Journal Menu
► ▼ Journal Menu-
- Metabolites Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserNeed Help?
Announcements
17 May 2024
Tu Youyou Award—Open for Nominations
In acknowledgment of the groundbreaking achievements of Professor Tu Youyou, the Nobel laureate credited with the discovery of artemisinin, MDPI is proud to announce the Tu Youyou Award. This award, inaugurated in 2016, seeks to honor scholars who excel in the fields of natural products chemistry and medicinal chemistry.
Prize:
- CHF 100,000 in total (If there is more than one winner, the prize will be divided equally amongst the winners);
- An award medal for each winner.
Who should be nominated?
- Scientists with outstanding achievements and contributions in the fields of natural products chemistry and medicinal chemistry.
Who can submit a nomination?
- Academic research institutes
- Universities
- Academic societies
Any personal nominations are not accepted.
How do I submit a nomination?
Candidates’ institutional affiliations need to submit their nominations for final candidates to the Tu Youyou Award Team directly by email after internal screening, and each institution can only nominate a maximum of two candidates for each edition of the Tu Youyou Award. Please note that the nominations cannot be modified once they are submitted and confirmed by the Tu Youyou Award Team.
Required nomination materials:
Nomination Form (Download)
Schedule:
Nomination deadline: 31 December 2024;
Winner announcement: 30 April 2025.
Contact:
Tu Youyou Award Team (tuyouyouaward@mdpi.com)
More information can be found on the Tu Youyou Award official website. For any inquiries, do not hesitate to contact the Tu Youyou Award Team.
16 May 2024
MDPI Romania Author Training Academic Events in April
MDPI Romania sponsored four events over the month of April, contributing author training sessions to each event.
The NutriSciLabs 2024 conference was held at the Carol Davila University of Medicine and Pharmacy on 12 April 2024. Organized by the Association of Students from the Faculty of Pharmacy Studies, the conference aimed to enhance students’ academic writing and research skills, and bolster their confidence in participating in the academic world. Ioana Paunescu, journal relations specialist for MDPI Romania, led the training session. Paunescu first outlined MDPI’s history and core values, then explained academic writing techniques, ethics, and similarity percentages. The presentation covered the entire editorial process, highlighting the amount of attention paid to details throughout manuscript processing. Paunescu also discussed common errors that authors make while writing, and how to avoid such errors.
On 13 April 2024, the NextGEN 2.0 Student Conference took place at the Babes-Bolyai University of Cluj-Napoca. NextGen Healthcare organized this event with the university’s European Students’ Society to discuss European and international current affairs. MDPI Romania contributed an author training session called Steps of the Publication Process. Irina Codruta Zaharia, journal relations specialist, began the session with a general introduction of MDPI before diving into different article types, different layout formats, possible journals to submit to, the MDPI submission checklist, and the MDPI editorial process. By explaining the fundamentals of writing and publishing academic papers, Zaharia imparted valuable industry knowledge to the curious minds present. Participants were eager to engage with Zaharia in the subsequent Q&A session.

Celebrating its 20th anniversary this year, the International Conference “Students for Students” has long been a gathering place for undergraduate and postgraduate students of all nationalities to present their work and exchange ideas with each other. This year’s conference was held on 18 April 2024, with MDPI Romania contributing an author training session called The World of Open Access. Key speakers included Dr. Norbert Kiss from MDPI Romania and Prof. Dragos Horvath from Strasbourg University. Dr. Kiss discussed the history of open access, differences between open access publishing and traditional publishing, and implications for open access in the future. Attendees expressed keen interest in the subject, approaching Dr. Kiss with questions about academic publishing as well as his career trajectory.

Most recently, on 26 April 2024, MDPI Romania sponsored the National Symposium of Students from Geology and Geophysics Faculties. The AAPG Students Chapter at the Babes-Bolyai University organized this event as an opportunity for geology students from all Romanian universities to present their work and confer with other researchers. Dr. Kiss gave his lecture The World of Open Access at this event as well.

MDPI is thankful to all the participants, speakers, and organizers who attended these events. Through their enthusiasm and dedication, these events were great successes.
11 May 2024
Metabolites | Highly Cited Papers in 2021–2023 in Web of Science
1. “L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine”
*Editor’s Choice Paper
by Marc R. McCann, Mery Vet George De la Rosa, Gus R. Rosania and Kathleen A. Stringer
Metabolites 2021, 11(1), 51; https://doi.org/10.3390/metabo11010051
Available online: https://www.mdpi.com/2218-1989/11/1/51
2. “Therapeutic and Toxic Effects of Valproic Acid Metabolites”
by Natalia A. Shnayder, Violetta V. Grechkina, Aiperi K. Khasanova, Elena N. Bochanova, Evgenia A. Dontceva, Marina M. Petrova, Azat R. Asadullin, German A. Shipulin, Kuanysh S. Altynbekov, Mustafa Al-Zamil et al.
Metabolites 2023, 13(1), 134; https://doi.org/10.3390/metabo13010134
Available online: https://www.mdpi.com/2218-1989/13/1/134
3. “Gut Microbiota Metabolites in Major Depressive Disorder—Deep Insights into Their Pathophysiological Role and Potential Translational Applications”
by Miguel A. Ortega, Miguel Angel Alvarez-Mon, Cielo García-Montero, Oscar Fraile-Martinez, Luis G. Guijarro, Guillermo Lahera, Jorge Monserrat, Paula Valls, Fernando Mora, Roberto Rodríguez-Jiménez et al.
Metabolites 2022, 12(1), 50; https://doi.org/10.3390/metabo12010050
Available online: https://www.mdpi.com/2218-1989/12/1/50
4. “Guide to Metabolomics Analysis: A Bioinformatics Workflow”
by Yang Chen, En min Li and Li-Yan Xu
Metabolites 2022, 12(4), 357; https://doi.org/10.3390/metabo12040357
Available online: https://www.mdpi.com/2218-1989/12/4/357
5. “The Potential of Metabolomics in Biomedical Applications”
by Vanessa Gonzalez-Covarrubias, Eduardo Martínez-Martínez and Laura del Bosque-Plata
Metabolites 2022, 12(2), 194; https://doi.org/10.3390/metabo12020194
Available online: https://www.mdpi.com/2218-1989/12/2/194
6. “Centella asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential”
by Lúcio Ricardo Leite Diniz, Leonardo Luiz Calado, Allana Brunna Sucupira Duarte and Damião Pergentino de Sousa
Metabolites 2023, 13(2), 276; https://doi.org/10.3390/metabo13020276
Available online: https://www.mdpi.com/2218-1989/13/2/276
7. “Obesity-Induced Brain Neuroinflammatory and Mitochondrial Changes”
by Luisa O. Schmitt and Joana M. Gaspar
Metabolites 2023, 13(1), 86; https://doi.org/10.3390/metabo13010086
Available online: https://www.mdpi.com/2218-1989/13/1/86
8. “Signatures of Mitochondrial Dysfunction and Impaired Fatty Acid Metabolism in Plasma of Patients with Post-Acute Sequelae of COVID-19 (PASC)”
by Vamsi P. Guntur, Travis Nemkov, Esther de Boer, Michael P. Mohning, David Baraghoshi, Francesca I. Cendali, Inigo San-Millán, Irina Petrache and Angelo D’Alessandro
Metabolites 2022, 12(11), 1026; https://doi.org/10.3390/metabo12111026
Available online: https://www.mdpi.com/2218-1989/12/11/1026
2 May 2024
MDPI Insights: The CEO's Letter #11 - 2023 Annual Report, MDPI Awards, STM

Welcome to the MDPI Insights: The CEO's Letter.
In these monthly letters, I will showcase two key aspects of our work at MDPI: our commitment to empowering researchers and our determination to facilitating open scientific exchange.
Opening Thoughts

2023 Annual Report
This is an exciting time of year at MDPI, as we have just released our annual report 2023, recapping the past year and sharing the progress and changes that took place during it.

