Role of Probiotics in Enhancing Immune Function and Improving the Effectiveness of Treatments for Pancreatic Cancer
Abstract
1. Introduction and Background
2. Probiotics May Help Fight Pancreatic Cancer, Either Directly or Indirectly: Insights from Preclinical Studies
2.1. Probiotics Directly Fight Pancreatic Cancer
| Probiotic Strains | Mechanism of Action | References |
|---|---|---|
| Direct inhibition of pancreatic tumor | ||
| Bifidobacterium longum Lactobacillus lactis Lactobacillus reutri Lactobacillus plantarum Lactobacillus paracasei/casei | -Inhibit pancreatic tumor proliferation | [59,60,68,69,70,71] |
| Lactobacillus paracasei/casei Lactobacillus rhamnosus | -Inhibit tumor growth by decreasing matrix metalloproteinase-9 (MMP-9) activity | [28,59,60,72,73,74,75,76,77] |
| Lactobacillus acidophilus Lactobacillus plantarum Lactobacillus rhamnosus | -Inactivated the NF-kB inflammatory pathway | [59,74,75,76,77,78,79,80,81] |
| Lactobacillus reutri Lactobacillus paracasei/casei | -Inhibit p53-p21-Cyclin B1/Cdk1 signaling pathway resulting in growth arrest at G2 growth phase of tumors (Ferrichrome-mediated apoptosis) | [60] |
| Lactobacillus paracasei/casei | -Activated c-jun N-terminal kinase (JNK)-mediated apoptosis of tumors | [28,59,60,72,73] |
| Lactobacillus plantarum Aspergillus oryzae | -P38 MAPK-mediated apoptosis | [64,82] |
| Bifidobacterium longum Lactobacillus paracasei/casei | -Increase efficacy of PD-1 therapy in pancreatic cancer | [28,59,60,68,69,70,71,72,73] |
| Indirect inhibition of pancreatic tumor | ||
| Clostridium butyricum Enterococcus faecalis Bacillus mesentericus | -Increase surface expression of CD11b, HLA-DR, CD4, CD45Ram CD25, CD44 and CD69 in PBMCs | [83] |
| Bifidobacterium longum Bifidobacterium breve Bifidobacterium infantis Lactobacillus acidophilus Lactobacillus lactis Lactobacillus plantarum Lactobacillus paracasei/casei Lactobacillus bulgaricus Lactobacillus rhamnosus | -Regulate cytokine secretion in PBMCs and NK cells | [59,74,75,76,77] |
| Streptococcus thermophilus Enterococcus faecalis Clostridium butyricum Bacillus mesentericus Lactobacillus plantarum Lactobacillus rhamnosus Enterococcus hirae | -Promote Th1-type cytokine profile, increasing IL-12 and IFN-γ in PBMCs, NK and T cells | [59,74,75,76,77,83,84,85,86,87] |
| Bifidobacterium infantis Bifidobacterium breve | -Support Th2 profile with higher IL-10 and IL-6 compared to IL-12 and IFN-γ | [59,74,75,76,77,88,89] |
| Lactobacillus lactis Lactobacillus rhamnosus Enterococcus hirae | -Enhance Th17 immune response against cancer | [86,87] |
| Streptococcus thermophilus Bifidobacterium longum Bifidobacterium breve Lactobacillus paracasei/casei Lactobacillus rhamnosus | -Boost cytotoxic activity in PBMCs, NK and T cells | [59,84,85] |
| Bifidobacterium longum Lactobacillus paracasei/casei Enterococcus hirae Bifidobacterium longum | -Increase the number of total T cells, NK cells, and increase the CD8+/CD4+ T ratio | [59,69,70,71,86,87] |
| Bifidobacterium breve Lactobacillus acidophilus Lactobacillus rhamnosus | -Encourage CD4+ and CD8+ T cell proliferation | [59,90,91] |
| Lactobacillus plantarum Lactobacillus paracasei/casei Bifidobacterium longum | -Increase tumor infiltration of CD4+ and CD8+ T cells | [59,64,92,93] |
| Lactobacillus casei
Lactobacillus reuteri | -Supression of TLR4 to promote macrophage M1 polarization | [60] |
| Bifidobacterium longum Lactobacillus lactis Bacillus mesentericus | -Increase anti-cancer gene expressions on dendritic cells | [59,69,70,71,83,94] |
| Boost efficacy or mimic the toxicity of other therapeutics | ||
| Bifidobacterium longum Lactobacillus acidophilus | -Protection against chemo- and radiotherapy-induced fever and diarrhea in pancreatic cancer patients | [59,69,70,71] |
| Lactobacillus acidophilus Lactobacillus paracasei/casei | -Sensitized tumor cells to chemotherapy by faster activation of caspase-3 and downregulation of p21 protein | [59,78,79,80,81] |
| Lactobacillus casei
Lactobacillus reuteri | -Regulate gut microbial homeostasis | [60] |
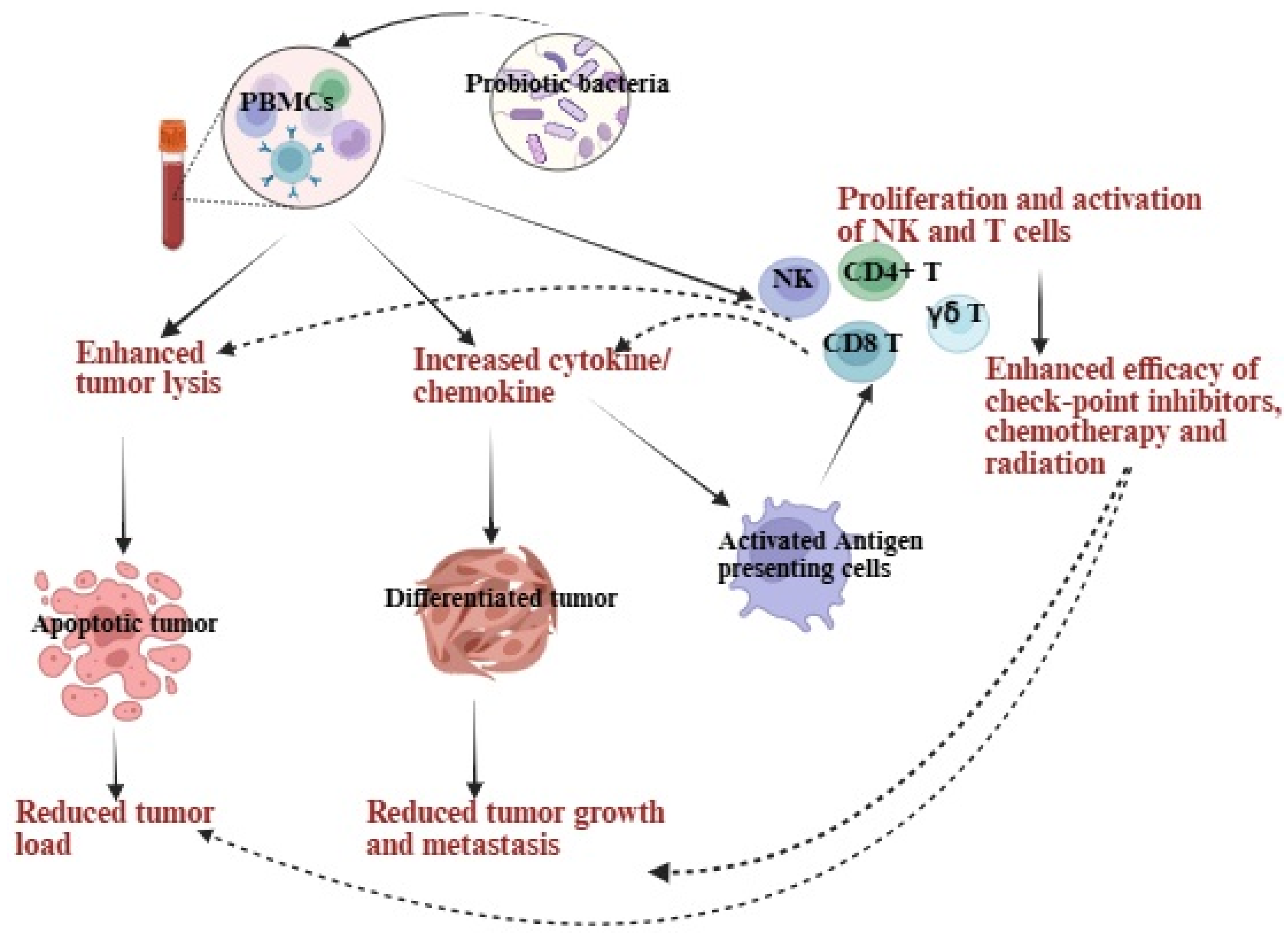
2.2. Probiotics Indirectly Fight Pancreatic Cancer
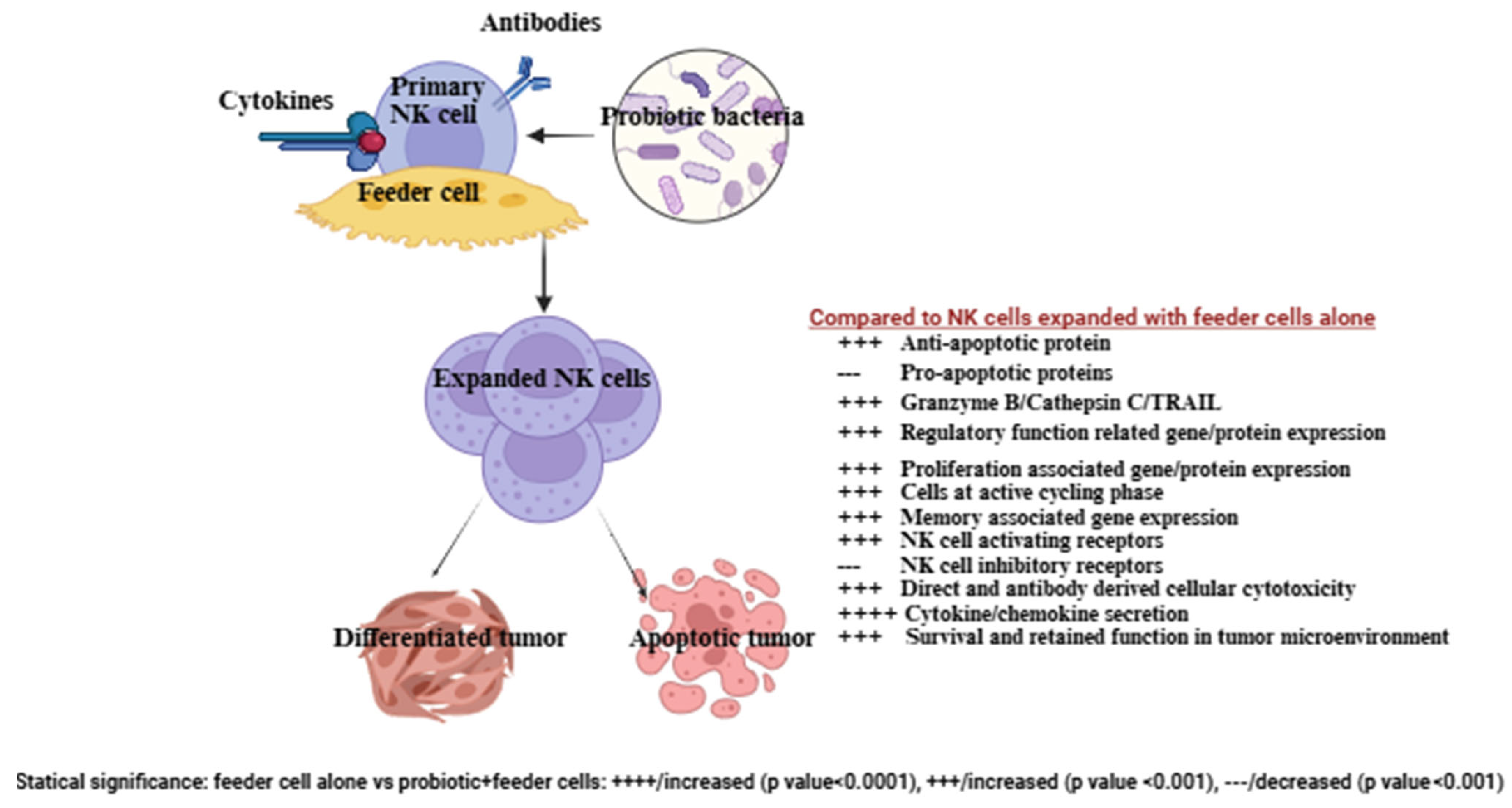
3. Probiotics, When Combined with Feeder Cells, Contribute to Improving the Development of NK Cell-Based Immunotherapies
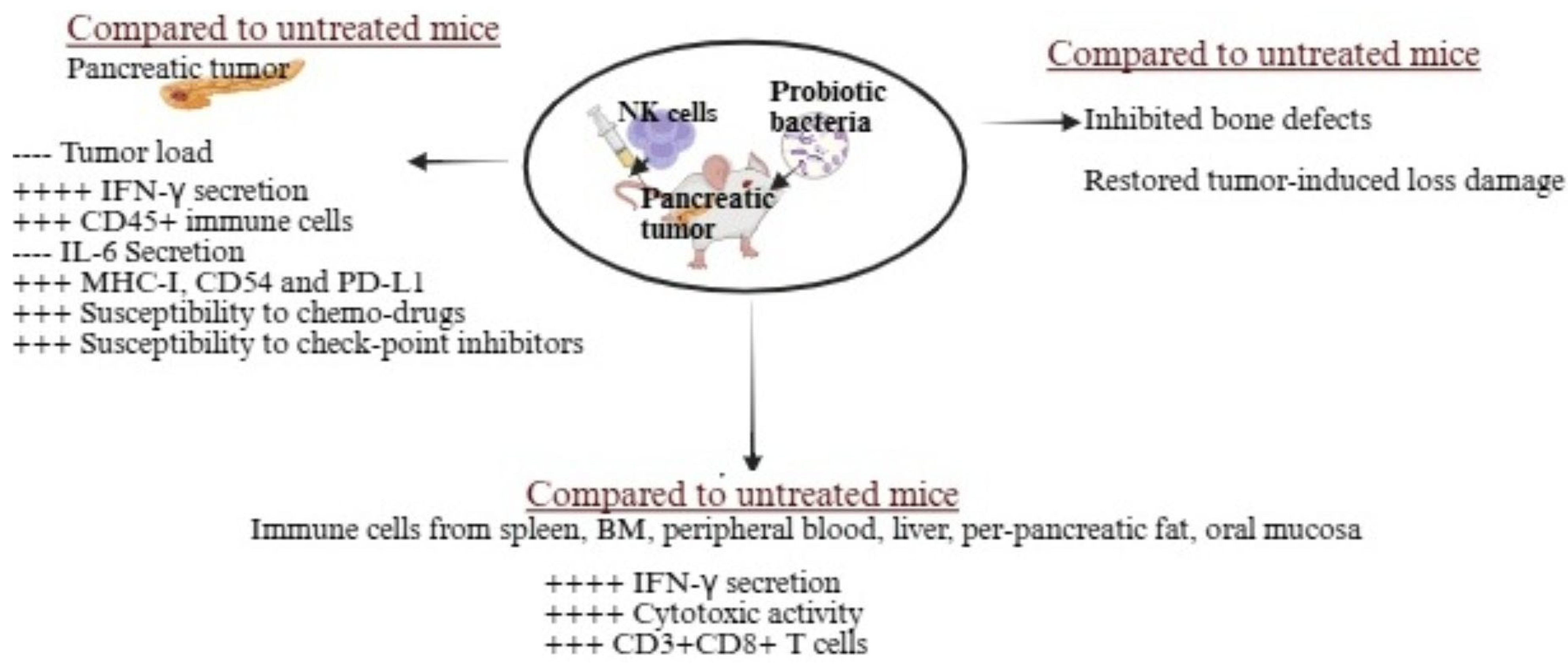
4. Exploring the Benefits of Adding Probiotics as a Supportive Therapy in Treating Pancreatic Cancer: Insights from Preclinical Studies
5. Clinical Studies on the Use of Probiotics in Human Pancreatic Cancer
6. Challenges and Limitations in Using Probiotics for Clinical Application in Pancreatic Cancer in Humans
7. Role of Microbiome Stratification in Ensuring and Boosting the Therapeutic Benefits of Probiotics or Combined NK Cell Therapies
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wenzel, P.; Mogler, C.; Görgülü, K.; Algül, H. Pancreatic Cancer: Current Concepts, Trends, and Future Directions. Turk. J. Gastroenterol. 2024, 36, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Stoop, T.F.; Javed, A.A.; Oba, A.; Koerkamp, B.G.; Seufferlein, T.; Wilmink, J.W.; Besselink, M.G. Pancreatic cancer. Lancet 2025, 405, 1182–1202. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, G.; Bungau, S.; Ilie, M.; Behl, T.; Vesa, C.M.; Brisc, C.; Bacalbasa, N.; Turi, V.; Costache, R.S.; Diaconu, C.C. Early Diagnosis of Pancreatic Cancer: The Key for Survival. Diagnostics 2020, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Borad, M.J.; Saadati, H.; Lakshmipathy, A.; Campbell, E.; Hopper, P.; Jameson, G.; Von Hoff, D.D.; Saif, M.W. Skeletal metastases in pancreatic cancer: A retrospective study and review of the literature. Yale J. Biol. Med. 2009, 82, 1–6. [Google Scholar]
