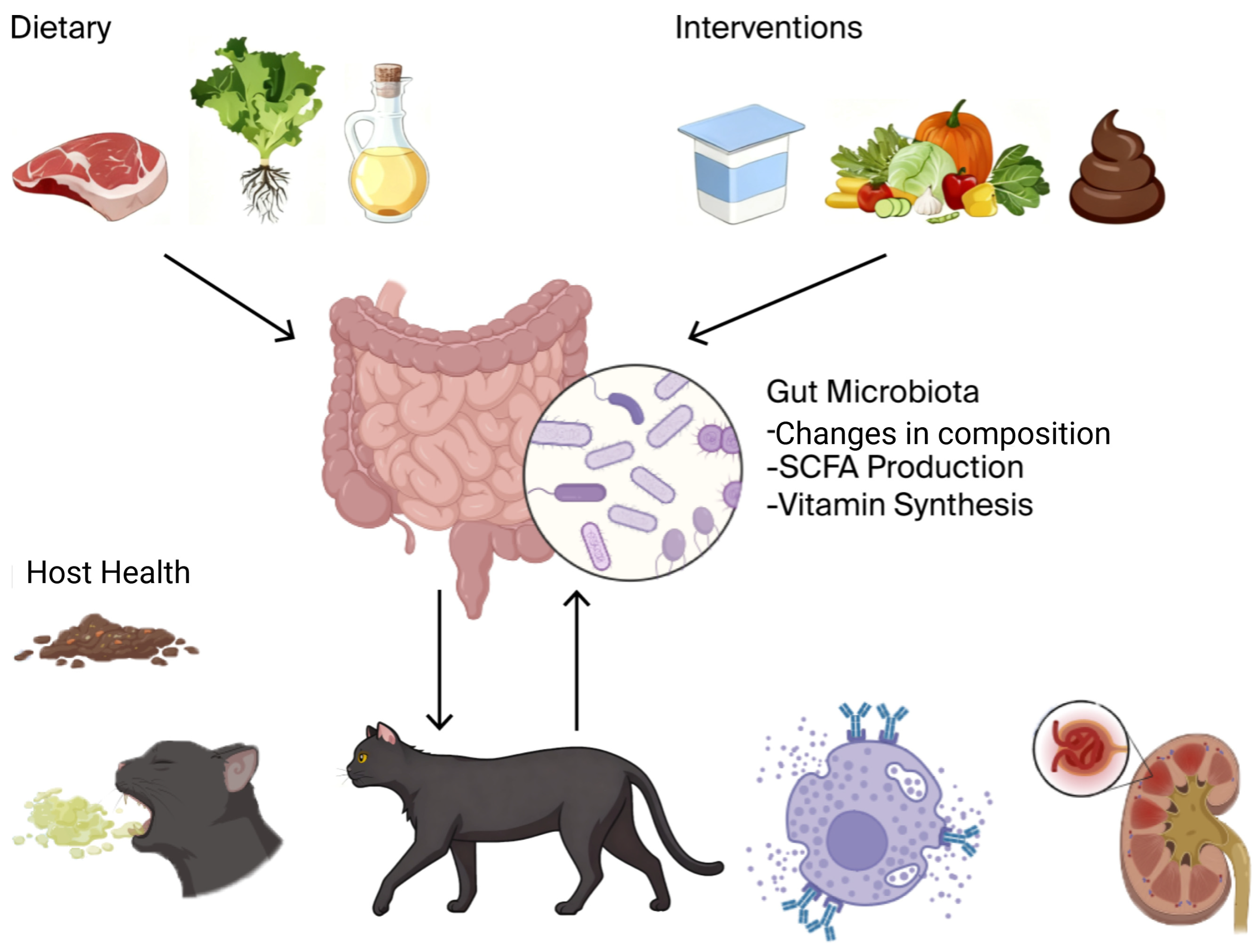
Dietary Modulation of the Gut Microbiota in Dogs and Cats and Its Role in Disease Management
Abstract
1. Introduction
2. Composition and Function of the Gut Microbiota in Companion Animals
2.1. Composition of the Gut Microbiome in Dogs and Cats
2.2. Metabolic Functions of the Gut Microbiome
2.3. Immune Regulatory Functions
2.4. Digestive Functions
2.5. Barrier Protective Functions
3. The Impact of Diet on the Gut Microbiome
3.1. Nutritional Components and Their Impact on Gut Microbiota
3.1.1. Protein
- High-Protein Diets: It has been shown that high-protein diets (and, in particular, those with a high animal protein content) can promote the growth of Bacteroidetes and Firmicutes [6,24]. These phyla are involved in protein metabolism and the breakdown of complex amino acids. Nonetheless, high-protein diets also favor the development of Clostridium perfringens, which is a potentially pathogenic bacterium, particularly in cats and dogs whose immunity is impaired [25].
- Protein Source: The origin of the protein also matters. Proteins derived from animal sources (e.g., fish, beef, and poultry) have been shown to stimulate the growth of useful microbes like Lactobacillus, which are beneficial for preservation of the gut barrier, as well as immune function. In contrast, plant-based proteins may support a different set of microbial taxa, including those associated with fiber fermentation and SCFA production [26,27].
3.1.2. Fat
- High-Fat Diets: Cats and dogs that consume high-fat diets demonstrate elevated numbers of Firmicutes, which are involved in fat catabolism. Omega-6 and omega-3 polyunsaturated fats, typically found in fish oils, can regulate the gut microbiome by elevating the numbers of anti-inflammatory microbes such as Lactobacillus and Bifidobacterium [28]. Fats also appear to regulate the gut barrier by elevating the numbers of microbes that are producers of SCFAs, elevating the integrity of the intestines.