Stefan Tochev (CEO, MDPI)
Reflecting on 2023, I can't help but think of the changes that have occurred not only at MDPI but also within our industry as a whole. In light of this, I’m reminded that change is the only constant, and that for a publishing enterprise that has experienced rapid growth, adapting to change becomes not only a necessity but also a catalyst for sustained success.
Looking back on the significant ground we covered in 2023, I am pleased to report that the year was marked by a rise in paper submissions, along with a range of initiatives aimed at improving our internal processes and delivering top services to our scientists.
2023 Top-line MDPI Numbers
An important priority for 2023 was to strengthen our editorial policies. Despite a notable increase in the number of papers submitted from 603,000 to 655,000 (+8.6%), there was a decrease in the number of papers published from 303,000 to 285,000 (-5.9%), consistent with the overall trend in the scholarly publishing market. MDPI’s market share in gold open access articles published reached 17% in 2023 (according to Dimensions data).
2023 Open Access Numbers
For over two decades, MDPI has been at the forefront of reshaping the academic publishing landscape, with OA surpassing subscription-based publishing in 2020. This trajectory is deeply rooted in our history and reflects our unwavering commitment to, and vision for, an open future. This momentum continued in 2023, with 39% of the 4.16 million articles and reviews published as gold full-OA, 15% as gold hybrid-OA, 8% as bronze-OA, and 3% as green-OA, relative to 35% behind a paywall. In terms of gold OA (full or hybrid) articles and reviews published, MDPI leads the way in terms of total articles published in 2023.
MDPI is the trusted and preferred OA publisher for the scholarly community
However, in 2023, we saw a downward trend in publication numbers compared to 2022. This trend was also seen in total OA publications. While the number of submissions increased, the decline in MDPI publications can be attributed to several factors, such as improved scrutiny in our peer-review process, including the evaluation of content scope and higher rejection rates, and a post-pandemic decrease in research related to COVID-19 papers.
Download 2023 MDPI Annual Report.
Access 2023 Digital Report.
Impactful Research

Recognizing Scholars – MDPI Awards
MDPI is committed to empowering young researchers as they embark on their careers. In 2023, our journals hosted a total of 400 awards, receiving 8,839 applications and nominations. The evaluation committees selected 959 winners, with the total budget for these awards amounting to CHF 546,500.
For more information about MDPI awards, applications, and winners, please click here
The following awards recognize scholars and the impact of their research, including Young Investigator Awards, Best PhD Thesis Awards, and Travel Awards for junior researchers.
MDPI Awards – The following awards require an application or a nomination.
Young Investigator Award (CHF 1000–2000 for each winner)
This award acknowledges the achievements of young investigators in research areas relevant to the journal’s scope. Candidates must have received their PhD no more than 10 years prior to the award announcement date and must be nominated.
Best PhD Thesis Award (CHF 500–800 for each winner)
This award recognizes young scholars who have completed outstanding PhD theses in research areas relevant to the journal’s scope. It aims to encourage them to continue their excellent work and make further contributions to their field.
Travel Award (CHF 500–800 for each winner)
This award encourages junior scientists to present their latest research at academic conferences relevant to the journal’s scope, thereby increasing their impact.
Distinguished Scholars
Additionally, 220 awards were granted to the most distinguished authors and reviewers in our journals through Best Paper Awards and Outstanding Reviewer Awards. The following awards are selected by the editorial staff of the journal and do not require an application or a nomination. They exemplify the excellent contributions made by our authors, reviewers, and editors.
These awards exemplify the excellent contributions made by our authors, reviewers, and editors
Best Paper Award (CHF 200–500 for each winner)
This award is granted annually to highlight publications of high quality, scientific significance, and extensive influence.
Outstanding Reviewer Award (CHF 500 for partial winners)
This award is given annually to recognize reviewers who generously contribute their time to reviewing papers and demonstrate thoroughness, professionalism, and timeliness in their reviews.
MDPI also offers awards for specific topics in various research fields:
Carbon Neutrality Award (CHF 500–800 for each winner)
Sponsored by MDPI journal Sustainability, this award recognizes applicants who have made exceptional academic or societal contributions to carbon neutrality, either in general or in relation to a specific carbon-neutrality-related issue.
Intelligent Manufacturing Award (CHF 500–800 for each winner)
Administered by MDPI journal Machines, this award is presented to an individual who has made outstanding academic or societal contributions to the field of intelligent manufacturing.
Granted: World Sustainability Awards
Inside MDPI

MDPI Opens Office in Seoul, Korea and Appoints Claude Seo as Office Manager
As CEO of the world’s leading OA publisher, I am pleased to announce the official opening of our Korean branch office. With this addition, MDPI now operates 21 offices in 12 countries worldwide, improving on our position as a truly global publisher. This expansion represents more than just an increase in our physical footprint; it is a pivotal step in our ongoing commitment to making scientific knowledge more accessible across the globe.
MDPI now operates 21 offices in 12 countries worldwide, improving on our position as a truly global publisher

Claude Seo (MDPI Korea Office Manager, Seoul)
Claude Seo, who has over 15 years of experience in the academic publishing industry, has been appointed as the Office Manager of the Seoul office. The launch of our Seoul office allows us to better support the Korean scientific community and to further promote the publication of OA journals in the region.
Reflecting on his role, Claude shared that he is:
“Delighted to have been entrusted with this position within MDPI, the no. 1 scholarly OA publisher. As we establish our presence in Korea, we are dedicated to integrating ourselves into the local community and contributing to its vibrant culture. Our commitment goes beyond business growth; it is about creating a more informed and innovative society by embracing and promoting diversity within the scientific community.”
Sungkyunkwan University Joins MDPI’s Institutional Open Access Program
Additionally, I am pleased to share that Sungkyunkwan University has joined MDPI’s Institutional Open Access Program (IOAP). This program offers free access to MDP’s online submission system for institutions and provides APC discounts to affiliated authors. Thirteen major Korean universities, including Sungkyunkwan University, Kyunghee University, Chung-Ang University, and the Catholic University of Korea, have adopted IOAP in Korea.
Learn more about MDPI’s collaboration in Korea in my previous CEO Letter, in which I recap our visit to Seoul, South Korea.
Coming Together for Science

4th MMCS: Harnessing the Power of New Drug Modalities
Our conference team successfully managed the 4th Molecules Medicinal Chemistry Symposium, held from 24–26 April in Barcelona, Spain. It was chaired by Prof. Dr. Diego Muñoz-Torrero from the Institute of Biomedicine (IBUB), University of Barcelona, Spain, and Prof. Dr. Simona Collina from the University of Pavia, Italy. In total, there were 84 accepted abstracts and 102 conference attendees from 22 different countries.

From left to right: Prof. Dr. Rino Ragno (Scientific Committee), Dr. Maria Emilia Sousa (Scientific Committee), Prof. Dr. Claudio Viegas Jr. (Scientific Committee), Prof. Dr. Simona Collina (Chair), Prof. Dr. Diego Muñoz-Torrero (Chair), Alvina Wu (Managing Editor, MDPI), Prof. Dr. Roman Dembinski (Scientific Committee), Prof. Dr. Mariana Spetea (Scientific Committee).
The event comprised 12 Invited Speakers, 35 Selected Talks, 10 Flash Poster Presentations, and 39 Posters. The overarching topic of the conference was the impact of the emergence of new drug modalities on drug discovery, with thematic sessions covering topics such as photoactivatable drugs, candidates targeting RNA and epigenetic targets, covalent modifiers, and the development of new anti-cancer agents, among other medicinal chemistry projects.