- Chen, W.; Xie, F.; Luong, T.Q.; Chang, J.; Lustigova, E.; Matrisian, L.M.; Shrader, E.E.; Wu, B.U. Identifying signs and symptoms of pancreatic cancer: A population-based study using electronic health records and natural language processing. Pancreatology 2025, 25, 1086–1094. [Google Scholar] [CrossRef]
- Mosalem, O.M.; Abdelhakeem, A.; Abdel-Razeq, N.H.; Babiker, H. Pancreatic ductal adenocarcinoma (PDAC): Clinical progress in the last five years. Expert Opin. Investig. Drugs 2025, 34, 149–160. [Google Scholar] [CrossRef]
- Lou, X.; Shi, Y.; Zhao, F.; Xu, X.; Wang, Y.; Qin, Y.; Zhang, W.; Ye, Z.; Wang, F.; Ding, T. Pancreatic Neuroendocrine Tumors Secrete Apolipoprotein E to Induce Tip Endothelial Cells That Remodel the Tumor-Stroma Ratio and Promote Cancer Progression. Cancer Res. 2025, 85, 2805–2819. [Google Scholar] [CrossRef]
- Singh, S.; Sawal, A. Comprehensive Review on Pancreatic Head Cancer: Pathogenesis, Diagnosis, and Treatment Challenges in the Quest for Improved Survival. Cureus 2024, 16, e54290. [Google Scholar] [CrossRef]
- Grigorescu, R.R.; Husar-Sburlan, I.A.; Gheorghe, C. Pancreatic Cancer: A Review of Risk Factors. Life 2024, 14, 980. [Google Scholar] [CrossRef]
- Javadrashid, D.; Baghbanzadeh, A.; Derakhshani, A.; Leone, P.; Silvestris, N.; Racanelli, V.; Solimando, A.G.; Baradaran, B. Pancreatic Cancer Signaling Pathways, Genetic Alterations, and Tumor Microenvironment: The Barriers Affecting the Method of Treatment. Biomedicines 2021, 9, 373. [Google Scholar] [CrossRef]
- Truong, L.H.; Pauklin, S. Pancreatic Cancer Microenvironment and Cellular Composition: Current Understandings and Therapeutic Approaches. Cancers 2021, 13, 5028. [Google Scholar] [CrossRef]
- Liu, Z.; Gou, A.; Wu, X. Liver metastasis of pancreatic cancer: The new choice at the crossroads. Hepatobiliary Surg. Nutr. 2023, 12, 88–91. [Google Scholar] [CrossRef]
- Avula, L.R.; Hagerty, B.; Alewine, C. Molecular mediators of peritoneal metastasis in pancreatic cancer. Cancer Metastasis Rev. 2020, 39, 1223–1243. [Google Scholar] [CrossRef]
- Yachida, S.; Iacobuzio-Donahue, C.A. The pathology and genetics of metastatic pancreatic cancer. Arch. Pathol. Lab. Med. 2009, 133, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, H.; Li, B.; Lyu, W.; Qian, W.; Han, L.; Wang, Z.; Wei, K. Characterization and treatment of brain metastases from pancreatic cancer: A systematic review. Discov. Oncol. 2025, 16, 1274. [Google Scholar] [CrossRef] [PubMed]
- Friedant, A.; Khurana, S.; Dissin, J. Rare Cerebral Manifestation of Pancreatic Adenocarcinoma: Insights from a Calcified Brain Metastasis (P1-6.009). Neurology 2025, 104 (Suppl. S1), 4549. [Google Scholar] [CrossRef]
- Wang, K.; He, H. Pancreatic Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1296, 243–257. [Google Scholar]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Carlomagno, S.; Setti, C.; Ortolani, F.; Sivori, S. Pancreatic ductal adenocarcinoma microenvironment: Soluble factors and cancer associated fibroblasts as modulators of NK cell functions. Immunol. Lett. 2024, 269, 106898. [Google Scholar] [CrossRef]
- Pan, D.; Li, X.; Qiao, X.; Wang, Q. Immunosuppressive tumor microenvironment in pancreatic cancer: Mechanisms and therapeutic targets. Front. Immunol. 2025, 16, 1582305. [Google Scholar] [CrossRef]
- Ahmed, S.; Bradshaw, A.-D.; Gera, S.; Dewan, M.Z.; Xu, R. The TGF-β/Smad4 Signaling Pathway in Pancreatic Carcinogenesis and Its Clinical Significance. J. Clin. Med. 2017, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.; Narang, A.; He, J.; Wolfgang, C.; Li, K.; Zheng, L. Consensus, debate, and prospective on pancreatic cancer treatments. J. Hematol. Oncol. 2024, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Hara, K.; Saito, A.; Cho, H. Adjuvant Treatment for Resectable Pancreatic Cancer. Anticancer. Res. 2025, 45, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, R.G.; Brower, S.T. Molecular Targets for the Diagnosis and Treatment of Pancreatic Cancer. Int. J. Mol. Sci. 2024, 25, 10843. [Google Scholar] [CrossRef]
- Baghel, K.; Mehrotra, S.; Prajapati, V.K. Revolutionizing pancreatic cancer treatment with CAR-T therapy. Adv. Protein Chem. Struct. Biol. 2025, 144, 331–353. [Google Scholar]
- Sexton, R.E.; Uddin, H.; Bannoura, S.; Khan, H.Y.; Mzannar, Y.; Li, Y.; Aboukameel, A.; Al-Hallak, M.N.; Al-Share, B.; Mohamed, A.; et al. Connecting the Human Microbiome and Pancreatic Cancer. Cancer Metastasis Rev. 2022, 41, 317–331, Erratum in Cancer Metastasis Rev. 2022, 41, 333. [Google Scholar] [CrossRef]
- Mohamed Elfadil, O.; Mundi, M.S.; Abdelmagid, M.G.; Patel, A.; Patel, N.; Martindale, R. Butyrate: More Than a Short Chain Fatty Acid. Curr. Nutr. Rep. 2023, 12, 255–262. [Google Scholar] [CrossRef]
- Shah, A.B.; Baiseitova, A.; Zahoor, M.; Ahmad, I.; Ikram, M.; Bakhsh, A.; Shah, M.A.; Ali, I.; Idress, M.; Ullah, R.; et al. Probiotic significance of Lactobacillus strains: A comprehensive review on health impacts, research gaps, and future prospects. Gut Microbes 2024, 16, 2431643. [Google Scholar] [CrossRef]
- Qin, W.; Wang, G.; Xia, Y.; Song, X.; Xiong, Z.; Huang, C.; Gong, C.; Zeng, Y.; Ai, L. The role of probiotic foods in acute pancreatitis: Current status and future directions. Curr. Opin. Food Sci. 2024, 60, 101231. [Google Scholar] [CrossRef]