- Obesity and Dysbiosis: High volumes of a high-fat diet with high caloric content can induce dysbiosis—a dis-equilibrium among obesity-related microbes and good bacteria. In such cases, the numbers of Bacteroides uniformis and Clostridium species can rise, resulting in inflammatory events in the gut [29].
3.1.3. Fiber
3.1.4. Carbohydrates
3.2. Food Formats and Their Effects on Gut Microbiota
3.2.1. Biologically Appropriate Raw Food (BARF) Diets
3.2.2. Home-Cooked Meals
3.2.3. Extruded Commercial Kibble
4. Links Between Gut Microbiota and Disease in Dogs and Cats
4.1. Gut Microbiota and Gastrointestinal Diseases
4.1.1. Gut Microbiota and IBD
4.1.2. Gut Microbiota and Gastrointestinal Inflammation
4.2. Gut Microbiota and Chronic Diseases
4.2.1. Gut Microbiota and Obesity
4.2.2. Gut Microbiota and Diabetes
4.3. Gut Microbiota and Immune-Related Diseases
4.3.1. Gut Microbiota and Allergies
4.3.2. Gut Microbiota and Autoimmune Diseases
4.4. Gut Microbiota and Liver and Kidney Diseases
4.4.1. Gut Microbiota and Liver Diseases
4.4.2. Gut Microbiota and Kidney Diseases
5. Dietary Interventions and Gut Health Management
5.1. Probiotics and Prebiotics
5.2. Specialized Dietary Interventions
5.3. Fecal Microbiota Transplantation (FMT)
6. Challenges and Research Gaps
7. Future Directions and Opportunities
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pilla, R.; Suchodolski, J. The Gut Microbiome of Dogs and Cats, and the Influence of Diet. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Honneffer, J.B.; Minamoto, Y.; Suchodolski, J.S. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J. Gastroenterol. 2014, 20, 16489–16497. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, G.; Argentini, C.; Milani, C.; Turroni, F.; Ossiprandi, M.C.; Van Sinderen, D.; Ventura, M. Catching a glimpse of the bacterial gut community of companion animals: A canine and feline perspective. Microb. Biotechnol. 2020, 13, 1708–1732. [Google Scholar] [CrossRef]
- Swanson, K. 0226 Dietary manipulation of canine and feline gut microbiome. J. Anim. Sci. 2016, 94, 107. [Google Scholar] [CrossRef]
- Li, Q.; Lauber, C.; Czarnecki-Maulden, G.; Pan, Y.; Hannah, S. Effects of the Dietary Protein and Carbohydrate Ratio on Gut Microbiomes in Dogs of Different Body Conditions. mBio 2017, 8, e01703-16. [Google Scholar] [CrossRef]
- You, I.; Kim, M.J. Comparison of Gut Microbiota of 96 Healthy Dogs by Individual Traits: Breed, Age, and Body Condition Score. Animals 2021, 11, 2432. [Google Scholar] [CrossRef]
- Moon, C.; Young, W.; Maclean, P.; Cookson, A.; Bermingham, E. Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats. Microbiology 2018, 7, e00677. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary Gut Microbial Metabolites, Short-chain Fatty Acids, and Host Metabolic Regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Chambers, E.; Preston, T.; Frost, G.; Morrison, D. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yao, J.; Yang, C.; Yu, S.; Yang, Z.; Wang, L.; Li, S.; He, N. Gut microbiota-derived short chain fatty acids act as mediators of the gut-liver-brain axis. Metab. Brain Dis. 2025, 40, 122. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Byndloss, M.X.; Bäumler, A.J. The germ-organ theory of non-communicable diseases. Nat. Rev. Microbiol. 2018, 16, 103–110. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Companion animals symposium: Microbes and gastrointestinal health of dogs and cats. J. Anim. Sci. 2011, 89, 1520–1530. [Google Scholar] [CrossRef]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 2015, 6, 33–47. [Google Scholar] [CrossRef]
- Song, S.J.; Lauber, C.; Costello, E.K.; Lozupone, C.A.; Humphrey, G.; Berg-Lyons, D.; Caporaso, J.G.; Knights, D.; Clemente, J.C.; Nakielny, S.; et al. Cohabiting family members share microbiota with one another and with their dogs. Elife 2013, 2, e00458. [Google Scholar] [CrossRef]
- Kamada, N.; Kim, Y.G.; Sham, H.P.; Vallance, B.A.; Puente, J.L.; Martens, E.C.; Núñez, G. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 2012, 336, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017, 357, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Gebreselassie, E.E.; Jewell, D. Long-Term Consumption of High Protein Disrupts Dog Gut Microbiome and Metabolites. FASEB J. 2019, 33, lb248. [Google Scholar] [CrossRef]
- Martínez-López, L.; Pepper, A.; Pilla, R.; Woodward, A.; Suchodolski, J.; Mansfield, C. Effect of sequentially fed high protein, hydrolyzed protein, and high fiber diets on the fecal microbiota of healthy dogs: A cross-over study. Anim. Microbiome 2021, 3, 42. [Google Scholar] [CrossRef]
- Li, Q.; Pan, Y. Differential Responses to Dietary Protein and Carbohydrate Ratio on Gut Microbiome in Obese vs. Lean Cats. Front. Microbiol. 2020, 11, 591462. [Google Scholar] [CrossRef]