We are pleased to have received feedback from attendees highlighting the professional approach of the conference chairs and participants, the high quality of the talks, and the overall outstanding organization on the part of the MDPI conference team.
Thank you to the conference sponsors, Fluorochem and IBUB, and to our partnering societies, The Spanish Society for Biochemistry and Molecular Biology (SEBBM), and the Spanish Society of Medicinal Chemistry (SEQT).
Upcoming In-Person Event

28–31 May, 2024
Polymers 2024 – Polymers for a Safe and Sustainable Future
Location: Athens, Greece
Conference Chairs: Prof. Dr. Dimitrios Bikiaris, Prof. Dr. Konstantinos Triantafyllidis, Dr. Ioanna Deligkiozi
We look forward to welcoming experts Prof. Damià Barceló, Prof. Minna Hakkareinen, and Prof. Armando J. D. Silvestre to this event.
Find more upcoming MDPI events here.
Organize Your Event with MDPI’s Sciforum
Sciforum is MDPI’s platform dedicated to the organization of scientific events. In line with our mission to promote science, Sciforum supports scholars, societies, research networks, and universities at all stages of organizing in-person events, virtual events and webinars. Our platforms are efficient, user-friendly, and cost-effective. We handle all steps related to event management. Contact us for details.
Closing Thoughts

A Report from the Future – STM US Annual Conference 2024

Stefan Tochev (CEO, MDPI), Dr. Giulia Stefenelli and Dr. Ioana Craciun from MDPI’s Scientific Office Board.
MDPI has for a long time been a sponsor of the STM Annual Conferences, held yearly in the US and Frankfurt, and is a trusted partner and supporter of the STM organization. While I have attended the Frankfurt conference for the past three years, this was my first time visiting the Washington, DC session, and I am glad I did.
Although it was a brief trip, I greatly appreciated the opportunity to attend in the company of my colleagues Dr. Giulia Stefenelli and Dr. Ioana Craciun from MDPI’s Scientific Office Board. They always help map some of the new industry trends against MDPI’s operational framework and guidelines. It’s a great group for kicking ideas back and forth on what we can apply at MDPI.
In my experience, STM never fails to deliver. It’s always a great conference for reconnecting with fellow publishers, industry friends, and vendors. As usual, STM this year curated a diverse range of speakers and panels, who proferred valuable knowledge and insights from outside our industry, delivering thought-provoking insights into our field. An example of this was the ‘Trust Panel’ session, which included Alan Schiffres (Managing Director, InfoLinx), who shared a number of the learnings about fraud and risk management from his 40 years in financial services, to help address some of the challenges we are currently facing in the area of publishing integrity.
Launch of STM Trends 2028 Panel
I particularly enjoyed the ‘Launch of STM Trends 2028 Panel’, which presented a report focused on the integration of humans and machines in scholarly publishing. The session highlighted themes such as AI, digitization, and the evolving research ecosystem. The report envisions a future where technology blurs the lines between human and machine involvement in research processes, with significant implications for trust, reputation, and equity. While this presents opportunities for connectivity and knowledge dissemination, it also poses challenges such as disinformation, fragmentation, and geopolitical tensions. We must rely on a wise combination of technology and human agency to navigate this complex landscape and will have to carefully examine its potential impacts on communication and scholarly publishing.
MDPI has joined the STM Integrity Hub. Click here to learn more.
While every presenter brought their own knowledge and personal touch, I was particularly pleased to encounter new speakers such as Heather Whitney (Research Assistant Professor, Radiology, University of Chicago) and Igor Grossmann (Professor of Psychology, University of Waterloo, Canada). Having studied Sociology at the University of Guelph, I have a soft spot for fellow social scientists from Canada, and I was very impressed by Igor’s presentation on ‘The Social Scientist: A View from the Future’ and his participation in the panel session ‘The Future Beyond the Article,’ which was one of my favourites from the conference, given the diversity of perspectives from the panel speakers.
Memorial Park in Washington, DC

On a personal note, this was my first-time visiting Washington, DC, and I’m glad we took an afternoon to visit the Memorial Park, with its incredible monuments to figures ranging from Martin Luther King, Jr. to Abraham Lincoln. The magnitude of the monuments is deeply impressive. I particularly appreciated the Thomas Jefferson memorial and the following quote, which I think speaks to the importance of change and adaptation. It’s something we can apply not only in our industry but also in our personal lives.
"I am not an advocate for frequent changes in laws and constitutions, but laws and institutions must go hand in hand with the progress of the human mind. As that becomes more developed, more enlightened, as new discoveries are made, new truths discovered and manners and opinions change, with the change of circumstances, institutions must advance also to keep pace with the times. We might as well require a man to wear still the coat which fitted him when a boy as a civilized society to remain ever under the regimen of their barbarous ancestors."
– Excerpted from a letter to Samuel Kercheval, July 12, 1816.
Chief Executive Officer
MDPI AG
30 April 2024
MDPI Opens Office in Seoul, Korea and Appoints Claude Seo as Office Manager

MDPI, the leading Open Access (OA) publisher, announced on Monday that it opened its Korean branch office on 29th April 2024. With this addition, MDPI now operates 21 offices in 12 countries worldwide. Claude Seo, with over 20 years of experience in the academic journal publishing industry, including Nature Publishing Group (NPG), has been appointed as the Office Manager of the Seoul office.
With the establishment of a Seoul office, MDPI aims to actively support Korea scientific communities to further promote the publication of OA journals in the region. As of the end of 2023, Korea ranked sixth worldwide in both submissions and publications of MDPI research papers. MDPI is the number one OA publisher in Korea.
Sungkyunkwan University Joins MDPI’s Institutional Open Access Program
In addition, MDPI announced that Sungkyunkwan University joined MDPI’s Institutional Open Access Program (IOAP). This program offers free access to MDPI online submission system for the institutions and APC discounts to affiliated authors. Thirteen major Korean universities, including Sungkyunkwan University, Kyung Hee University, Chung-Ang University and Catholic University of Korea, have adopted IOAP in Korea.
OA aims to break down barriers that have traditionally restricted access to science, ensuring that knowledge is available to all, regardless of financial situation or institutional affiliations. Authors, academia, and scientific communities are rapidly moving toward OA. MDPI has been at the forefront of reshaping the academic publishing landscape, with OA surpassing subscription-based publishing in 2020.
MDPI CEO Visits Seoul, Korea
In March, Stefan Tochev, Chief Executive Officer (CEO) of MDPI, visited Korea and met with various stakeholders including government agencies, research and academic institutions, universities. During his visit, Stefan highlighted that MDPI continues to pave the way for a world where science is accessible to all, supporting a global community of inclusive innovation and collaborative solutions.
Stefan expressed excitement about the new venture, stating, "Today marks a significant milestone for MDPI as we celebrate the opening of our new office in Seoul, Korea. This expansion is more than just an increase in our physical footprint, it is a pivotal step in our ongoing commitment to making scientific knowledge more accessible, participatory, and inclusive across the globe."
Claude Seo, reflecting on his new role, commented, "I am delighted to have been offered this position of trust within MDPI, the no. 1 scholarly OA publisher. As we establish our presence in Korea, we are dedicated to integrating into the local community and contributing to its vibrant culture. Our commitment goes beyond business growth; it is about creating a more informed and innovative society by embracing and promoting diversity within the scientific community.”
For further inquiries, please contact our Seoul office directly.
About MDPI
A pioneer in scholarly, open access publishing, MDPI has supported academic communities since 1996. MDPI is leading the transition to open science by making more research free and accessible to everyone. Over 3.3 million researchers have entrusted MDPI with publishing their scientific discoveries. MDPI’s editorial process is bolstered by a network of dedicated reviewers, a team of 6000 diligent, well-trained staff members, and an in-house article submission platform that was designed to ensure efficient processes within its 430 fully OA titles.
2 April 2024
MDPI Insights: The CEO's Letter #10 - South Korea, IWD, U2A, Japan