- Werawatganon, D.; Vivatvakin, S.; Somanawat, K.; Tumwasorn, S.; Klaikeaw, N.; Siriviriyakul, P.; Chayanupatkul, M. Effects of probiotics on pancreatic inflammation and intestinal integrity in mice with acute pancreatitis. BMC Complement. Med. Ther. 2023, 23, 166. [Google Scholar] [CrossRef]
- Nobels, A.; van Marcke, C.; Jordan, B.F.; Van Hul, M.; Cani, P.D. The gut microbiome and cancer: From tumorigenesis to therapy. Nat. Metab. 2025, 7, 895–917. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xie, L.; Huang, L. Regulation of host immune responses by Lactobacillus through aryl hydrocarbon receptors. Med. Microecol. 2023, 16, 100081. [Google Scholar] [CrossRef]
- Mackowiak, P.A. Recycling metchnikoff: Probiotics, the intestinal microbiome and the quest for long life. Front. Public Health 2013, 1, 52. [Google Scholar] [CrossRef]
- Mowat, A.M. Historical Perspective: Metchnikoff and the intestinal microbiome. J. Leukoc. Biol. 2021, 109, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Guo, H.; Wen, C.; Sun, G.; Tang, F.; Li, Y. The dual role of gut microbiota in pancreatic cancer: New insights into onset and treatment. Ther. Adv. Med. Oncol. 2025, 17, 17588359251324882. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Fu, Z.-J.; Wang, Y.-Z.; Zhang, C.; Chen, Q.-W.; An, J.-X.; Zhang, X.-Z. Probiotics functionalized with a gallium-polyphenol network modulate the intratumor microbiota and promote anti-tumor immune responses in pancreatic cancer. Nat. Commun. 2024, 15, 7096. [Google Scholar] [CrossRef]
- Li, P.; Zhang, H.; Dai, M. Current status and prospect of gut and oral microbiome in pancreatic cancer: Clinical and translational perspectives. Cancer Lett. 2024, 604, 217274. [Google Scholar] [CrossRef]
- Kaur, K.; Reese, P.; Chiang, J.; Jewett, A. Natural Killer Cell Therapy Combined with Probiotic Bacteria Supplementation Restores Bone Integrity in Cancer by Promoting IFN-γ Production. Cells 2025, 14, 1347. [Google Scholar] [CrossRef]
- Kaur, K.; Kozlowska, A.K.; Topchyan, P.; Ko, M.-W.; Ohanian, N.; Chiang, J.; Cook, J.; Maung, P.O.; Park, S.-H.; Cacalano, N.; et al. Probiotic-Treated Super-Charged NK Cells Efficiently Clear Poorly Differentiated Pancreatic Tumors in Hu-BLT Mice. Cancers 2020, 12, 63. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; Babalghith, A.O.; Faidah, H.; Ahmed, F.; Khanam, A.; Mozaffar, B.; Kambal, N.; et al. Probiotics and Cancer: Mechanistic Insights and Organ-Specific Impact. Biomolecules 2025, 15, 879. [Google Scholar] [CrossRef]
- Chen, S.-M.; Chieng, W.-W.; Huang, S.-W.; Hsu, L.-J.; Jan, M.-S. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci. Rep. 2020, 10, 20319. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Carriço, M.; Capelas, M.L.; Pimenta, N.; Santos, T.; Ganhão-Arranhado, S.; Mäkitie, A.; Ravasco, P. The impact of pre-, pro- and synbiotics supplementation in colorectal cancer treatment: A systematic review. Front. Oncol. 2024, 14, 1395966. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, S.; Ying, L.; Zhang, W.; Chen, X.; Liang, Y.; Chen, R.; Yao, K.; Li, C.; Yu, C.; et al. The effect of probiotics supplementation on cancer-treatment complications: A critical umbrella review of interventional meta-analyses. Crit. Rev. Food Sci. Nutr. 2025, 65, 3702–3727. [Google Scholar] [CrossRef]
- Palmer, J.M.; Rajasekaran, K.; Thakar, M.S.; Malarkannan, S. Clinical relevance of natural killer cells following hematopoietic stem cell transplantation. J. Cancer 2013, 4, 25–35. [Google Scholar] [CrossRef]
- Fildes, J.E.; Yonan, N.; Leonard, C.T. Natural killer cells and lung transplantation, roles in rejection, infection, and tolerance. Transpl. Immunol. 2008, 19, 1–11. [Google Scholar] [CrossRef]
- Farag, S.S.; Caligiuri, M.A. Human natural killer cell development and biology. Blood Rev. 2006, 20, 123–137. [Google Scholar] [CrossRef]
- Türkseven, M.R.; Oygür, T. Evaluation of natural killer cell defense in oral squamous cell carcinoma. Oral Oncol. 2010, 46, e34–e37. [Google Scholar] [CrossRef]
- Accomando, W.P.; Wiencke, J.K.; Houseman, E.A.; Butler, R.A.; Zheng, S.; Nelson, H.H.; Kelsey, K.T. Decreased NK cells in patients with head and neck cancer determined in archival DNA. Clin. Cancer Res. 2012, 18, 6147–6154. [Google Scholar] [CrossRef]
- Mickel, R.A.; Kessler, D.; Taylor, J.; Lichtenstein, A. Natural killer cell cytotoxicity in the peripheral blood, cervical lymph nodes, and tumor of head and neck cancer patients. Cancer Res. 1988, 48, 5017–5022. [Google Scholar] [PubMed]
- Kaur, K.; Cook, J.; Park, S.-H.; Topchyan, P.; Kozlowska, A.; Ohanian, N.; Fang, C.; Nishimura, I.; Jewett, A. Novel Strategy to Expand Super-Charged NK Cells with Significant Potential to Lyse and Differentiate Cancer Stem Cells: Differences in NK Expansion and Function between Healthy and Cancer Patients. Front. Immunol. 2017, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Wynberg, J.; Srinivasan, R.; Becknell, B.; McCoy, J.P.; Takahashi, Y.; Suffredini, D.A.; Linehan, W.M.; Caligiuri, M.A.; Childs, R.W. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood 2004, 104, 170–177. [Google Scholar] [CrossRef]