- Lyu, Y.; Xu, J.; Verdoodt, F.; Vanhaecke, L.; Hemeryck, L.; Hesta, M. Faecal metabolome responses to an altered dietary protein:carbohydrate ratio in adult dogs. Vet. Q. 2023, 43, 1–10. [Google Scholar] [CrossRef]
- Cândido, F.; Valente, F.X.; Grześkowiak, Ł.; Moreira, A.; Rocha, D.; Alfenas, R. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: Mechanisms and clinical implications on obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar] [CrossRef]
- Schauf, S.; De La Fuente, G.; Newbold, C.; Salas-Mani, A.; Torre, C.; Abecia, L.; Castrillo, C. Effect of dietary fat to starch content on fecal microbiota composition and activity in dogs. J. Anim. Sci. 2018, 96, 3684–3698. [Google Scholar] [CrossRef]
- Fagundes, R.; Bourgonje, A.; Saeed, A.; Vila, V.; Plomp, N.; Blokzijl, T.; Sadabad, S.; Von Martels, J.; Van Leeuwen, S.; Weersma, R.; et al. Inulin-grown Faecalibacterium prausnitzii cross-feeds fructose to the human intestinal epithelium. Gut Microbes 2021, 13, 1993582. [Google Scholar] [CrossRef]
- Lindstad, L.; Lo, G.; Leivers, S.; Lu, Z.; Michalak, L.; Pereira, G.; Røhr, Å.; Martens, E.; McKee, L.; Louis, P.; et al. Human Gut Faecalibacterium prausnitzii Deploys a Highly Efficient Conserved System To Cross-Feed on β-Mannan-Derived Oligosaccharides. mBio 2021, 12, e0362820. [Google Scholar] [CrossRef] [PubMed]
- Salas-Mani, A.; Jeusette, I.; Castillo, I.; Manuelian, C.L.; Lionnet, C.; Iraculis, N.; Sanchez, N.; Fernández, S.; Vilaseca, L.; Torre, C. Fecal microbiota composition changes after a BW loss diet in Beagle dogs. J. Anim. Sci. 2018, 96, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Bhosle, A.; Jackson, M.I.; Walsh, A.M.; Franzosa, E.A.; Badri, D.V.; Huttenhower, C. Response of the gut microbiome and metabolome to dietary fiber in healthy dogs. mSystems 2025, 10, e0045224. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Cui, X.; Guo, M.; Tian, Y.; Xu, W.; Huang, K.; Zhang, Y.-X. Insoluble Dietary Fiber from Pear Pomace Can Prevent High-Fat Diet-Induced Obesity in Rats Mainly by Improving the Structure of the Gut Microbiota. J. Microbiol. Biotechnol. 2017, 27, 856–867. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.; Yadava, K.; Sichelstiel, A.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.; Harris, N.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Breton, J.; Tu, V.; Tanes, C.; Quinn, R.; Conrad, M.; Kachelries, K.; Bittinger, K.; Baldassano, R.; Compher, C.; Albenberg, L. Dietary Inflammatory Potential and Food Patterns in Relation to Gut Microbiome Among Children with Crohn’s Disease: A Comparative Study with Healthy Controls. Gastroenterology 2021, 160, S54. [Google Scholar] [CrossRef]
- Larke, J.; Bacalzo, N.; Castillo, J.; Couture, G.; Chen, Y.; Xue, Z.; Alkan, Z.; Kable, M.; Lebrilla, C.; Stephensen, C.; et al. Dietary Intake of Monosaccharides from Foods is Associated with Characteristics of the Gut Microbiota and Gastrointestinal Inflammation in Healthy US Adults. J. Nutr. 2022, 153, 106–119. [Google Scholar] [CrossRef]
- Tachon, S.; Zhou, J.; Keenan, M.; Martin, R.; Marco, M. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol. Ecol. 2013, 83, 299–309. [Google Scholar] [CrossRef]
- Scott, K.; Duncan, S.; Flint, H. Dietary fibre and the gut microbiota. Nutr. Bull. 2008, 33, 201–211. [Google Scholar] [CrossRef]
- Castañeda, S.; Ariza, G.; Rincón-Riveros, A.; Muñoz, M.; Ramírez, J. Diet-induced changes in fecal microbiota composition and diversity in dogs (Canis lupus familiaris): A comparative study of BARF-type and commercial diets. Comp. Immunol. Microbiol. Infect. Dis. 2023, 98, 102007. [Google Scholar] [CrossRef]
- Kim, J.; An, J.; Kim, W.-H.; Lee, S.; Cho, S. Differences in the gut microbiota of dogs (Canis lupus familiaris) fed a natural diet or a commercial feed revealed by the Illumina MiSeq platform. Gut Pathog. 2017, 9, 68. [Google Scholar] [CrossRef]
- Hellgren, J.; Hästö, L.S.; Wikström, C.; Fernström, L.L.; Hansson, I. Occurrence of Salmonella, Campylobacter, Clostridium and Enterobacteriaceae in raw meat-based diets for dogs. Vet. Rec. 2019, 184, 442. [Google Scholar] [CrossRef]
- Schmidt, M.; Unterer, S.; Suchodolski, J.S.; Honneffer, J.B.; Guard, B.C.; Lidbury, J.A.; Steiner, J.M.; Fritz, J.; Kölle, P. The fecal microbiome and metabolome differs between dogs fed Bones and Raw Food (BARF) diets and dogs fed commercial diets. PLoS ONE 2018, 13, e0201279. [Google Scholar] [CrossRef]
- Viegas, F.M.; Ramos, C.P.; Xavier, R.G.C.; Lopes, E.O.; Júnior, C.A.O.; Bagno, R.M.; Diniz, A.N.; Lobato, F.C.F.; Silva, R.O.S. Fecal shedding of Salmonella spp., Clostridium perfringens, and Clostridioides difficile in dogs fed raw meat-based diets in Brazil and their owners’ motivation. PLoS ONE 2020, 15, e0231275. [Google Scholar] [CrossRef]