Welcome to the MDPI Insights: The CEO's Letter.
In these monthly letters, I will showcase two key aspects of our work at MDPI: our commitment to empowering researchers and our determination to facilitating open scientific exchange.
Opening Thoughts

Left to right: Dr. Jisuk Kang (Scientific Officer, MDPI), Stefan Tochev (CEO, MDPI), and Dr. Giulia Stefenelli (Chair of Scientific Office Board, MDPI), during media meetings at Prain Agency office in Seoul, South Korea.
Visit to Seoul, South Korea
During my recent visit to South Korea, I had the privilege of meeting various stakeholders, including representatives of government, research institutions, and academia, to understand their needs and communicate MDPI’s commitment to accessible science. Accompanied by my colleagues Dr. Giulia Stefenelli and Dr. Jisuk Kang, I engaged with the Korean scientific community, which is increasingly embracing open access (OA).
As the leading OA publisher in South Korea, MDPI is trusted by local authors and in 2023 enjoyed an approximately 30% share of the OA market. South Korea ranks sixth globally for MDPI in terms of research papers submitted and published.
MDPI and South Korea by Numbers
As at 30 March, over 76,000 MDPI articles have been authored by individuals affiliated with Korean institutions. We have over 1,800 active editorial board members (EBMs) from South Korea, with more than 880 EBMs having an H-index between 26 and 50, including 10 serving as Chief Editors.
“South Korea is the sixth-largest contributor to our total publications”
Over the past five years (2019–2023), nearly 120,000 authors affiliated with South Korean institutes have published with MDPI. Specifically in 2023, we received approximately 25,000 submissions from South Korean authors, publishing close to 13,000 articles, resulting in a rejection rate of 47.4%, which is not far below MDPI’s overall rejection rate of 56.4% in 2023.
Institutional Partnerships with South Korea
I am pleased to share that MDPI has more than 825 institutional partnerships worldwide, with 12 in South Korea, including Kyunghee University, Chung-Ang University, and Inha University, among others.

Left to right: Dr. Jisuk Kang (Scientific Officer, MDPI), Dr. Giulia Stefenelli (Chair of Scientific Office Board, MDPI), and Stefan Tochev (CEO, MDPI) fielding media questions at Prain PR Agency office in Seoul, South Korea.
Over the past three years (2021–2023), we have had some of the most prestigious academic universities ranked among the top 10 Korean institutions publishing with MDPI. Seoul National University had the highest number of publications with MDPI during those three years, publishing nearly 6,000 papers. Universities such as Korea University and Yonsei University also rank among the top 10 Korean institutions publishing with MDPI.
MDPI Hosts Seminar for Academia and Media
As the world’s leading OA publisher, MDPI is actively democratizing science. This is reflected in the seminars we hosted on 21 March to address questions about our editorial processes and ethical standards. The visit garnered media coverage, reflecting our mission to providing high-quality services and fostering open dialogue in the community.
“MDPI is actively democratizing science”
MDPI in the News

Stefan Tochev (CEO, MDPI) leading a seminar on OA and MDPI at Prain PR Agency office in Seoul, South Korea.
Media coverage generated by our visit to Seoul included the following stories:
“Open access is an unstoppable trend…it will lead the development of the knowledge ecosystem.”
I greatly appreciate the contributions of everyone who took the time to meet with us, share their stories, and hold us accountable for continuing to provide high-quality publishing services while identifying areas for improvement. I am also excited to announce that we have opened an MDPI office in Seoul and will release a press release on MDPI.com, with details, by the end April 2024. The purpose of the office is to establish a local presence to connect with and support the South Korean academic community through institutional partnerships, conferences, author workshops, stakeholder communications, and more.
Impactful Research
Featured Articles on Women’s Leadership and Healthcare
In celebration of International Women’s Day (8 March 2024), MDPI curated a collection of research articles on various topics, including women’s leadership, reproductive health, preventive healthcare, and a selection of articles from our journal Women.
Women’s Leadership
- Women Entrepreneurship and Sustainable Development: Bibliometric Analysis and Emerging Research Trends
Sustainability 2022, 14, 9160. https://doi.org/10.3390/su14159160 - Refugee Women Business Mentors: New Evidence for Women’s Empowerment
Sustainability 2022, 14, 9154. https://doi.org/10.3390/su14159154 - Women and Leadership in Higher Education: A Systematic Review
Soc. Sci. 2023, 12, 555. https://doi.org/10.3390/socsci12100555 - Understanding Needs and Potentials for Gender-Balanced Empowerment and Leadership in Climate Change Adaptation and Mitigation in Africa
Sustainability 2022, 14, 9410. https://doi.org/10.3390/su14159410 - Challenges Women Experience in Leadership Careers: An Integrative Review
Merits 2023, 3, 366-389. https://doi.org/10.3390/merits3020021
Women’s Reproductive Health
- Recreational Female Athletes’ Understanding of and Perceived Impact of the Menstrual Cycle on Physical Performance, Mood, and Sleeping Behaviour
Women 2023, 3, 445-456. https://doi.org/10.3390/women3030034 - Difficulties in Adaptation of the Mother and Newborn via Cesarean Section versus Natural Birth—A Narrative Review
Life 2023, 13, 300. https://doi.org/10.3390/life13020300 - The Main Theories on the Pathogenesis of Endometriosis
Int. J. Mol. Sci. 2023, 24, 4254. https://doi.org/10.3390/ijms24054254
Women’s Preventive Healthcare
- Insulin Metabolism in Polycystic Ovary Syndrome: Secretion, Signaling, and Clearance
Int. J. Mol. Sci. 2023, 24, 3140. https://doi.org/10.3390/ijms24043140 - Assessing Barriers Encountered by Women in Cervical Cancer Screening and Follow-Up Care in Urban Bolivia, Cochabamba
Healthcare 2022, 10, 1604. https://doi.org/10.3390/healthcare10091604 - Updates on HPV Vaccination
Diagnostics 2023, 13, 243. https://doi.org/10.3390/diagnostics13020243
Featured Articles in MDPI Journal Women
Below are a few articles from Women, our journal focused on women’s health, the social determinants of health, and the healthcare system that serves women. The aim of Women is to encourage academics to publish their experimental and theoretical results in detail, to aid reproducibility, and in an engaging style, to aid comprehensibility.
- Premenstrual Syndrome and Exercise: A Narrative Review
Women 2023, 3, 348-364. https://doi.org/10.3390/women3020026 - Increasing Awareness of the Human Papillomavirus (HPV) Vaccine for Women 18–45 Years of Age
Women 2023, 3, 365-373. https://doi.org/10.3390/women3030027 - Addressing Women’s Needs with Human Immunodeficiency Virus (HIV) and Enhancing the Visibility of Pharmacists in the Public Health Arena
Women 2022, 2, 346-352. https://doi.org/10.3390/women2040032
Inside MDPI