- Alici, E.; Sutlu, T.; Björkstrand, B.; Gilljam, M.; Stellan, B.; Nahi, H.; Quezada, H.C.; Gahrton, G.; Ljunggren, H.-G.; Dilber, M.S. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood 2008, 111, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, H.; Kakuda, H.; Shimasaki, N.; Imai, C.; Ma, J.; Lockey, T.; Eldridge, P.; Leung, W.H.; Campana, D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009, 69, 4010–4017. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Lundqvist, A.; McCoy, P.; Samsel, L.; Fan, Y.; Tawab, A.; Childs, R. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy 2009, 11, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Martín-Antonio, B.; Yang, H.; Ku, S.; Lee, D.A.; Cooper, L.J.N.; Decker, W.K.; Li, S.; Robinson, S.N.; Sekine, T.; et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS ONE 2013, 8, e76781. [Google Scholar] [CrossRef]
- Kaur, K.; Ko, M.-W.; Ohanian, N.; Cook, J.; Jewett, A. Osteoclast-expanded super-charged NK-cells preferentially select and expand CD8+ T cells. Sci. Rep. 2020, 10, 20363. [Google Scholar] [CrossRef]
- Kaur, K.; Chen, P.-C.; Ko, M.-W.; Mei, A.; Senjor, E.; Malarkannan, S.; Kos, J.; Jewett, A. Sequential therapy with supercharged NK cells with either chemotherapy drug cisplatin or anti-PD-1 antibody decreases the tumor size and significantly enhances the NK function in Hu-BLT mice. Front. Immunol. 2023, 14, 1132807. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Bui, V.T.; Tseng, H.-C.; Kozlowska, A.; Maung, P.O.; Kaur, K.; Topchyan, P.; Jewett, A. Augmented IFN-γ and TNF-α Induced by Probiotic Bacteria in NK Cells Mediate Differentiation of Stem-Like Tumors Leading to Inhibition of Tumor Growth and Reduction in Inflammatory Cytokine Release; Regulation by IL-10. Front. Immunol. 2015, 6, 576. [Google Scholar] [CrossRef]
- Zhu, Z.; Yi, B.; Tang, Z.; Chen, X.; Li, M.; Xu, T.; Zhao, Z.; Tang, C. Lactobacillus casei combined with Lactobacillus reuteri alleviate pancreatic cancer by inhibiting TLR4 to promote macrophage M1 polarization and regulate gut microbial homeostasis. BMC Cancer 2023, 23, 1044. [Google Scholar] [CrossRef]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Zhang, R.; Danshiitsoodol, N.; Noda, M.; Yonezawa, S.; Kanno, K.; Sugiyama, M. Stevia Leaf Extract Fermented with Plant-Derived Lactobacillus plantarum SN13T Displays Anticancer Activity to Pancreatic Cancer PANC-1 Cell Line. Int. J. Mol. Sci. 2025, 26, 4186. [Google Scholar] [CrossRef] [PubMed]
- Farrow, B.; Rychahou, P.; O’Connor, K.L.; Evers, B.M. Butyrate inhibits pancreatic cancer invasion. J. Gastrointest. Surg. 2003, 7, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133 (Suppl. S7), 2485s–2493s. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, S.; Zang, D.; Sun, H.; Sun, Y.; Chen, J. Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic (Review). Int. J. Oncol. 2024, 64, 44. [Google Scholar] [CrossRef]
- Chen, S.M.; Hsu, L.-J.; Lee, H.-L.; Lin, C.-P.; Huang, S.-W.; Lai, C.J.-L.; Lin, C.-W.; Chen, W.-T.; Chen, Y.-J.; Lin, Y.-C.; et al. Lactobacillus Attenuate the Progression of Pancreatic Cancer Promoted by Porphyromonas Gingivalis in K-rasG12D Transgenic Mice. Cancers 2020, 12, 3522. [Google Scholar] [CrossRef]
- Mandelbaum, N.; Zhang, L.; Carasso, S.; Ziv, T.; Lifshiz-Simon, S.; Davidovich, I.; Luz, I.; Berinstein, E.; Gefen, T.; Cooks, T.; et al. Extracellular vesicles of the Gram-positive gut symbiont Bifidobacterium longum induce immune-modulatory, anti-inflammatory effects. NPJ Biofilms Microbiomes 2023, 9, 30. [Google Scholar] [CrossRef]
- Pei, B.; Peng, S.; Huang, C.; Zhou, F. Bifidobacterium modulation of tumor immunotherapy and its mechanism. Cancer Immunol. Immunother. 2024, 73, 94. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, W.; Liang, Z.; Wang, J.; Zeng, Z.; Kołat, D.; Li, X.; Zhou, D.; Xu, X.; Zhao, L. Critical role of the gut microbiota in immune responses and cancer immunotherapy. J. Hematol. Oncol. 2024, 17, 33. [Google Scholar] [CrossRef]
- Zhang, S.L.; Han, B.; Mao, Y.-Q.; Zhang, Z.-Y.; Li, Z.-M.; Kong, C.-Y.; Wu, Y.; Chen, G.-Q.; Wang, L.-S. Lacticaseibacillus paracasei sh2020 induced antitumor immunity and synergized with anti-programmed cell death 1 to reduce tumor burden in mice. Gut Microbes 2022, 14, 2046246. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Ma, Y.; Wang, Y.; Hou, X.; Yu, L. Contribution of Lactobacilli on Intestinal Mucosal Barrier and Diseases: Perspectives and Challenges of Lactobacillus casei. Life 2022, 12, 1910. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Zhao, X.; Li, R.; Li, Y.; Ma, C.; Zhao, X.; Bugno, J.; Qin, Y.; Zhang, J.; Liu, H.; et al. Lactobacillus rhamnosus GG induces STING-dependent IL-10 in intestinal monocytes and alleviates inflammatory colitis in mice. J. Clin. Investig. 2025, 135, e174910. [Google Scholar] [CrossRef] [PubMed]
- Fong, F.L.Y.; Kirjavainen, P.; Wong, V.H.Y.; El-Nezami, H. Immunomodulatory effects of Lactobacillus rhamnosus GG on dendritic cells, macrophages and monocytes from healthy donors. J. Funct. Foods 2015, 13, 71–79. [Google Scholar] [CrossRef]
- Wang, L.; Liang, H.L.; Weichselbaum, R. Lactobacillus rhamnosus GG re-shapes gut microbiota and triggers STING-type I IFN-dependent antitumor immunity. J. Immunol. 2022, 208 (Suppl. 1), 120.04. [Google Scholar] [CrossRef]
- Ludwig, I.S.; Broere, F.; Manurung, S.; Lambers, T.T.; van der Zee, R.; van Eden, W. Lactobacillus rhamnosus GG-Derived Soluble Mediators Modulate Adaptive Immune Cells. Front. Immunol. 2018, 9, 1546. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, Y.; Choi, J.-W.; Park, S.-H.; Cho, M.-L.; Kwok, S.-K. Lactobacillus acidophilus Supplementation Exerts a Synergistic Effect on Tacrolimus Efficacy by Modulating Th17/Treg Balance in Lupus-Prone Mice via the SIGNR3 Pathway. Front. Immunol. 2021, 12, 696074. [Google Scholar] [CrossRef]