- Aizpurua, O.; Botnen, A.B.; Eisenhofer, R.; Odriozola, I.; Santos-Bay, L.; Bjørnsen, M.B.; Gilbert, M.T.P.; Alberdi, A. Functional Insights Into the Effect of Feralisation on the Gut Microbiota of Cats Worldwide. Mol. Ecol. 2025, 34, e17695. [Google Scholar] [CrossRef] [PubMed]
- Tanprasertsuk, J.; Shmalberg, J.; Maughan, H.; Tate, D.; Perry, L.; Jha, A.; Honaker, R. Heterogeneity of gut microbial responses in healthy household dogs transitioning from an extruded to a mildly cooked diet. PeerJ 2021, 9, e11648. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, G.; Milani, C.; Mancabelli, L.; Longhi, G.; Anzalone, R.; Lugli, G.; Duranti, S.; Turroni, F.; Ossiprandi, M.; Van Sinderen, D.; et al. Deciphering the Bifidobacterial Populations within the Canine and Feline Gut Microbiota. Appl. Environ. Microbiol. 2020, 86, e02875-19. [Google Scholar] [CrossRef] [PubMed]
- Weeth, L.P. Focus on nutrition: Home-prepared diets for dogs and cats. Compend. Contin. Educ. Vet. 2013, 35, E3. [Google Scholar]
- Hiney, K.; Sypniewski, L.; DeSilva, U.; Pezeshki, A.; Rudra, P.; Goodarzi, P.; Willis, E.; McFarlane, D. Fecal microbiota composition, serum metabolomics, and markers of inflammation in dogs fed a raw meat-based diet compared to those on a kibble diet. Front. Vet. Sci. 2024, 11, 1328513. [Google Scholar] [CrossRef]
- Mason, B.; Sahoo, D.K.; Iennarella-Servantez, C.A.; Kathrani, A.; Morgan, S.M.; Bourgois-Mochel, A.; Bray, A.M.; Gabriel, V.; Zdyrski, C.; Groeltz, J.M.; et al. Effects of a Western Diet on Colonic Dysbiosis, Bile Acid Dysmetabolism and Intestinal Inflammation in Clinically Healthy Dogs. J. Vet. Intern. Med. 2025, 39, e70035. [Google Scholar] [CrossRef]
- Tanprasertsuk, J.; Perry, L.M.; Tate, D.E.; Honaker, R.W.; Shmalberg, J. Apparent total tract nutrient digestibility and metabolizable energy estimation in commercial fresh and extruded dry kibble dog foods. Transl. Anim. Sci. 2021, 5, txab071. [Google Scholar] [CrossRef] [PubMed]
- Gizzarelli, M.; Calabrò, S.; Vastolo, A.; Molinaro, G.; Balestrino, I.; Cutrignelli, M.I. Clinical Findings in Healthy Dogs Fed with Diets Characterized by Different Carbohydrates Sources. Front. Vet. Sci. 2021, 8, 667318. [Google Scholar] [CrossRef] [PubMed]
- Yamka, R.; Sires, R.; Wakshlag, J.; Huson, H.J. Serum Metabolomics of Senior Dogs Fed a Fresh, Human-Grade Food or an Extruded Kibble Diet. Metabolites 2025, 15, 676. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Grieshop, C.M.; Flickinger, E.A.; Bauer, L.L.; Chow, J.; Wolf, B.W.; Garleb, K.A.; Fahey, G.C. Fructooligosaccharides and Lactobacillus acidophilus Modify Gut Microbial Populations, Total Tract Nutrient Digestibilities and Fecal Protein Catabolite Concentrations in Healthy Adult Dogs. J. Nutr. 2002, 132, 3721–3731. [Google Scholar] [CrossRef]
- Suchodolski, J.; Dowd, S.; Wilke, V.; Steiner, J.; Jergens, A. 16S rRNA Gene Pyrosequencing Reveals Bacterial Dysbiosis in the Duodenum of Dogs with Idiopathic Inflammatory Bowel Disease. PLoS ONE 2012, 7, e39333. [Google Scholar] [CrossRef]
- Marsilio, S.; Pilla, R.; Sarawichitr, B.; Chow, B.; Hill, S.L.; Ackermann, M.R.; Estep, J.S.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Characterization of the fecal microbiome in cats with inflammatory bowel disease or alimentary small cell lymphoma. Sci. Rep. 2019, 9, 19208. [Google Scholar] [CrossRef]
- Guard, B.C.; Honneffer, J.B.; Jergens, A.E.; Jonika, M.M.; Toresson, L.; Lawrence, Y.A.; Webb, C.B.; Hill, S.; Lidbury, J.A.; Steiner, J.M.; et al. Longitudinal assessment of microbial dysbiosis, fecal unconjugated bile acid concentrations, and disease activity in dogs with steroid-responsive chronic inflammatory enteropathy. J. Vet. Intern. Med. 2019, 33, 1295–1305. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef]
- Suchodolski, J. The importance of the microbiome and metabolome in health and disease of dogs and cats. Acta Vet. Scand. 2015, 57, K6. [Google Scholar] [CrossRef]
- Yeon, K.-D.; Kim, S.-M.; Kim, J.-H. Association between Gut Microbiota and Metabolic Health and Obesity Status in Cats. Animals 2024, 14, 2524. [Google Scholar] [CrossRef]
- Zhong, X.; Harrington, J.; Millar, S.; Perry, I.; O’Toole, P.; Phillips, C. Gut Microbiota Associations with Metabolic Health and Obesity Status in Older Adults. Nutrients 2020, 12, 2364. [Google Scholar] [CrossRef] [PubMed]
- Phungviwatnikul, T.; Lee, A.H.; Belchik, S.E.; Suchodolski, J.S.; Swanson, K.S. Weight loss and high-protein, high-fiber diet consumption impact blood metabolite profiles, body composition, voluntary physical activity, fecal microbiota, and fecal metabolites of adult dogs. J. Anim. Sci. 2021, 100, skab379. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhu, D.; Lu, J.; Liu, J.; Wu, Z.; Liu, L. Dietary supplementation with low and high polymerization inulin ameliorates adipose tissue inflammation via the TLR4/NF-κB pathway mediated by gut microbiota disturbance in obese dogs. Res. Vet. Sci. 2022, 152, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, Y.; Wu, Z. Programming of metabolic and autoimmune diseases in canine and feline: Linkage to the gut microbiome. Microb. Pathog. 2023, 185, 106436. [Google Scholar] [CrossRef]
