- Article
Repercussions of Symbiotic Bacteria Associated with Entomopathogenic Nematodes and Their Biogenic Silver Nanoparticles on Immune Responses at Root-Knot Nematode Suppression
- Rehab Y. Ghareeb,
- Shawky M. Eid and
- Hanan Alfy
- + 1 author
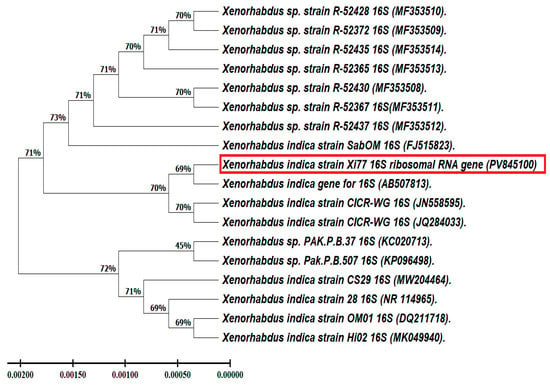
Root-knot nematodes (RKNs) of the Meloidogyne genus impact various plants, including crops, fruits, and vegetables. Few chemical control options exist globally, and many nematicides are banned due to health and environmental risks. This study tested a new nematicidal agent, the symbiotic bacterium Xenorhabdus indica, which was molecularly identified (PV845100). Cell-free culture supernatants of Xenorhabdus spp. and their biogenic Ag-NPs were used in nematicidal assays. Meloidogyne incognita showed high mortality rates of 95.3%, 74.6%, and 72.6% after 72 h of treatment with the X. indica filtrate at three concentrations. At the same concentrations, biogenic Ag-NPs resulted in 82.0%, 90.0%, and 85.3% mortality rates, respectively. After 72 h, hatchability decreased by 53%, 74.6%, and 72.6% for the X. indica filtrate and 82.0%, 90.0%, and 85.3% for Ag-NPs. Quantitative real-time PCR (Q-PCR) revealed that Mi-Ache1 expression was lower in M. incognita second-stage juveniles (J2s) treated with the filtrate and Ag-NPs after 72 h compared to controls. Mi-Ache2 expression was also decreased, but only slightly. Furthermore, both the X. indica filtrate and biogenic Ag-NPs were safe in human lung (WI-38) and skin (HFB4) cell lines. These findings suggest that bacterial filtrates and their biogenic Ag-NPs could serve as cost-effective, environmentally friendly alternatives to commercial nematicides.
31 December 2025