- Vissers, Y.M.; Snel, J.; Zuurendonk, P.F.; Kleerebezem, M.; Wichers, H.J.; Savelkoul, H.F. Lactobacillus strains differentially modulate cytokine production by hPBMC from pollen-allergic patients. FEMS Immunol. Med. Microbiol. 2011, 61, 28–40. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, S.; Shi, J.; Xie, Q.; Li, N.; Guan, J.; Evivie, S.E.; Liu, F.; Li, B.; Huo, G. Effects of Lactobacillus acidophilus KLDS1.0901 on Proliferation and Apoptosis of Colon Cancer Cells. Front. Microbiol. 2022, 12, 788040. [Google Scholar] [CrossRef]
- Arasu, K.A.; Rajasekar, T. Immunomodulatory Activity of Postbiotics from Lactobacillus. In Postbiotics; Dharumadurai, D., Ed.; Springer: New York, NY, USA, 2024; pp. 181–186. [Google Scholar]
- Konishi, H.; Isozaki, S.; Kashima, S.; Moriichi, K.; Ichikawa, S.; Yamamoto, K.; Yamamura, C.; Ando, K.; Ueno, N.; Akutsu, H.; et al. Probiotic Aspergillus oryzae produces anti-tumor mediator and exerts anti-tumor effects in pancreatic cancer through the p38 MAPK signaling pathway. Sci. Rep. 2021, 11, 11070. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.C.; Lin, T.-Y.; Lai, M.-W.; Kong, M.-S.; Chang, H.-J.; Chen, C.-C. Probiotic Bio-Three induces Th1 and anti-inflammatory effects in PBMC and dendritic cells. World J. Gastroenterol. 2010, 16, 3529–3540. [Google Scholar] [CrossRef] [PubMed]
- Latvala, S.; Miettinen, M.; Kekkonen, R.A.; Korpela, R.; Julkunen, I. Lactobacillus rhamnosus GG and Streptococcus thermophilus induce suppressor of cytokine signalling 3 (SOCS3) gene expression directly and indirectly via interleukin-10 in human primary macrophages. Clin. Exp. Immunol. 2011, 165, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Dargahi, N.; Johnson, J.C.; Apostolopoulos, V. Immune Modulatory Effects of Probiotic Streptococcus thermophilus on Human Monocytes. Biologics 2021, 1, 396–415. [Google Scholar] [CrossRef]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef]
- Goubet, A.-G.; Wheeler, R.; Fluckiger, A.; Qu, B.; Lemaître, F.; Iribarren, K.; Mondragón, L.; Alou, M.T.; Pizzato, E.; Durand, S.; et al. Multifaceted modes of action of the anticancer probiotic Enterococcus hirae. Cell Death Differ. 2021, 28, 2276–2295. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; Plaza-Díaz, J.; de Almagro, M.C.; Gil, Á.; Moreno-Muñoz, J.A.; Fontana, L. Bifidobacterium longum subsp. infantis CECT 7210 Reduces Inflammatory Cytokine Secretion in Caco-2 Cells Cultured in the Presence of Escherichia coli CECT 515. Int. J. Mol. Sci. 2022, 23, 10813. [Google Scholar]
- You, J.; Yaqoob, P. Evidence of immunomodulatory effects of a novel probiotic, Bifidobacterium longum bv. infantis CCUG 52486. FEMS Immunol. Med. Microbiol. 2012, 66, 353–362. [Google Scholar] [CrossRef]
- Gavzy, S.J.; Kensiski, A.; Lee, Z.L.; Mongodin, E.F.; Ma, B.; Bromberg, J.S. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 2023, 15, 2291164. [Google Scholar] [CrossRef]
- Fanning, S.; Hall, L.J.; Cronin, M.; Zomer, A.; Mac Sharry, J.; Goulding, D.; O’Connell, M.; Shanahan, F.; Nally, K.; Dougan, G.; et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc. Natl. Acad. Sci. USA 2012, 109, 2108–2113. [Google Scholar] [CrossRef]
- Hao, R.; Liu, Q.; Wang, L.; Jian, W.; Cheng, Y.; Zhang, Q.; Hayer, K.; Idris, R.K.R.; Zhang, Y.; Lu, H.; et al. Anti-inflammatory effect of Lactiplantibacillus plantarum T1 cell-free supernatants through suppression of oxidative stress and NF-κB- and MAPK-signaling pathways. Appl. Environ. Microbiol. 2023, 89, e00608-23. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Lu, Y.-C.; Ou, C.-C.; Lin, S.-L.; Tsai, C.-C.; Huang, C.-T.; Lin, M.-Y. Lactobacillus plantarum MYL26 induces endotoxin tolerance phenotype in Caco-2 cells. BMC Microbiol. 2013, 13, 190. [Google Scholar] [CrossRef]
- Takagi, A.; Ikemura, H.; Matsuzaki, T.; Sato, M.; Nomoto, K.; Morotomi, M.; Yokokura, T. Relationship between the in vitro response of dendritic cells to Lactobacillus and prevention of tumorigenesis in the mouse. J. Gastroenterol. 2008, 43, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Fink, L.N.; Zeuthen, L.H.; Christensen, H.R.; Morandi, B.; Frokiaer, H.; Ferlazzo, G.; Frøkiær, H. Distinct gut-derived lactic acid bacteria elicit divergent dendritic cell-mediated NK cell responses. Int. Immunol. 2007, 19, 1319–1327. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Mohamadzadeh, M.; Olson, S.; Kalina, W.V.; Ruthel, G.; Demmin, G.L.; Warfield, K.L.; Bavari, S.; Klaenhammer, T.R. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc. Natl. Acad. Sci. USA 2005, 102, 2880–2885. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lozano-Ruiz, B.; Yang, F.M.; Fan, D.D.; Shen, L.; González-Navajas, J.M. The Multifaceted Role of Th1, Th9, and Th17 Cells in Immune Checkpoint Inhibition Therapy. Front. Immunol. 2021, 12, 625667. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, S.-Z.; Du, P.; Cheng, Z.-B.; Hu, H.; Wang, S.-Y. Probiotics Can Boost the Antitumor Immunity of CD8+T Cells in BALB/c Mice and Patients with Colorectal Carcinoma. J. Immunol. Res. 2020, 2020, 4092472. [Google Scholar] [CrossRef]
- Fan, S.; Li, Y.; Huang, S.; Wang, W.; Zhang, B.; Zhang, J.; Jian, X.; Song, Z.; Wu, M.; Tu, H.; et al. Microbiota-Derived L-SeMet Potentiates CD8+ T Cell Effector Functions and Facilitates Anti-Tumor Responses. Int. J. Mol. Sci. 2025, 26, 2511. [Google Scholar] [CrossRef]
- Römer, P.S.; Berr, S.; Avota, E.; Na, S.-Y.; Battaglia, M.; Berge, I.T.; Einsele, H.; Hünig, T. Preculture of PBMCs at high cell density increases sensitivity of T-cell responses, revealing cytokine release by CD28 superagonist TGN1412. Blood 2011, 118, 6772–6782. [Google Scholar] [CrossRef]