- Rostaher, A.; Morsy, Y.; Favrot, C.; Unterer, S.; Schnyder, M.; Scharl, M.; Fischer, N.M. Comparison of the Gut Microbiome between Atopic and Healthy Dogs—Preliminary Data. Animals 2022, 12, 2377. [Google Scholar] [CrossRef]
- Noli, C.; Varina, A.; Barbieri, C.; Pirola, A.; Olivero, D. Analysis of Intestinal Microbiota and Metabolic Pathways before and after a 2-Month-Long Hydrolyzed Fish and Rice Starch Hypoallergenic Diet Trial in Pruritic Dogs. Vet. Sci. 2023, 10, 478. [Google Scholar] [CrossRef]
- Dargahi, N.; Johnson, J.; Donkor, O.; Vasiljevic, T.; Apostolopoulos, V. Immunomodulatory effects of probiotics: Can they be used to treat allergies and autoimmune diseases? Maturitas 2019, 119, 25–38. [Google Scholar] [CrossRef]
- Haase, S.; Haghikia, A.; Wilck, N.; Müller, D.N.; Linker, R.A. Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology 2018, 154, 230–238. [Google Scholar] [CrossRef]
- Bartel, S.; Jatzlauk, G.; Kepert, I.; Fonseca, J.; Müller, C.; Milger, K.; Hochwind, K.; Kostric, M.; Eickelberg, O.; Schloter, M.; et al. D-Tryptophan is a Probiotic Substance Influencing the Gut Microbiome and in vitro Th2 Differentiation. Eur. Respir. J. 2016, 48 (Suppl. S60), PA3985. [Google Scholar]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Kim, S.M.; Kim, J.H. A pilot study of alterations of the gut microbiome in canine chronic kidney disease. Front. Vet. Sci. 2023, 10, 1241215. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.; Quimby, J. Insights into the gut-kidney axis and implications for chronic kidney disease management in cats and dogs. Vet. J. 2024, 306, 106181. [Google Scholar] [CrossRef]
- Koyama, K.; Akiyama, R.; Oda, H.; Komiya, T.; Gokita, K.; Sako, T.; Mori, A. Effect of commercial prescription diets containing prebiotics on clinical signs and fecal microbiome in dogs with intestinal disease. Pol. J. Vet. Sci. 2024, 27, 599–610. [Google Scholar] [CrossRef]
- Wilson, S.; Swanson, K. The influence of ‘biotics’ on the gut microbiome of dogs and cats. Vet. Rec. 2024, 195, 2–12. [Google Scholar] [CrossRef]
- Wang, W.; Dong, H.; Chang, X.; Chen, Q.; Wang, L.; Chen, S.; Chen, L.; Wang, R.; Ge, S.; Wang, P.; et al. Bifidobacterium lactis and Lactobacillus plantarum Enhance Immune Function and Antioxidant Capacity in Cats through Modulation of the Gut Microbiota. Antioxidants 2024, 13, 764. [Google Scholar] [CrossRef]
- O’Mahony, D.; Murphy, K.; Macsharry, J.; Boileau, T.; Sunvold, G.; Reinhart, G.; Kiely, B.; Shanahan, F.; O’Mahony, L. Portrait of a canine probiotic Bifidobacterium—From gut to gut. Vet. Microbiol. 2009, 139, 106–112. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, W.; Zhong, S.; Chen, W.; Xie, S.; Chen, M.; Yu, Q. The alleviating effects and mechanisms of Enterococcus faecium Kimate-X and Lactobacillus plantarum Kimate-F combination on canine inflammatory bowel disease. Front. Vet. Sci. 2025, 12, 1534665. [Google Scholar] [CrossRef]
- Han, B.; Liang, S.; Sun, J.; Tao, H.; Wang, Z.; Liu, B.; Wang, X.; Liu, J.; Wang, J. The Effect of Lactobacillus plantarum on the Fecal Microbiota, Short Chain Fatty Acids, Odorous Substances, and Blood Biochemical Indices of Cats. Microorganisms 2024, 12, 91. [Google Scholar] [CrossRef]
- Van Den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Villar, A.; Zangara, A.; Smidt, C.; Risco, E. In Vitro Evaluation of Prebiotic Properties of a Commercial Artichoke Inflorescence Extract Revealed Bifidogenic Effects. Nutrients 2020, 12, 1552. [Google Scholar] [CrossRef]
- Alexander, C.; Cross, T.-W.; Devendran, S.; Neumer, F.; Theis, S.; Ridlon, J.; Suchodolski, J.; De Godoy, M.; Swanson, K. Effects of prebiotic inulin-type fructans on blood metabolite and hormone concentrations and faecal microbiota and metabolites in overweight dogs. Br. J. Nutr. 2018, 120, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Tanprasertsuk, J.; Jha, A.; Shmalberg, J.; Jones, R.; Perry, L.; Maughan, H.; Honaker, R. The microbiota of healthy dogs demonstrates individualized responses to synbiotic supplementation in a randomized controlled trial. Anim. Microbiome 2021, 3, 36. [Google Scholar] [CrossRef]
- Laflamme, D. Nutritional care for aging cats and dogs. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 769–791. [Google Scholar] [CrossRef] [PubMed]
- Stockman, J. Nutrition and Aging in Dogs and Cats. Adv. Exp. Med. Biol. 2024, 1446, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Ing, N.; Steiner, J. The Use of Diets in the Diagnosis and Treatment of Common Gastrointestinal Diseases in Dogs and Cats. Adv. Exp. Med. Biol. 2024, 1446, 39–53. [Google Scholar] [CrossRef]
