]
- Sutharchanadevi, M.; Murugan, R. 9.18—Eight-membered Rings with One Nitrogen Atom; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Comprehensive Heterocyclic Chemistry II: Pergamon, Turkey, 1996; pp. 403–428. [Google Scholar]
- Listratova, A.V.; Voskressensky, L.G. Recent Advances in the Synthesis of Hydrogenated Azocine-Containing Molecules. Synthesis 2017, 49, 3801. [Google Scholar]
- Mack, R.A.; Zazulak, W.I.; Radov, L.A.; Baer, J.E.; Stewart, J.D.; Elzer, P.H. Drug-induced modifications of the immune response. 12. 4,5-Dihydro-4-oxo-2-(substituted amino)-3-furancarboxylic acids and derivatives as novel antiallergic agents. J. Med. Chem. 1988, 31, 1918. [Google Scholar] [CrossRef]
- Choi, H.Y.; Chi, D.Y. Nonselective bromination-selective debromination strategy: Selective bromination of unsymmetrical ketones on singly activated carbon against doubly activated carbon. Org. Lett. 2003, 5, 411. [Google Scholar] [CrossRef]
- Takaaki, K.; Katsumasa, H. Preparation of γ-Halogeno-β-keto esters, Japan. Patent Application No. JP1999-56383; Patent No. JP2000256262, 19 September 2000. [Google Scholar]
- Hu, D.; Grice, P.; Ley, S.V. Rotamers or diastereomers? An overlooked NMR solution. J. Org. Chem. 2012, 77, 5198. [Google Scholar]

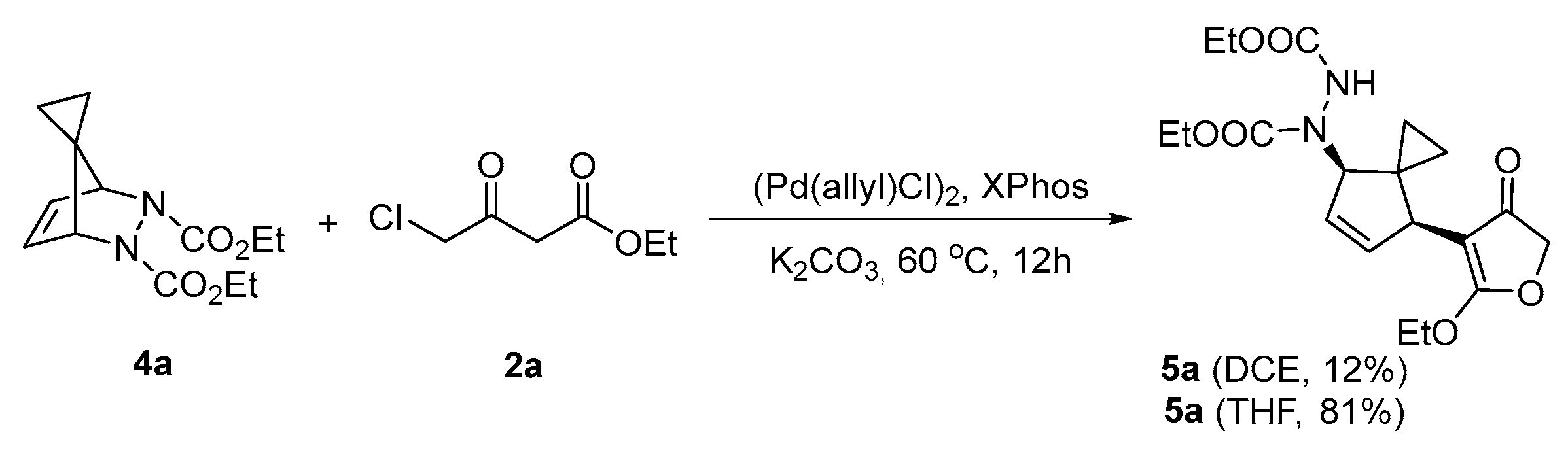

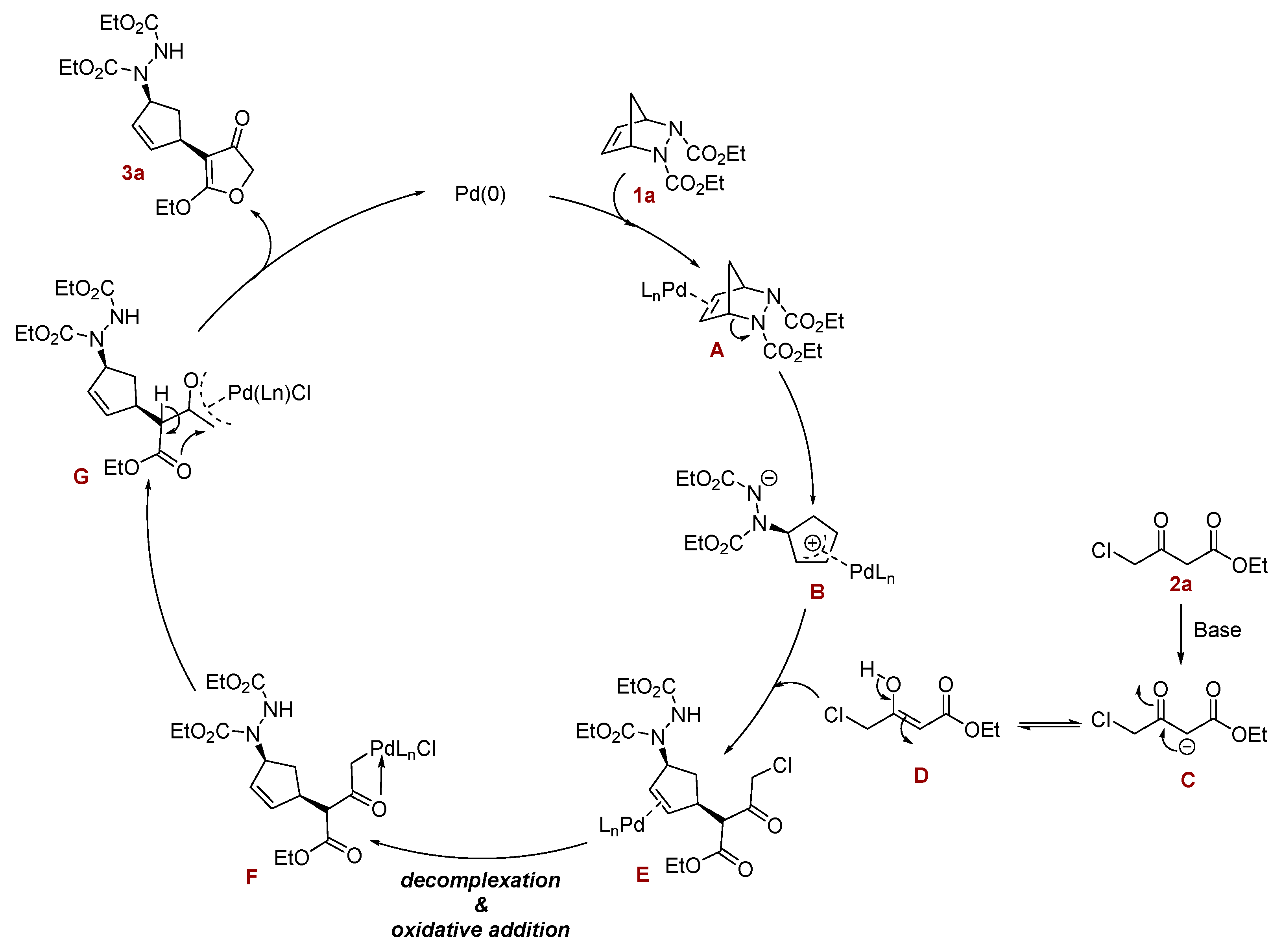
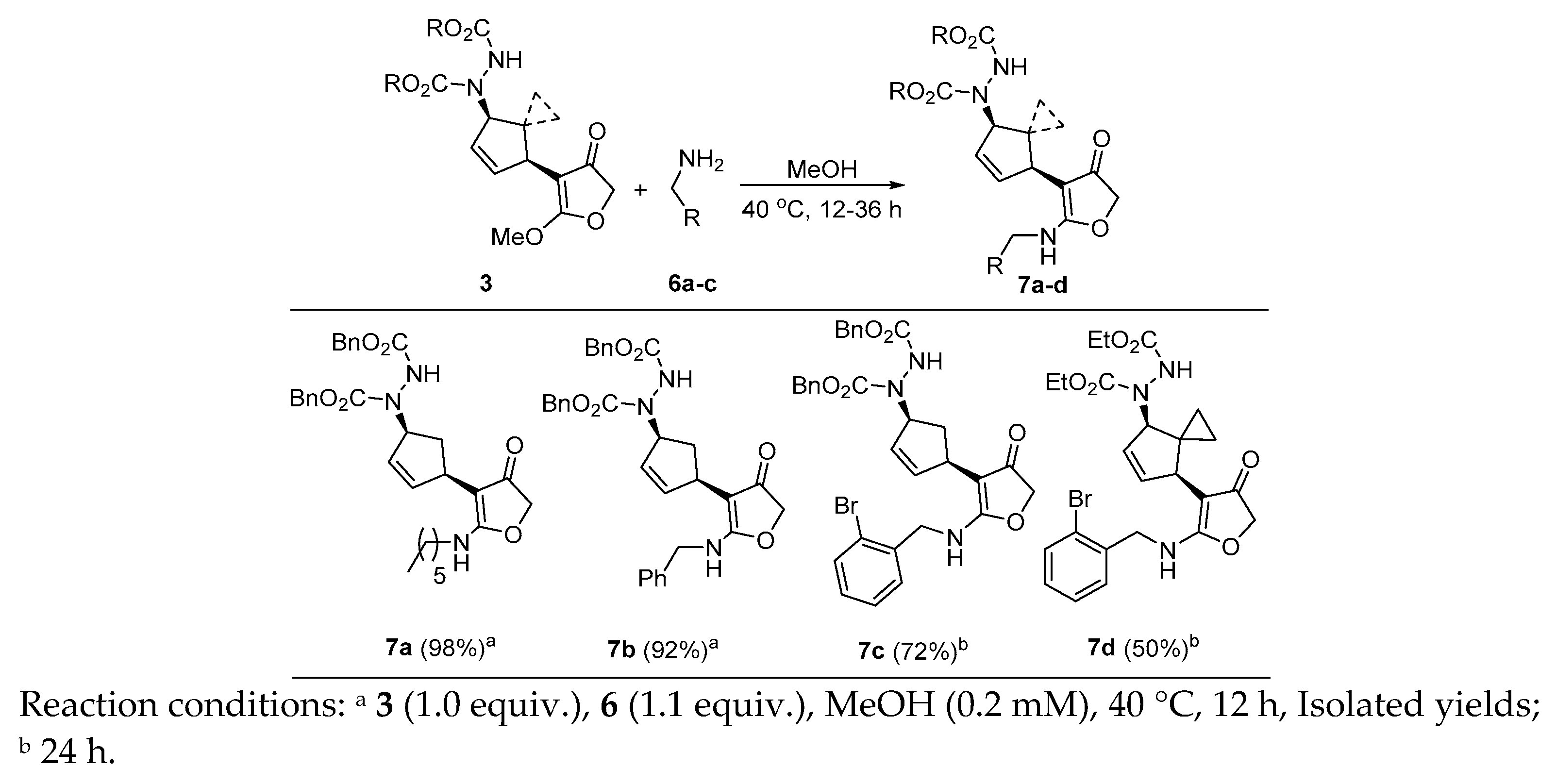
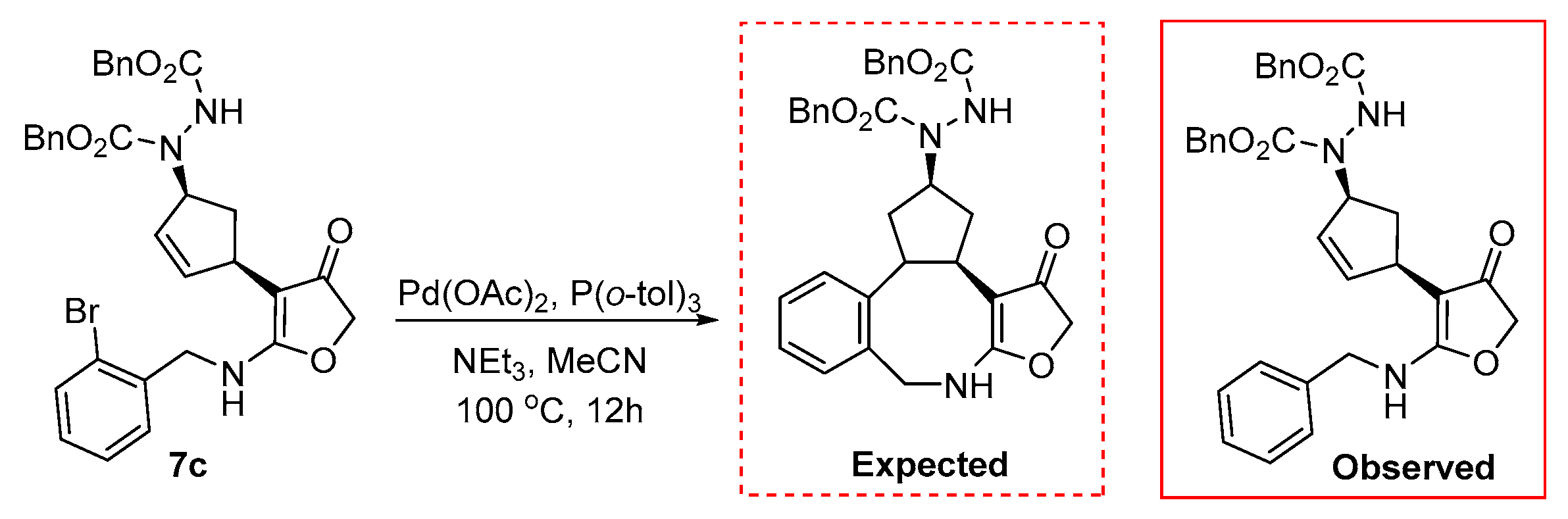
| Entry | Catalyst | Ligand | Base | Solvent | Yield |
|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | dppf | K2CO3 | THF | 10 |
| 2 | Pd(OCOCF3)2 | dppf | K2CO3 | THF | 20 |
| 3 | Pd(PPh3)4 | dppf | K2CO3 | THF | 15 |
| 4 | (Pd(allyl)Cl)2 | dppf | K2CO3 | THF | 32 |
| 5 | PdCl2 | dppf | K2CO3 | THF | 28 |
| 6 | Pd(dba)3.CHCl3 | dppf | K2CO3 | THF | 23 |