- Coënon, L.; Geindreau, M.; Ghiringhelli, F.; Villalba, M.; Bruchard, M. Natural Killer cells at the frontline in the fight against cancer. Cell Death Dis. 2024, 15, 614. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Chen, C.; Zhou, W.; Hao, J.; He, S.; Liu, Y.; Ku, Y.; Huang, L.; Zhang, C.; Shu, Y.; et al. Natural Killer Cell-Mediated Antitumor Immunity: Molecular Mechanisms and Clinical Applications. MedComm 2025, 6, e70387. [Google Scholar] [CrossRef] [PubMed]
- Morcillo-Martín-Romo, P.; Valverde-Pozo, J.; Ortiz-Bueno, M.; Arnone, M.; Espinar-Barranco, L.; Espinar-Barranco, C.; García-Rubiño, M.E. The Role of NK Cells in Cancer Immunotherapy: Mechanisms, Evasion Strategies, and Therapeutic Advances. Biomedicines 2025, 13, 857. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.-H.; Zhang, J.; Li, H.-S.; Zhou, Y.; Guan, C.-X. Nature killer cell for solid tumors: Current obstacles and prospective remedies in NK cell therapy and beyond. Crit. Rev. Oncol./Hematol. 2025, 205, 104553. [Google Scholar] [CrossRef]
- Fincham, R.E.A.; Delvecchio, F.R.; Goulart, M.R.; Yeong, J.P.S.; Kocher, H.M. Natural killer cells in pancreatic cancer stroma. World J. Gastroenterol. 2021, 27, 3483–3501. [Google Scholar] [CrossRef]
- Kumar, V.; Mahato, R.I. Natural killer cells for pancreatic cancer immunotherapy: Role of nanoparticles. Cancer Lett. 2023, 579, 216462. [Google Scholar] [CrossRef]
- Fanijavadi, S.; Thomassen, M.; Jensen, L.H. Targeting Triple NK Cell Suppression Mechanisms: A Comprehensive Review of Biomarkers in Pancreatic Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 515. [Google Scholar] [CrossRef]
- Kaur, K.; Ko, M.-W.; Chen, F.; Jewett, A. Defective NK cell expansion, cytotoxicity, and lack of ability to differentiate tumors from a pancreatic cancer patient in a long term follow-up: Implication in the progression of cancer. Cancer Immunol. Immunother. 2022, 71, 1033–1047. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Jacobs, M.T.; Fehniger, T.A. Allogeneic natural killer cell therapy. Blood 2023, 141, 856–868. [Google Scholar] [CrossRef]
- Fang, F.; Wang, W.; Chen, M.; Tian, Z.; Xiao, W. Technical advances in NK cell-based cellular immunotherapy. Cancer Biol. Med. 2019, 16, 647–654. [Google Scholar] [CrossRef]
- Liu, S.; Galat, V.; Galat4, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.; Tarannum, M.; Lérias, J.R.; Piccinelli, S.; Borrego, L.M.; Maeurer, M.; Romee, R.; Castillo-Martin, M. Building a Better Defense: Expanding and Improving Natural Killer Cells for Adoptive Cell Therapy. Cells 2024, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Perussia, B.; Ramoni, C.; Anegon, I.; Cuturi, M.; Faust, J.; Trinchieri, G. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat. Immun. Cell Growth Regul. 1987, 6, 171–188. [Google Scholar] [PubMed]
- Rabinowich, H.; Sedlmayr, P.; Herberman, R.B.; Whiteside, T.L. Increased proliferation, lytic activity, and purity of human natural killer cells cocultured with mitogen-activated feeder cells. Cell Immunol. 1991, 135, 454–470. [Google Scholar] [CrossRef]
- Srivastava, S.; Lundqvist, A.; Childs, R.W. Natural killer cell immunotherapy for cancer: A new hope. Cytotherapy 2008, 10, 775–783. [Google Scholar] [CrossRef]
- Gras Navarro, A.; Björklund, A.; Chekenya, M. Therapeutic potential and challenges of Natural killer cells in treatment of solid tumors. Front. Immunol. 2015, 6, 202. [Google Scholar] [CrossRef]
- Garg, T.K.; Szmania, S.M.; Khan, J.A.; Hoering, A.; Malbrough, P.A.; Moreno-Bost, A.; Greenway, A.D.; Lingo, J.D.; Li, X.; Yaccoby, S.; et al. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica 2012, 97, 1348–1356. [Google Scholar] [CrossRef]
- Gurney, M.; Kundu, S.; Pandey, S.; O’dWyer, M. Feeder Cells at the Interface of Natural Killer Cell Activation, Expansion and Gene Editing. Front. Immunol. 2022, 13, 802906. [Google Scholar] [CrossRef]
- Michen, S.; Frosch, J.; Füssel, M.; Schackert, G.; Momburg, F.; Temme, A. Artificial feeder cells expressing ligands for killer cell immunoglobulin-like receptors and CD94/NKG2A for expansion of functional primary natural killer cells with tolerance to self. Cytotherapy 2020, 22, 354–368. [Google Scholar] [CrossRef]
- Tran, T.B.T.; Bui, T.V.A.; Tran, T.M.T.; Nguyen, N.M.; Nguyen, H.T.P.; Tran, T.P.D.; Nguyen, D.M.Q.; Ngo, T.M.Q.; Nguyen, T.B.; Verhoeyen, E.; et al. In Vitro Expansion and Transduction of Primary NK Cells Using Feeder Cells Expressing Costimulatory Molecules and IL-21. Cancer Sci. 2025, 116, 1847–1860. [Google Scholar] [CrossRef]
- Marr, B.; Jo, D.; Jang, M.; Lee, S.-H. Cytokines in Focus: IL-2 and IL-15 in NK Adoptive Cell Cancer Immunotherapy. Immune Netw. 2025, 25, e17. [Google Scholar] [CrossRef] [PubMed]
- Denman, C.J.; Senyukov, V.V.; Somanchi, S.S.; Phatarpekar, P.V.; Kopp, L.M.; Johnson, J.L.; Singh, H.; Hurton, L.; Maiti, S.N.; Huls, M.H.; et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 2012, 7, e30264. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ye, Y.; Gao, Y.; Huang, H.; Zhao, Y. Influence of KIR and NK Cell Reconstitution in the Outcomes of Hematopoietic Stem Cell Transplantation. Front. Immunol. 2020, 11, 2022. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Rezvani, K. Can we make a better match or mismatch with KIR genotyping? Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 106–118. [Google Scholar] [CrossRef]