- Elliott, D. Nutritional management of chronic renal disease in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 1377–1384. [Google Scholar] [CrossRef]
- Pugliese, A.; Gruppillo, A.; Pietro, S. Clinical Nutrition in Gerontology: Chronic Renal Disorders of the Dog and Cat. Vet. Res. Commun. 2005, 29, 57–63. [Google Scholar] [CrossRef]
- Streeter, R.M.; Wakshlag, J.J. Nutritional support in hepatic failure in dogs and cats. In Nutritional Management of Hospitalized Small Animals; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 199–209. [Google Scholar]
- Stickel, F.; Hoehn, B.; Schuppan, D.; Seitz, H.K. Review article: Nutritional therapy in alcoholic liver disease. Aliment. Pharmacol. Ther. 2003, 18, 357–373. [Google Scholar] [CrossRef]
- Masuda, T.; Shirabe, K.; Yoshiya, S.; Matono, R.; Morita, K.; Hashimoto, N.; Ikegami, T.; Yoshizumi, T.; Baba, H.; Maehara, Y. Nutrition support and infections associated with hepatic resection and liver transplantation in patients with chronic liver disease. JPEN. J. Parenter. Enter. Nutr. 2013, 37, 318–326. [Google Scholar] [CrossRef]
- Mathew, A.; Seymour, E.; Byun, J.; Pennathur, S.; Hummel, S. Altered Metabolic Profile With Sodium-Restricted Dietary Approaches to Stop Hypertension Diet in Hypertensive Heart Failure With Preserved Ejection Fraction. J. Card. Fail. 2015, 21, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, S.; Gross, K.; Ogburn, P.; Calvert, C.; Jacobs, G.; Lowry, S.; Bird, K.; Koehler, L.; Swanson, L. Effects of dietary fat and L-carnitine on plasma and whole blood taurine concentrations and cardiac function in healthy dogs fed protein-restricted diets. Am. J. Vet. Res. 2001, 62, 1616–1623. [Google Scholar] [CrossRef]
- Maia, A.; Batista, T.; Victorio, J.; Clerici, S.; Delbin, M.; Carneiro, E.; Davel, A. Taurine Supplementation Reduces Blood Pressure and Prevents Endothelial Dysfunction and Oxidative Stress in Post-Weaning Protein-Restricted Rats. PLoS ONE 2014, 9, e105851. [Google Scholar] [CrossRef]
- Ahmadi, M.; Askari, V.; Shahri, B.; Noghab, S.M.M.; Jarahi, L.; Rahimi, V.B. Omega-3 fatty acids effectively mitigate high-sensitivity C-reactive protein (hs-CRP) biomarker of inflammation in acute myocardial infarction patients: A randomized, double-blind, placebo-controlled clinical trial. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 881–890. [Google Scholar] [CrossRef]
- Anderson, J.; Randles, K.; Kendall, C.; Jenkins, D. Carbohydrate and Fiber Recommendations for Individuals with Diabetes: A Quantitative Assessment and Meta-Analysis of the Evidence. J. Am. Coll. Nutr. 2004, 23, 17–25. [Google Scholar] [CrossRef]
- Silva, F.; Kramer, C.; Crispim, D.; Azevedo, M. A high-glycemic index, low-fiber breakfast affects the postprandial plasma glucose, insulin, and ghrelin responses of patients with type 2 diabetes in a randomized clinical trial. J. Nutr. 2015, 145, 736–741. [Google Scholar] [CrossRef]
- Moreira, F.D.; Reis, C.E.G.; Gallassi, A.; Welker, A. Postprandial hyperglycemia in patients with type 2 diabetes is reduced by raw insoluble fiber: A randomized trial. Nutr. Metab. Cardiovasc. Dis. NMCD 2023, 34, 2673–2679. [Google Scholar] [CrossRef]
- Blanchard, T.; Eppe, J.; Mugnier, A.; Delfour, F.; Meynadier, A. Enhancing cognitive functions in aged dogs and cats: A systematic review of enriched diets and nutraceuticals. GeroScience 2025, 47, 2925–2947. [Google Scholar] [CrossRef]
- Taha, A.; Henderson, S.; Burnham, W. Dietary Enrichment with Medium Chain Triglycerides (AC-1203) Elevates Polyunsaturated Fatty Acids in the Parietal Cortex of Aged Dogs: Implications for Treating Age-Related Cognitive Decline. Neurochem. Res. 2009, 34, 1619–1625. [Google Scholar] [CrossRef]
- Hall, J.; Jewell, D. Feeding Healthy Beagles Medium-Chain Triglycerides, Fish Oil, and Carnitine Offsets Age-Related Changes in Serum Fatty Acids and Carnitine Metabolites. PLoS ONE 2012, 7, e49510. [Google Scholar] [CrossRef]
- Stavrinou, P.; Andreou, E.; Aphamis, G.; Pantzaris, M.; Ioannou, M.; Patrikios, I.; Giannaki, C. The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment. Nutrients 2020, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.C.; Moore, H.B.; Overbey, D.M.; Morton, A.P.; Harnke, B.; Gerich, M.E.; Vogel, J.D. Fecal microbiota transplant in patients with Clostridium difficile infection: A systematic review. J. Trauma. Acute Care Surg. 2016, 81, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Green, J.E.; Davis, J.A.; Berk, M.; Hair, C.; Loughman, A.; Castle, D.; Athan, E.; Nierenberg, A.A.; Cryan, J.F.; Jacka, F.; et al. Efficacy and safety of fecal microbiota transplantation for the treatment of diseases other than Clostridium difficile infection: A systematic review and meta-analysis. Gut Microbes 2020, 12, 1854640. [Google Scholar] [CrossRef] [PubMed]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Improvement in Clinical Symptoms and Fecal Microbiome After Fecal Microbiota Transplantation in a Dog with Inflammatory Bowel Disease. Vet. Med. 2019, 10, 197–201. [Google Scholar] [CrossRef]