| 7 | (Pd(allyl)Cl)2 | dppe | K2CO3 | THF | 10 |
| 8 | (Pd(allyl)Cl)2 | dppp | K2CO3 | THF | 34 |
| 9 | (Pd(allyl)Cl)2 | XPhos | K2CO3 | THF | 55 |
| 10 | (Pd(allyl)Cl)2 | DevPhos | K2CO3 | THF | 43 |
| 11 | (Pd(allyl)Cl)2 | XPhos | Na2CO3 | THF | 51 |
| 12 | (Pd(allyl)Cl)2 | XPhos | Cs2CO3 | THF | 32 |
| 13 | (Pd(allyl)Cl)2 | XPhos | NaH | THF | NR |
| 14 | (Pd(allyl)Cl)2 | XPhos | NaOtBu | THF | 25 |
| 15 | (Pd(allyl)Cl)2 | XPhos | K2CO3 | CH3CN | 68 |
| 16 | (Pd(allyl)Cl)2 | XPhos | K2CO3 | Toluene | 47 |
| 17 | (Pd(allyl)Cl)2 | XPhos | K2CO3 | 1,4-Dioane | 58 |
| 18 | (Pd(allyl)Cl)2 | XPhos | K2CO3 | DCE | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omanakuttan, V.K.; Valsan, A.; Hopf, H.; John, J. Palladium Catalyzed Ring-Opening of Diazabicylic Olefins with 4-Halo-1,3-Dicarbonyl Compounds: Accessing 3(2H)-Furanone-Appended Cyclopentenes. Organics 2023, 4, 70-85. https://doi.org/10.3390/org4010006
Omanakuttan VK, Valsan A, Hopf H, John J. Palladium Catalyzed Ring-Opening of Diazabicylic Olefins with 4-Halo-1,3-Dicarbonyl Compounds: Accessing 3(2H)-Furanone-Appended Cyclopentenes. Organics. 2023; 4(1):70-85. https://doi.org/10.3390/org4010006
Chicago/Turabian StyleOmanakuttan, Vishnu K., Alisha Valsan, Henning Hopf, and Jubi John. 2023. "Palladium Catalyzed Ring-Opening of Diazabicylic Olefins with 4-Halo-1,3-Dicarbonyl Compounds: Accessing 3(2H)-Furanone-Appended Cyclopentenes" Organics 4, no. 1: 70-85. https://doi.org/10.3390/org4010006
APA StyleOmanakuttan, V. K., Valsan, A., Hopf, H., & John, J. (2023). Palladium Catalyzed Ring-Opening of Diazabicylic Olefins with 4-Halo-1,3-Dicarbonyl Compounds: Accessing 3(2H)-Furanone-Appended Cyclopentenes. Organics, 4(1), 70-85. https://doi.org/10.3390/org4010006