Championing Women’s Healthcare and Access to Healthcare Information
MDPI colleagues from our offices joined in celebrating #IWD2024. In doing so, we emphasized key missions that encompass:
- Empowering women to assume leadership and decision-making roles in both business and science.
- Helping women and girls make informed decisions about their health.
- Recruiting and developing female talent and fostering inclusive workplace environments.
“We are thrilled to recognize the accomplishments of women scientists”
I am proud to see our colleagues enthusiastically supporting the International Women’s Day call to ‘Inspire Inclusion!’ The heart-hands in the collage below symbolize our appreciation of the achievements of women researchers and the recognition of the trailblazers who have courageously made a mark on societies past and present.
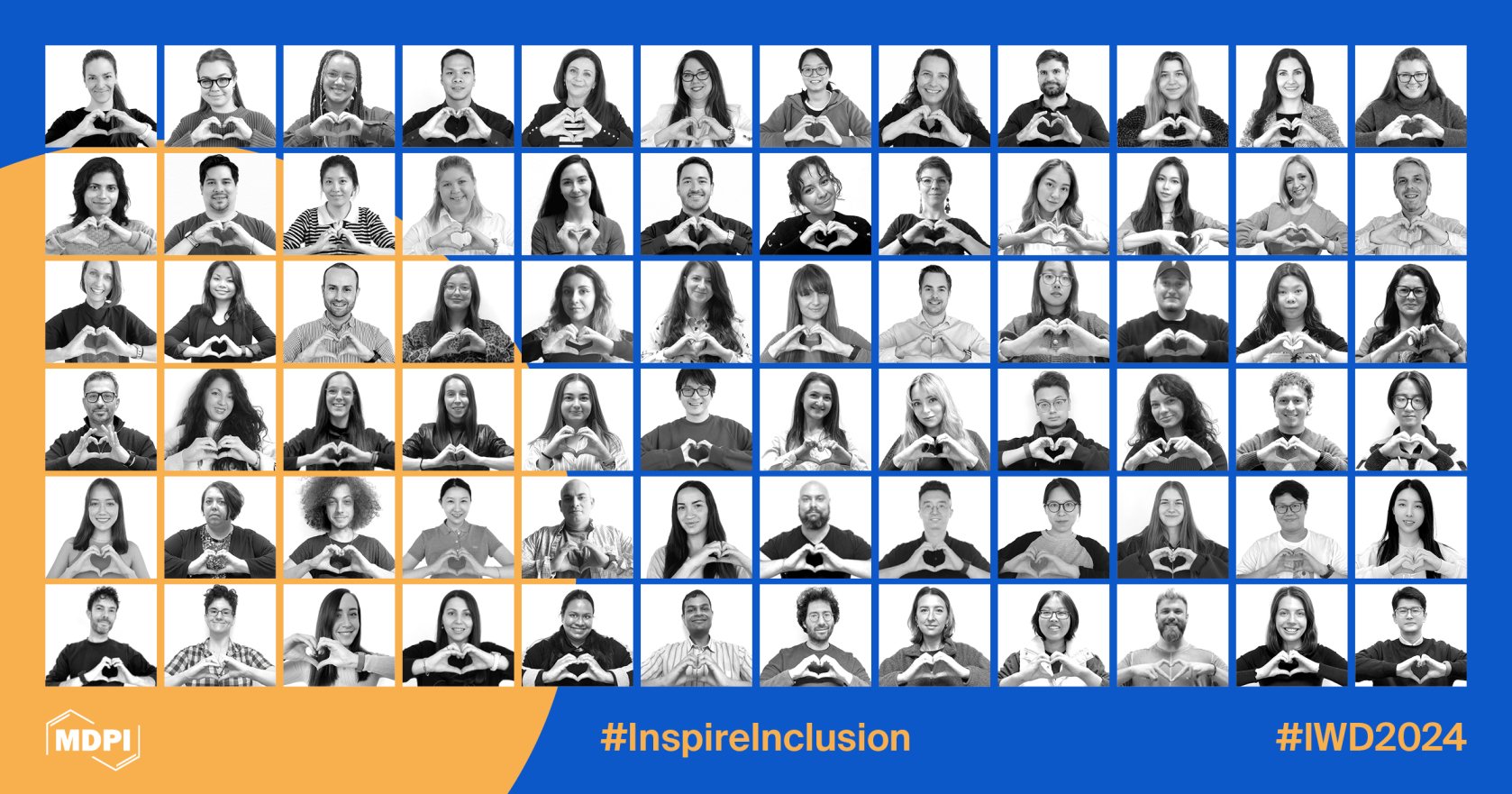
We are thrilled to recognize the accomplishments of women scientists through our many MDPI awards and by highlighting success stories. As inspirational figures, female scientists are paving the way for the next generation of women aspiring to pursue careers in engineering, life sciences, computing, and various other STEM fields.
“I consider myself lucky because I work with incredibly talented women who inspire me every day.”
– Dr. Alessandra Pasut, Winner of MDPI's ‘Biology 2023 Young Investigator Award’
“It’s really important to find a supportive and enabling environment in which to do your science; it would have a big impact on you as a person and on your scientific outputs.”
– Dr. Rhea Longley, Winner of the ‘Pathogens 2023 Young Investigator Award’
Open-access publishing, in particular, allows early-career women researchers to share their work more widely, potentially attracting mentorship opportunities and collaborations. This support is crucial for career development and advancement.
Coming Together for Science

MDPI Joins United2Act in Collective Fight to Stop Paper Mills
In my February 2024 CEO Letter, I highlighted some of our recent initiatives aimed at bolstering our commitment to research integrity, including joining the STM Integrity Hub and expanding our Research Integrity and Publication Ethics team (RIPE). Continuing our efforts in coming together for science, I am pleased to share our participation in the United2Act initiative.
The text below is taken from our official announcement:
United2Act represents an international group of stakeholders in the publishing industry committed to addressing the collective challenge posed to research integrity by paper mills.
Scientists and academic publishers have increasingly noted the alarming proliferation of paper mills, recognized as fraudulent entities seeking to manipulate the publication process for financial profit. These entities engage in fraudulent practices such as falsifying or fabricating data, selling co-authorship of fake papers, manipulating peer review, and including inappropriate citations. These actions pose a significant threat to the integrity of the scholarly record, prompting widespread concern among those involved in the academic community.
MDPI has been actively contributing to combat the undermining of the scientific record. Our editors employ a set of tools to detect potential ethical breaches within a manuscript and to tackle the issue of fake papers.
United2Act’s consensus statement is the outcome of a virtual summit held in May 2023. It involved the participation of research bodies, publishers, researchers/sleuths, universities, and publishing infrastructure from 15 countries and resulted in a Consensus Statement outlining five key areas of action for all stakeholders:
- Education and awareness
- Improve post-publication corrections
- Facilitate and organise research on paper mills
- Enable the development of trust markers
- Facilitate dialogue between stakeholders
MDPI is committed to promoting transparency and integrity in scholarly publishing and is continuing to work closely with the scientific community toward this goal.
Closing Thoughts

Left to right: Ryo Hirayama (Marketing Specialist, MDPI), Takashi Sasabe (Marketing Specialist, MDPI), Dr. Giulia Stefenelli (Chair of Scientific Office Board, MDPI), Dr. Izumi Yamamoto (Marketing Manager, MDPI), and Stefan Tochev (CEO, MDPI), at MDPI’s office in Tokyo, Japan.
Visit to Tokyo and Kyoto, Japan
In March, I had the opportunity to visit our Tokyo office and engage with stakeholders in Tokyo and Kyoto. During the visit, I also recorded a video message to welcome Japanese scholars working with MDPI and to highlight our operations in Japan.
We held meetings with Editors-in-Chief, librarians, scholars, and external consultants to gather feedback on our efforts to enhance our reputation and explore additional steps we can take in that direction. Japan's rich cultural heritage, characterized by tradition, respect, and formality, provided valuable insights into meeting the publishing needs specific to Japan.
Japan’s Open Access statistics
Over the years, we have seen a shift from subscription-only to gold OA publishing in Japan, despite the lack of an official mandate. Here are some statistics:
- 2012: 68% of articles were subscription-only, 6% were green Open Access, and 8% were gold Open Access.
- 2016: 55% of articles were subscription-only, 6% were green Open Access, and 20% were gold Open Access.
- 2022: 43% of articles were subscription-only, 7% were green Open Access, and 39% were gold Open Access.
To learn more about the history of OA in Japan as well as about future trends, please read this blog post.
“Japan is the ninth-largest contributor to our total publications”