- Fehily, S.R.; Basnayake, C.; Wright, E.K.; Kamm, M.A. Fecal microbiota transplantation therapy in Crohn’s disease: Systematic review. J. Gastroenterol. Hepatol. 2021, 36, 2672–2686. [Google Scholar] [CrossRef]
- Pereira, G.Q.; Gomes, L.A.; Santos, I.S.; Alfieri, A.F.; Weese, J.S.; Costa, M.C. Fecal microbiota transplantation in puppies with canine parvovirus infection. J. Vet. Intern. Med. 2018, 32, 707–711. [Google Scholar] [CrossRef]
- Karra, D.A.; Suchodolski, J.S.; Newman, S.J.; Flouraki, E.; Lidbury, J.A.; Steiner, J.M.; Xenoulis, P.G. Single Enema Fecal Microbiota Transplantation in Cats With Chronic Enteropathy. J. Vet. Intern. Med. 2025, 39, e70054. [Google Scholar] [CrossRef]
- Rojas, C.; Entrolezo, Z.; Jarett, J.; Jospin, G.; Kingsbury, D.; Martin, A.; Eisen, J.; Ganz, H. Microbiome Responses to Fecal Microbiota Transplantation in Cats with Chronic Digestive Issues. Vet. Sci. 2023, 10, 561. [Google Scholar] [CrossRef]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Fecal microbiota transplantation as a new treatment for canine inflammatory bowel disease. Biosci. Microbiota Food Health 2020, 40, 98–104. [Google Scholar] [CrossRef]
- Li, K.; Zhou, X.; Zhong, Z.; Liu, H.; Li, M.; Peng, G.; Zhou, Z. Indications for canine fecal microbiota transplantation. Thai J. Vet. Med. 2022, 52, 13–21. [Google Scholar] [CrossRef]
- Sugita, K.; Shima, A.; Takahashi, K.; Ishihara, G.; Kawano, K.; Ohmori, K. Pilot evaluation of a single oral fecal microbiota transplantation for canine atopic dermatitis. Sci. Rep. 2023, 13, 8824. [Google Scholar] [CrossRef]
- Lee, M.A.; Questa, M.; Wanakumjorn, P.; Kol, A.; McLaughlin, B.; Weimer, B.; Buono, A.; Suchodolski, J.; Marsilio, S. Safety profile and effects on the peripheral immune response of fecal microbiota transplantation in clinically healthy dogs. J. Vet. Intern. Med. 2024, 38, 1425–1436. [Google Scholar] [CrossRef]
- Hanifeh, M.; Scarsella, E.; Rojas, C.; Ganz, H.; Huhtinen, M.; Laine, T.; Spillmann, T. Oral Fecal Microbiota Transplantation in Dogs with Tylosin-Responsive Enteropathy—A Proof-of-Concept Study. Vet. Sci. 2024, 11, 439. [Google Scholar] [CrossRef]
- Kalita, J.; Prasad, A.; Shukla, S.K.; Verma, P.; Arora, N.; Singh, J.L. Faecal Microbiota Transplantation in Canine Parvoviral Diarrhoea. Indian J. Anim. Res. 2024, 58, 1799–1803. [Google Scholar] [CrossRef]
- Brown, R.; Barko, P.; Romero, J.R.; Williams, D.; Gochenauer, A.; Nguyen-Edquilang, J.; Suchodolski, J.; Pilla, R.; Ganz, H.; Lopez-Villalobos, N.; et al. The effect of lyophilised oral faecal microbial transplantation on functional outcomes in dogs with diabetes mellitus. J. Small Anim. Pract. 2025, 66, 567–581. [Google Scholar] [CrossRef]
- Collier, A.; Gómez, D.; Monteith, G.; Plattner, B.; Verbrugghe, A.; Webb, J.; Weese, J.; Blois, S. Investigating fecal microbial transplant as a novel therapy in dogs with inflammatory bowel disease: A preliminary study. PLoS ONE 2022, 17, e0276295. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, A.; Stübing, H.; Suchodolski, J.; Pilla, R.; Unterer, S.; Busch, K. Comparing treatment effects on dogs with acute hemorrhagic diarrhea syndrome: Fecal microbiota transplantation, symptomatic therapy, or antibiotic treatment. J. Am. Vet. Med. Assoc. 2024, 262, 1657–1665. [Google Scholar] [CrossRef]
- Cerquetella, M.; Marchegiani, A.; Rossi, G.; Trabalza-Marinucci, M.; Passamonti, F.; Isidori, M.; Rueca, F. Case Report: Oral Fecal Microbiota Transplantation in a Dog Suffering From Relapsing Chronic Diarrhea—Clinical Outcome and Follow-Up. Front. Vet. Sci. 2022, 9, 893342. [Google Scholar] [CrossRef] [PubMed]
- Vecchiato, C.; Sabetti, M.; Sung, C.; Sportelli, F.; Delsante, C.; Pinna, C.; Alonzo, M.; Erba, D.; Suchodolski, J.; Pilla, R.; et al. Effect of faecal microbial transplantation on clinical outcome, faecal microbiota and metabolome in dogs with chronic enteropathy refractory to diet. Sci. Rep. 2025, 15, 11957. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.; Entrolezo, Z.; Jarett, J.; Jospin, G.; Martin, A.; Ganz, H. Microbiome Responses to Oral Fecal Microbiota Transplantation in a Cohort of Domestic Dogs. Vet. Sci. 2024, 11, 42. [Google Scholar] [CrossRef]
- Van Mulders, L.; Vanden Broecke, E.; De Paepe, E.; Mortier, F.; Vanhaecke, L.; Daminet, S. Metabolomics reveals alterations in gut-derived uremic toxins and tryptophan metabolism in feline chronic kidney disease. Vet. Q. 2025, 45, 1–15. [Google Scholar] [CrossRef]
- Association of American Feed Control Officials (AAFCO). AAFCO Official Publication; Association of American Feed Control Officials: Champaign, IL, USA, 2024; Available online: https://www.aafco.org/resources/publications/ (accessed on 20 October 2025).