- Ko, M.W.; Mei, A.; Senjor, E.; Nanut, M.P.; Gao, L.W.; Wong, P.; Chen, P.-C.; Cohn, W.; Whitelegge, J.P.; Kos, J.; et al. Osteoclast-expanded supercharged NK cells perform superior antitumour effector functions. BMJ Oncol. 2025, 4, e000676. [Google Scholar] [CrossRef]
- Kaur, K.; Safaie, T.; Ko, M.-W.; Wang, Y.; Jewett, A. ADCC against MICA/B Is Mediated against Differentiated Oral and Pancreatic and Not Stem-Like/Poorly Differentiated Tumors by the NK Cells; Loss in Cancer Patients due to Down-Modulation of CD16 Receptor. Cancers 2021, 13, 239. [Google Scholar] [CrossRef]
- Kaur, K.; Chen, P.-C.; Jewett, A. Supercharged NK cells: A unique population of NK cells capable of differentiating stem cells and lysis of MHC class I high differentiated tumors. Cell Death Dis. 2025, 16, 665. [Google Scholar] [CrossRef]
- Huerta-Yepez, S.; Chen, P.-C.; Kaur, K.; Jain, Y.; Singh, T.; Esedebe, F.; Liao, Y.J.; DiBernardo, G.; A Moatamed, N.; Mei, A.; et al. Supercharged NK cells, unlike primary activated NK cells, effectively target ovarian cancer cells irrespective of MHC-class I expression. BMJ Oncol. 2025, 4, e000618. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Kerbauy, L.N.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Becker, P.S.; Suck, G.; Nowakowska, P.; Ullrich, E.; Seifried, E.; Bader, P.; Tonn, T.; Seidl, C. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol. Immunother. 2016, 65, 477–484. [Google Scholar] [CrossRef]
- Leivas, A.; Perez-Martínez, A.; Blanchard, M.J.; Martín-Clavero, E.; Fernández, L.; Lahuerta, J.J.; Martinez-Lopez, J. Novel treatment strategy with autologous activated and expanded natural killer cells plus anti-myeloma drugs for multiple myeloma. Oncoimmunology 2016, 5, e1250051. [Google Scholar] [CrossRef]
- Kita, A.; Fujiya, M.; Konishi, H.; Tanaka, H.; Kashima, S.; Iwama, T.; Ijiri, M.; Murakami, Y.; Takauji, S.; Goto, T.; et al. Probiotic-derived ferrichrome inhibits the growth of refractory pancreatic cancer cells. Int. J. Oncol. 2020, 57, 721–732. [Google Scholar] [CrossRef]
- Maher, S.; Elmeligy, H.A.; Aboushousha, T.; Helal, N.S.; Ossama, Y.; Rady, M.; Hassan, A.M.A.; Kamel, M. Synergistic immunomodulatory effect of synbiotics pre- and postoperative resection of pancreatic ductal adenocarcinoma: A randomized controlled study. Cancer Immunol. Immunother. 2024, 73, 109. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, P.; Huang, Y.; Zhang, W.; Nie, Y.; Gao, C. Metabolic engineering of Lactobacilli spp. for disease treatment. Microb. Cell Factories 2025, 24, 53. [Google Scholar] [CrossRef]
- Rocha-Ramírez, L.M.; Pérez-Solano, R.A.; Castañón-Alonso, S.L.; Guerrero, S.S.M.; Pacheco, A.R.; Garibay, M.G.; Eslava, C. Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages. J. Immunol. Res. 2017, 2017, 4607491. [Google Scholar] [CrossRef]
- Musazadeh, V.; Zarezadeh, M.; Faghfouri, A.H.; Keramati, M.; Jamilian, P.; Jamilian, P.; Mohagheghi, A.; Farnam, A. Probiotics as an effective therapeutic approach in alleviating depression symptoms: An umbrella meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 8292–8300. [Google Scholar] [CrossRef]
- Ko, M.-W.; Kaur, K.; Safaei, T.; Chen, W.; Sutanto, C.; Wong, P.; Jewett, A. Defective Patient NK Function Is Reversed by AJ2 Probiotic Bacteria or Addition of Allogeneic Healthy Monocytes. Cells 2022, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Sobocki, B.K.; Kaźmierczak-Siedlecka, K.; Folwarski, M.; Hawryłkowicz, V.; Makarewicz, W.; Stachowska, E. Pancreatic Cancer and Gut Microbiome-Related Aspects: A Comprehensive Review and Dietary Recommendations. Nutrients 2021, 13, 4425. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S. Are Probiotics Beneficial or Harmful for Pancreatic Cancer Outcomes? Probiotics Antimicrob. Proteins 2025, 17, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5, 73–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whelan, K.; Alexander, M.; Gaiani, C.; Lunken, G.; Holmes, A.; Staudacher, H.M.; Theis, S.; Marco, M.L. Design and reporting of prebiotic and probiotic clinical trials in the context of diet and the gut microbiome. Nat. Microbiol. 2024, 9, 2785–2794. [Google Scholar] [CrossRef]
- Butel, M.J. Probiotics, gut microbiota and health. Med. Mal. Infect. 2014, 44, 1–8. [Google Scholar] [CrossRef]
- Kamareddine, L.; Najjar, H.; Sohail, M.U.; Abdulkader, H.; Al-Asmakh, M. The Microbiota and Gut-Related Disorders: Insights from Animal Models. Cells 2020, 9, 2401. [Google Scholar] [CrossRef] [PubMed]
- Schlienger de Alba, B.N.; Andrews, H.E. Benefits and Challenges of Encapsulating Bifidobacterium Probiotic Strains with Bifidogenic Prebiotics. Probiotics Antimicrob. Proteins 2024, 16, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Gajjar, D.; Seshadri, S. Understanding the role of gut microfloral bifidobacterium in cancer and its potential therapeutic applications. Microbiome Res. Rep. 2024, 3, 3. [Google Scholar] [CrossRef]
- Mejía-Caballero, A.; Salas-Villagrán, V.A.; Jiménez-Serna, A.; Farrés, A. Challenges in the production and use of probiotics as therapeuticals in cancer treatment or prevention. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab052. [Google Scholar] [CrossRef]
- Lu, K.; Dong, S.; Wu, X.; Jin, R.; Chen, H. Probiotics in Cancer. Front. Oncol. 2021, 11, 638148. [Google Scholar] [CrossRef]
- De Lucia, S.S.; Nista, E.C.; Candelli, M.; Archilei, S.; Deutschbein, F.; Capuano, E.; Gasbarrini, A.; Franceschi, F.; Pignataro, G. Microbiota and Pancreatic Cancer: New Therapeutic Frontiers Between Engineered Microbes, Metabolites and Non-Bacterial Components. Cancers 2025, 17, 3618. [Google Scholar] [CrossRef]