Left to right: Stefan Tochev (CEO, MDPI), Dr. Izumi Yamamoto (Marketing Manager, MDPI), and Dr. Giulia Stefenelli (Chair of Scientific Office Board, MDPI) visiting Kyoto University in Kyoto, Japan.
MDPI and Japan by Numbers
As at 1 April, over 50,000 MDPI articles have been authored by scholars affiliated with Japanese institutions, making the country the ninth-largest contributor to our total publications. Over the past three years (2020–2023), nearly 90,000 authors affiliated with Japanese institutes have published with MDPI, and we have collaborated with over 4,600 Guest Editors from Japan.
In 2023, we published over 8,200 papers from authors associated with Japanese institutions. MDPI collaborates with 41 institutional partnerships in Japan, including the University of Tokyo, Hokkaido University, and Nagoya University. We have over 2,100 active EBMs from Japan, more than 1,050 EBMs having an H-index between 26 and 50, including 13 serving as Editors-in-Chief.
General Feedback – a side note
A general takeaway from our discussions with stakeholders from around the world is that negative perceptions of MDPI often stem from misinformation, misconceptions, or misunderstandings about MDPI and our practices. While we acknowledge our mistakes and work diligently to address them, maintaining a strong editorial procedure and robust peer-review process, I find that educating stakeholders about our how we do what we do and our ongoing improvements tends to help shift opinions.
That said, we recognize the importance of addressing individual concerns. We take feedback seriously and are continuously working to get better while not compromising the core principles that millions of authors appreciate about MDPI.
Chief Executive Officer
MDPI AG
29 March 2024
Meet Us at the 20th Annual International Conference of the Metabolomics Society (Metabolomics 2024), 16–20 June 2024, Osaka, Japan

MDPI will be attending the 20th Annual International Conference of the Metabolomics Society (Metabolomics 2024) held in Osaka, Japan, from 16 to 20 June 2024. The conference is the official annual meeting of the Metabolomics Society, and the largest metabolomics meeting worldwide. This is the third time that the conference will be held in Japan, following the 2005 and 2014 conferences held in Tsuruoka. We look forward to welcoming the metabolomics community, building strong connections, and discussing world-class research in a relaxed collegial environment. Let us build on the momentum of the incredible Niagara Falls and Valencia conferences that brought us back together in person, and make it the best meeting of 2024 together!
During this conference, MDPI will welcome researchers from different backgrounds to visit and share their latest views and research with us.
The following MDPI journals will be represented:
- Metabolites;
- Biomolecules;
- Plants;
- IJMS;
- Life;
- Cells;
- Agronomy;
- Nutrients;
- Cancers;
- CIMB;
- Genes;
- Antioxidants.
If you plan on attending this conference, feel free to stop by our booth. Our delegates look forward to meeting you in person to answer any questions you may have. For more information about the conference, please visit the following link: https://www.metabolomics2024.org/.
19 March 2024
Metabolites | Issue Cover Articles in 2023
The articles below have been selected as the 2023 Issue Cover Articles by the Editorial Office of Metabolites (ISSN: 2218-1989). These articles are from multiple fields within the scope of Metabolites, and we hope they can provide insights and references for scholars in related fields.
|
|
1. “Maternal Metabolites Indicative of Mental Health Status during Pregnancy” by Katarina Laketic, Sophie Lalonde-Bester, Kim Smyth, Donna M. Slater, Suzanne C. Tough, Hiroaki Ishida, Hans J. Vogel, Gerald F. Giesbrecht, Chunlong Mu and Jane Shearer Metabolites 2023, 13(1), 24; https://doi.org/10.3390/metabo13010024 Available online: https://www.mdpi.com/2218-1989/13/1/24 |
|
|
2. “Association between Circulating Amino Acids and COVID-19 Severity” by Ina Maltais-Payette, Fannie Lajeunesse-Trempe, Philippe Pibarot, Laurent Biertho and André Tchernof Metabolites 2023, 13(2), 201; https://doi.org/10.3390/metabo13020201 Available online: https://www.mdpi.com/2218-1989/13/2/201 |
|
|
3. “Primary Treatment Effects for High-Grade Serous Ovarian Carcinoma Evaluated by Changes in Serum Metabolites and Lipoproteins” *Editor’s Choice Paper by Cecilie Fredvik Torkildsen, Marie Austdal, Ann-Charlotte Iversen, Tone Frost Bathen, Guro Fanneløb Giskeødegård, Elisabeth Berge Nilsen, Grete Alræk Iversen, Ragnar Kvie Sande, Line Bjørge and Liv Cecilie Vestrheim Thomsen Metabolites 2023, 13(3), 417; https://doi.org/10.3390/metabo13030417 Available online: https://www.mdpi.com/2218-1989/13/3/417 |
|
|
4. “Amino Acid Profiles in Older Adults with Frailty: Secondary Analysis from MetaboFrail and BIOSPHERE Studies” *Editor’s Choice Paper by Riccardo Calvani, Anna Picca, Leocadio Rodriguez-Mañas, Matteo Tosato, Hélio José Coelho-Júnior, Alessandra Biancolillo, Olga Laosa, Jacopo Gervasoni, Aniello Primiano, Lavinia Santucci et al. Metabolites 2023, 13(4), 542; https://doi.org/10.3390/metabo13040542 Available online: https://www.mdpi.com/2218-1989/13/4/542 |
|
|
5. “Metabolomic Profiling of Bipolar Disorder by 1H-NMR in Serbian Patients” by Katarina Simić, Zoran Miladinović, Nina Todorović, Snežana Trifunović, Nataša Avramović, Aleksandra Gavrilović, Silvana Jovanović, Dejan Gođevac, Ljubodrag Vujisić, Vele Tešević et al. Metabolites 2023, 13(5), 607; https://doi.org/10.3390/metabo13050607 Available online: https://www.mdpi.com/2218-1989/13/5/607 |
|
|
6. “Systemic Metabolomics in a Framework of Genetics and Lifestyle in Age-Related Macular Degeneration” by Eric F. Thee, İlhan E. Acar, Johanna M. Colijn, Magda A. Meester-Smoor, Timo Verzijden, Sara J. Baart, Mohamed A. Jarboui, Sascha Fauser, Carel B. Hoyng, Marius Ueffing et al. Metabolites 2023, 13(6), 701; https://doi.org/10.3390/metabo13060701 Available online: https://www.mdpi.com/2218-1989/13/6/701 |
|
|
7. “Crosstalk between Breast Milk N-Acetylneuraminic Acid and Infant Growth in a Gut Microbiota-Dependent Manner” by Runze Ouyang, Sijia Zheng, Xiaolin Wang, Qi Li, Juan Ding, Xiao Ma, Zhihong Zhuo, Zhen Li, Qi Xin, Xin Lu et al. Metabolites 2023, 13(7), 846; https://doi.org/10.3390/metabo13070846 Available online: https://www.mdpi.com/2218-1989/13/7/846 |
|
|
8. “Untargeted Metabolomics and Body Mass in Adolescents: A Cross-Sectional and Longitudinal Analysis” by Amarnath Singh, Garrett Kinnebrew, Ping-Ching Hsu, Daniel Y. Weng, Min-Ae Song, Sarah A. Reisinger, Joseph P. McElroy, Brittney Keller-Hamilton, Amy K. Ferketich, Jo L. Freudenheim et al. Metabolites 2023, 13(8), 899; https://doi.org/10.3390/metabo13080899 Available online: https://www.mdpi.com/2218-1989/13/8/899 |
|
|
9. “Metabolic Profiling Early Post-Allogeneic Haematopoietic Cell Transplantation in the Context of CMV Infection” by Kirstine K. Rasmussen, Quenia dos Santos, Cameron Ross MacPherson, Adrian G. Zucco, Lars Klingen Gjærde, Emma E. Ilett, Isabelle Lodding, Marie Helleberg, Jens D. Lundgren, Susanne D. Nielsen et al. Metabolites 2023, 13(9), 968; https://doi.org/10.3390/metabo13090968 Available online: https://www.mdpi.com/2218-1989/13/9/968 |
|
|
10. “Urinary Metabolite Profiling to Non-Invasively Monitor the Omega-3 Index: An Exploratory Secondary Analysis of a Randomized Clinical Trial in Young Adults” by Brittany C. MacIntyre, Meera Shanmuganathan, Shannon L. Klingel, Zachary Kroezen, Erick Helmeczi, Na-Yung Seoh, Vanessa Martinez, Adrian Chabowski, Zeny Feng, Philip Britz-McKibbin et al. Metabolites 2023, 13(10), 1071; https://doi.org/10.3390/metabo13101071 Available online: https://www.mdpi.com/2218-1989/13/10/1071 |
|
|
11. “Cord Blood Metabolite Profiles and Their Association with Autistic Traits in Childhood” by Christin S. Kaupper, Sophia M. Blaauwendraad, Charlotte A. M. Cecil, Rosa H. Mulder, Romy Gaillard, Romy Goncalves, Ingo Borggraefe, Berthold Koletzko and Vincent W. V. Jaddoe Metabolites 2023, 13(11), 1140; https://doi.org/10.3390/metabo13111140 Available online: https://www.mdpi.com/2218-1989/13/11/1140 |
|
|
12. “Sex-Specific Relationships between HDL-Cholesterol Levels and 10-Year Mortality in Individuals with Atherosclerotic Cardiovascular Disease: A Nationwide Cohort Study of South Koreans” by Hyun Suk Yang, Ho Jin Jeong, Hyeongsu Kim, Seungho Lee and Mina Hur Metabolites 2023, 13(12), 1175; https://doi.org/10.3390/metabo13121175 Available online: https://www.mdpi.com/2218-1989/13/12/1175 |
4 March 2024
MDPI Insights: The CEO's Letter #9 - Romania, Research Integrity, Viruses