- FEDIAF European Pet Food Industry Federation. FEDIAF Nutritional Guidelines; European Pet Food Industry Federation: Brussels, Belgium, 2023; Available online: https://europeanpetfood.org/self-regulation/nutritional-guidelines/ (accessed on 20 October 2025).
| Species | Indication | Delivery Route | Dose and Frequency | Donor Screening (Pathogens/AMR) | Preparation (Fresh/Frozen; Vehicle; Anaerobiosis) | Peri-Procedural Regimen (Pre-Treatment and Co-Therapy) | Follow-Up (Weeks) | Engraftment/Biomarkers | Adverse Events | Clinical Outcomes and Durability |
|---|---|---|---|---|---|---|---|---|---|---|
| Cat [109] | Chronic digestive signs | Oral capsule | 50 capsules over 2 weeks | Not detailed | Lyophilized, oral capsule; frozen | None reported | 2 weeks | ASVs shared with donor; taxa shifts | None reported | Microbiota shifted toward healthy; varied by signs/diet |
| Dog [112] | Atopic dermatitis | Oral | Single dose | Yes (not detailed) | Not specified | None | 8 weeks | Shared ASVs correlated with clinical improvement | None reported | Improved CADESI and PVAS scores |
| Dog [113] | Healthy (safety study) | Rectal enema | Single 5 g/kg dose | Yes | Fresh, enema | None | 4 weeks | No change in dysbiosis index or immune markers | Mild (vomiting, diarrhea) | Safe, no immunologic impact |
| Dog [105] | IBD | Rectal enema | Repeated over months | Yes | Fresh, enema | Steroids previously | Long-term | Microbiota resembled donor | None | Symptoms improved; no adverse events |
| Dog [110] | IBD | Rectal enema | Single (unclear frequency) | Not specified | Fresh, enema | Not specified | Weeks | Fusobacterium increased | None | Clinical signs improved |
| Dog [114] | Tylosin-responsive enteropathy | Oral capsule | Daily ×4 weeks | Yes | Oral capsules; frozen | Tylosin restarted prior | 8 weeks | 30% donor strain engraftment | None reported | Higher response vs. placebo, not significant |
| Dog [115] | Parvoviral diarrhea | Rectal enema | Daily ×8 days | Not detailed | Not specified | Symptomatic therapy | 8 days + 2 month | Improved transplant retention | 1 fever, 1 epistaxis | Faster resolution, fewer relapses |
| Dog [116] | Diabetes mellitus | Oral capsule | Daily (duration unclear) | Yes | Lyophilized oral capsules | Insulin | 60 days | SCFA, bile acids, Faecalibacterium ↑ | None | Decreased water intake; mild benefit |
| Dog [117] | IBD | Rectal enema | Single | Yes | Frozen enema | Steroid + diet | 30 days | No microbiota shift; safe | None | CCECAI↓ in both FMT and placebo |
| Dog [118] | AHDS | Rectal enema | Single | Yes | Not detailed | None | 42 days | More stable DI than antibiotics | None | FMT not faster but more microbiota-stable |
| Dog [119] | Chronic diarrhea | Oral capsule | Multiple over time | Not detailed | Frozen capsules | Steroids | 18 months | Symptoms improved; steroid-free | None | Long-term control, no relapses |
| Dog [120] | Chronic enteropathy | Rectal enema | Single | Yes | Not specified | None | 12 months | CIBDAI ↓; propionate & bile acid metabolism ↑ | None | Durable improvement in 50% |
| Dog [121] | Chronic GI signs | Oral capsule | 50 capsules | Yes | Lyophilized oral capsules | Not specified | 2 weeks | 18% donor ASV engraftment | None | SCFA producers ↑; donor overlap affected outcome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Zhong, S.; Wang, J.; Yu, W. Dietary Modulation of the Gut Microbiota in Dogs and Cats and Its Role in Disease Management. Microorganisms 2025, 13, 2669. https://doi.org/10.3390/microorganisms13122669
Yang B, Zhong S, Wang J, Yu W. Dietary Modulation of the Gut Microbiota in Dogs and Cats and Its Role in Disease Management. Microorganisms. 2025; 13(12):2669. https://doi.org/10.3390/microorganisms13122669
Chicago/Turabian StyleYang, Benlu, Shengwei Zhong, Jue Wang, and Wanting Yu. 2025. "Dietary Modulation of the Gut Microbiota in Dogs and Cats and Its Role in Disease Management" Microorganisms 13, no. 12: 2669. https://doi.org/10.3390/microorganisms13122669
APA StyleYang, B., Zhong, S., Wang, J., & Yu, W. (2025). Dietary Modulation of the Gut Microbiota in Dogs and Cats and Its Role in Disease Management. Microorganisms, 13(12), 2669. https://doi.org/10.3390/microorganisms13122669