- Abe, S.; Masuda, A.; Matsumoto, T.; Inoue, J.; Toyama, H.; Sakai, A.; Kobayashi, T.; Tanaka, T.; Tsujimae, M.; Yamakawa, K.; et al. Impact of intratumoral microbiome on tumor immunity and prognosis in human pancreatic ductal adenocarcinoma. J. Gastroenterol. 2024, 59, 250–262. [Google Scholar] [CrossRef]
- Decker-Farrell, A.R.; Sastra, S.A.; Harimoto, T.; Hasselluhn, M.C.; Palermo, C.F.; Ballister, E.R.; Badgley, M.A.; Danino, T.; Olive, K.P. Tumor-selective treatment of metastatic pancreatic cancer with an engineered, probiotic living drug. bioRxiv 2024. [Google Scholar] [CrossRef]
- Fanijavadi, S.; Jensen, L.H. Dysbiosis–NK Cell Crosstalk in Pancreatic Cancer: Toward a Unified Biomarker Signature for Improved Clinical Outcomes. Int. J. Mol. Sci. 2025, 26, 730. [Google Scholar] [CrossRef]
- Kim, N.; Ma, J.; Kim, W.; Kim, J.; Belenky, P.; Lee, I. Genome-resolved metagenomics: A game changer for microbiome medicine. Exp. Mol. Med. 2024, 56, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Elkrief, A.; Pidgeon, R.; Vareki, S.M.; Messaoudene, M.; Castagner, B.; Routy, B. The gut microbiome as a target in cancer immunotherapy: Opportunities and challenges for drug development. Nat. Rev. Drug Discov. 2025, 24, 685–704. [Google Scholar] [CrossRef] [PubMed]
- Pourali, G.; Kazemi, D.; Chadeganipour, A.S.; Arastonejad, M.; Kashani, S.N.; Pourali, R.; Maftooh, M.; Akbarzade, H.; Fiuji, H.; Hassanian, S.M.; et al. Microbiome as a biomarker and therapeutic target in pancreatic cancer. BMC Microbiol. 2024, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, B.; Abdulabbas, H.T.; Alturki, A.D.; Mansouri, P.; Zarenezhad, E.; Nasiri-Ghiri, M.; Ghasemian, A. Synergistic Interactions Between Probiotics and Anticancer Drugs: Mechanisms, Benefits, and Challenges. Probiotics Antimicrob. Proteins 2025, 17, 2791–2804. [Google Scholar] [CrossRef]
- Tavanaeian, S.; Feizabadi, M.M.; Falsafi, S.; Aghdaei, H.A.; Houri, H. Oral and fecal microbiome alterations in pancreatic cancer: Insights into potential diagnostic biomarkers. BMC Microbiol. 2025, 25, 624. [Google Scholar] [CrossRef]
- Dean, A.P.; Comito, O.R.; Yusoff, I.; Rao, S.; Johansson, M.; Navadgi, S.; Webber, L.; Watanabe, Y. Potential effect of the gut microbiome on tumour response to anti-cancer treatment in patients diagnosed with pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2025, 43 (Suppl. S4), 757. [Google Scholar] [CrossRef]
| Probiotics | Combined Therapy | Dose | Trial Phase | ClinicalTrials.gov Identifier | Patients Enrolled |
|---|---|---|---|---|---|
| Clostridium butyricum | NA | NCT06998823 | 120 | ||
| Lactobacillus reuteri ATG-F4 | oxaliplatin-based chemotherapy | 4 × 1010 CFU daily for 12 weeks | Phase 2 | NCT06436976 | 30 |
| Lactobacillus acidophilus, Bifidobacterium lactis, Lactobacillus plantarum, Lactobacillus paracasei, Bifidobacterium breve, Streptococcus thermophiles, Lactobacillus salivarius, and Bifidobacterium longum | Surgical | Total: 2.5 × 1010 CFU | Phase 4 | NCT06199752 | 90 |
| Strengths | Limitations |
|---|---|
| Probiotics can impact a wide range of immune system functions linked to pancreatic cancer. Probiotics can affect innate immunity by influencing macrophages, dendritic cells, and natural killer (NK) cell activity, as well as adaptive immunity by shaping B-cell and T-cell responses, cytokine production, and the development of regulatory T cells (Tregs). Probiotics may also boost antigen presentation, adjust inflammatory signaling pathways, and support gut barrier health, all of which help maintain immune balance and could indirectly influence the progression of pancreatic tumors. Probiotics may boost the efficacy of NK cell-based and other conventional pancreatic cancer therapeutics. Probiotics may reduce tumor load, restore immune function, and restore bone defects in a mouse model of pancreatic cancer. Probiotics may increase the tolerance of chemotherapy in pancreatic cancer patients. | There is a lack of solid research and quality clinical trials, with most of the current findings coming from early lab work or small, mixed groups of human participants. It is still uncertain which strains work best, what the right dosage is, or how safe probiotics are for people with pancreatic cancer. Studies so far just do not provide enough proof to confirm benefits or set clear guidelines. Sometimes, taking probiotics could raise the toxicity associated with immunotherapy, perhaps due to their unpredictable effects on the body’s immune response. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, K. Role of Probiotics in Enhancing Immune Function and Improving the Effectiveness of Treatments for Pancreatic Cancer. Microorganisms 2025, 13, 2687. https://doi.org/10.3390/microorganisms13122687
Kaur K. Role of Probiotics in Enhancing Immune Function and Improving the Effectiveness of Treatments for Pancreatic Cancer. Microorganisms. 2025; 13(12):2687. https://doi.org/10.3390/microorganisms13122687
Chicago/Turabian StyleKaur, Kawaljit. 2025. "Role of Probiotics in Enhancing Immune Function and Improving the Effectiveness of Treatments for Pancreatic Cancer" Microorganisms 13, no. 12: 2687. https://doi.org/10.3390/microorganisms13122687
APA StyleKaur, K. (2025). Role of Probiotics in Enhancing Immune Function and Improving the Effectiveness of Treatments for Pancreatic Cancer. Microorganisms, 13(12), 2687. https://doi.org/10.3390/microorganisms13122687