Welcome to the MDPI Insights: The CEO's Letter.
In these monthly letters, I will showcase two key aspects of our work at MDPI: our commitment to empowering researchers and our determination to facilitating open scientific exchange.
Opening Thoughts

Reka Kovacs (Deputy Office Manager, MDPI), Stefan Tochev (CEO, MDPI), and Sandra Ana Spatariu (Office Manager, MDPI) at the MDPI office in Cluj, Romania.
MDPI’s Impact on Romania
In February, I visited our office in Cluj, Romania. I worked closely with our senior office managers and various teams, including the departments of training, marketing and conferences, as well as our journal relationship specialists, reviewing our service to the local scholarly community. During the visit, I also met with representatives from Babes-Bolyai University and the Technical University of Cluj-Napoca. Our multifunctional Romanian office plays an important role in supporting our collaborations with the local market as well as helping to meet MDPI’s overall business needs.

Feedback and strategy meeting with a group of MDPI’s Journal Relationship Specialists at the MDPI office in Cluj, Romania.
With 22,436 articles, Romania ranks as a top 20 contributing country to MDPI’s total number of papers published as at 28 February 2024. This highlights the importance of our collaboration with Romanian-affiliated authors and the growing opportunity to support their publishing needs. MDPI is one of the few academic publishers with a significant presence in Romania, boasting over 360 colleagues across our offices in Bucharest and Cluj. We are also proud to hire colleagues from local institutions to launch their careers within publishing.
Romania ranks as a top 20 contributing country.
The Numbers: 2019–2023
MDPI has seen a healthy increase in submissions from Romanian authors over the past three years, from 8,439 in 2021 to 11,866 by end of 2023, with most submissions going to journals such as Sustainability, Medicina, Diagnostics, IJMS, Applied Sciences, and JCM. From 2019 to 2023, MDPI published articles from 32,145 authors affiliated with Romanian institutions. Over those years, we have worked with Romanian Guest Editors on nearly 3,000 occasions to support their Special Issue and Topical collections.
With more than 300 Editorial Board Members from Romania, 34 appear on the board of Mathematics, 27 on Materials, 19 on Polymers, 18 on Coatings, and 16 on Molecules, while three serve as Section Editors-in-Chief (SEiC) on our journals Coatings (3.4 IF, 4.6 Citescore), Magnetochemistry (2.7 IF, 3.5 Citescore), and Chemosensors (4.2 IF, 3.9 Citescore).
Institutional Open Access Programs
Our commitment to working with institutions is evident in Romania, where we have established eight Institutional Open Access Programs (IOAP) with esteemed institutions such as the University of Bucharest, the University of Medicine and Pharmacy Cluj-Napoca, and most recently the National Institute for Laser, Plasma and Radiation Physics.
Our growth and presence in Romania are a true testament.
We also have IOAP agreements with Babes-Bolyai University and the Technical University of Cluj-Napoca, where I had the opportunity to meet senior stakeholders during my visit. Below are a few photos capturing our meeting with Prof. Radu Silaghi-Dumitrescu (Head of Faculty of Chemistry, Babes-Bolyai University) at the MDPI office in Cluj, Romania, along with a photo from our meeting with Vice Deans Nicoleta Cobarzan, Nicoleta Ilies, and Hoda Gavril, from the faculty of Civil Engineering at the Technical University of Cluj, Romania.


Our growth and presence in Romania are a true testament to the service we provide to the scholarly community and the relationships we foster in that region. We look forward to continuing to support Romanian scholars and institutions by providing a valuable and trusted experience with MDPI, the leader in open access publishing.
Impactful Research
MDPI Joins the STM Integrity Hub
MDPI has long been a supporter and partner of STM, with our involvement ranging from sponsoring and attending events to helping organize event programs. By joining the STM Integrity Hub, we aim to further our commitment to STM initiatives aimed at safeguarding the integrity of science.
“We are pleased to welcome MDPI as the 35th organisation participating in the Hub. This expansion is critical, as every new member enhances our capacity to prevent fraudulent submissions from entering the academic record.”
Joris van Rossum, Director of Research Integrity, STM
MDPI operates in full alignment with STM Integrity Hub's values of shared data and experiences. We strongly believe in collaboration and open exchange for the purposes of creating a holistic approach to support research integrity at MDPI itself and across the entire academic publishing industry. The Integrity Hub is an excellent example of how publishers can come together to jointly address industry-wide challenges related to research integrity, such as manuscripts that breach research integrity standards and paper-mills.
I look forward to our Research Integrity and Publication Ethics Team (RIPE) team immersing themselves in this initiative, exchanging information, best practices, and tools for the benefit of the entire scholarly ecosystem. We believe that ethical publishing standards should be implemented across the board, and we aim to be rigorous in our approach, addressing research integrity issues and improving the impact of published research.
Inside MDPI

MDPI Expands Research Integrity and Publication Ethics Team (RIPE)
In addition to external collaborations and joint initiatives aimed at further strengthening our commitment to research integrity, we are also enhancing our internal efforts. This includes improving our processes and guidelines and expanding our teams and departments to ensure quality assurance throughout our publishing process.
We are pleased to announce the expansion of our Research Integrity and Publication Ethics Team (RIPE) at MDPI. The RIPE team has recently welcomed new colleagues, each bringing unique skills and a personal commitment to prioritize ethical considerations in all our work.
The demand for research integrity and high ethical standards in academic publishing is steadily rising across our industry. Our expanded RIPE team will work to enhance and align our practices with industry best practices, ensuring excellence in research integrity and publication ethics.

Stefan Tochev (CEO, MDPI) introduces Dr. Tim Tait-Jamieson (Research Integrity Lead, MDPI) for his presentation on MDPI’s Retraction and Approval Process to a group of Journal Relationship Specialists at the MDPI office in Cluj, Romania: “The demand for research integrity and publication ethics is steadily rising across our industry.”
Introducing our Research Integrity and Publication Ethics Team
Led by Dr. Tim Tait-Jamieson (Research Integrity Lead), the RIPE team comprises Dr. Ivana Resanovic (Research Integrity Manager), Dr. Lavinia Rogojina (Research Integrity Manager), Ms. Diana Apodaritei (Research Integrity Specialist), Dr. Zoltan Mihaly (Research Integrity Specialist), Mr. Aleksandar Đukić (Research Integrity Specialist), Ms. Ana Stankovic (Research Integrity Specialist), and Ms. Anna Pena (Publication Ethics Assistant).
Please click here to access everything that you need to know about MDPI’s Research and Publication Ethics.
With this span of complementary roles, the RIPE team collaborates directly with journal editorial teams and works closely with various departments, including our Scientific Office Board and our Journal Relationship Specialists. The team’s primary objectives are to help prevent issues regarding research integrity and publication ethics during peer review, uphold MDPI’s ethics policies, adhere to industry standards, and resolve publication ethics and research integrity issues and complaints.
Quality Updates to Special Issues Oversight
At MDPI, we are committed to reviewing policies pertaining to the quality of research. In this blog post, Shaheena Patel (Communications Associate, MDPI), outlines two recent updates to MDPI journal processes. These updates pertain to Special Issue (SI) quality guidelines, in line with criteria provided by COPE and DOAJ. Alongside the SI updates, details regarding the new minor corrections policy introduced in 2024 are provided in the blog.
The two updates we implemented include greater oversight and the verification of Guest Editor credentials. These guidelines require that Editors-in-Chief (EiCs) and Editorial Board Members (EBMs) take responsibility for overseeing SIs.
PS. Thank you, James Butcher, for featuring this up in your 67th issue of the Journalogy newsletter.
Read more:
Coming Together for Science

Viruses 2024 – A World of Viruses
I am pleased to share the success of our MDPI conference Viruses 2024 – A World of Viruses, held 14-16 February, in Barcelona. With 240 registrations, this event brought together top scientists, researchers, and industry experts from 40 countries to share their findings on the latest developments in viral pathogenesis and immune responses.
Attendees gathered for the 5th edition of the Viruses’ conference, where we hosted influential keynote speeches from Nobel Prize laureate Dr. Charles M. Rice and ‘Distinguished Senior Virologist’ Prof. Luis Enjuanes, along with 14 invited speakers, 47 selected speakers, and nine flash poster presenters, to discuss the most significant issues in virology today.
Recap on the #Viruses2024 Conference
Take a look at the key moments from MDPI’s Viruses event and please join us in commemorating a gathering for global knowledge and cooperation. A heartfelt thank-you to all attendees; their passion and engagement played a crucial role in making this event an engaging success!
Below are calls to action from the keynote speakers encouraging collaboration and communication:
“There’s never been a better time than now to really take the power that we have both in terms of basic research and also in biotech and pharma to develop antiviral agents.” - Dr. Charles M. Rice, The Rockefeller University, New York, USA
“The collaboration between labs is absolutely essential. Improving initial detection and improving communication is a must for all of us working in science.” - Prof. Dr. Luis Enjuanes, National Center of Biotechnology (CNB-CSIC), Madrid, Spain

Our thanks go to our sponsors and partnering societies, our Viruses journal and editorial team, our Barcelona colleagues, and the social media, conference and other MDPI teams for making this event a memorable occasion. View the event gallery here.
Upcoming In-Person Event

24–26 April, 2024
4th MMCS – Harnessing the Power of New Drug Modalities
Location: Barcelona, Spain
Esteemed speakers at MMCS 2024 include Prof. Arun K. Ghosh, the mind behind the Darunavir molecule, and Prof. Paul Brennan, CSO of Alzheimer's Research UK Oxford Drug Discovery Institute.
Find more upcoming MDPI events here.
Organize Your Event with MDPI’s Sciforum
Sciforum is MDPI’s platform dedicated to the organization of scientific events. In line with our mission to promote science, Sciforum supports scholars, societies, research networks, and universities at all stages of organizing in-person events, virtual events and webinars. Our platforms are efficient, user-friendly, and cost-effective. We handle all steps related to event management. Contact us for details.
Closing Thoughts

Researcher to Reader (R2R) Conference
From 20–21 February 2024, I had the pleasure of attending the Researcher to Reader (R2R) conference in London, which MDPI has proudly sponsored over the years. The conference programme offered a variety of session formats, including workshops, panel discussions, debates, interviews, presentations, and lightning talks, with opportunities to discuss relevant topics.
We take pride in supporting the scientific community, bringing researchers across the world together to network, exchange ideas and share the latest in science and publishing. In 2023, MDPI invested close to 2 million CHF in sponsoring over 2,000 scientific and publishing-related conferences worldwide.
R2R Peer Review Innovations Workshop
I found the R2R conference to be engaging, with the workshops being particularly enjoyable. My colleague Giulia Stefenelli (Chair of Scientific Office Board) and I participated in the “Peer Review Innovations” workshop, which spanned four sessions over the two days. These sessions explored the future of peer review and how we can improve the peer review process for everyone involved. Notably, the large majority of attendees expressed their opinion that peer review, as currently practiced, requires significant improvement. Together, we collaborated on potential immediate and long-term improvements and innovative processes, aiming to create an ecosystem beneficial to all stakeholders by strengthening submission systems with the aim of reducing threats and making authors more responsible for their work. We also discussed the opportunity for academic institutions to better scrutinize the quality of the work produced and submitted to journals.
Our group comprised publishers, software providers, librarians, and more, bringing diverse perspectives to the discussions. These interactions were relevant to MDPI’s ongoing conversations, providing insights to our efforts. The session also made me appreciate that MDPI is doing well, as the group discussions included the subject of various quality checks that we have already embedded in our processes, ensuring that we keep abreast of industry standards.
The need for an optimized system to incentivize the activities of editors and reviewers was also a focus of discussion, as well as the support that reviewers need from publishers via the provision of strong reports through fixed forms, questionnaires and training.
At MDPI, we are currently auditing our reviewer program to improve reviewer recognition, guidelines, and methods for identifying suitable reviewers, while maintaining our commitment to quality and timeliness.
Congratulations to Mark Carden, Conference Director, and the R2R team for organizing a productive and successful event. PS: The break times were greatly appreciated as well!
Chief Executive Officer
MDPI AG
4 February 2024
Metabolites | Highly Cited Papers in 2022 in the Section “Food Metabolomics”
1. “Mechanism of Soy Isoflavone Daidzein-Induced Female-Specific Anorectic Effect”
by Mina Fujitani, Takafumi Mizushige, Sudhashree Adhikari, Keshab Bhattarai and Taro Kishida
Metabolites 2022, 12(3), 252; https://doi.org/10.3390/metabo12030252
Available online: https://www.mdpi.com/2218-1989/12/3/252
2. “The Effects of Dietary Supplements, Nutraceutical Agents, and Physical Exercise on Myostatin Levels: Hope or Hype?”
by Heitor O. Santos, Henrique S. Cerqueira and Grant M. Tinsley
Metabolites 2022, 12(11), 1146; https://doi.org/10.3390/metabo12111146
Available online: https://www.mdpi.com/2218-1989/12/11/1146
3. “Functional Nutrients to Ameliorate Neurogenic Muscle Atrophy”
by Viviana Moresi, Alessandra Renzini, Giorgia Cavioli, Marilia Seelaender, Dario Coletti, Giuseppe Gigli and Alessia Cedola
Metabolites 2022, 12(11), 1149; https://doi.org/10.3390/metabo12111149
Available online: https://www.mdpi.com/2218-1989/12/11/1149
4. “Natural Products Targeting Hsp90 for a Concurrent Strategy in Glioblastoma and Neurodegeneration”
by Sarmistha Mitra, Raju Dash, Yeasmin Akter Munni, Nusrat Jahan Selsi, Nasrin Akter, Md Nazim Uddin, Kishor Mazumder and II Soo Moon
Metabolites 2022, 12(11), 1153; https://doi.org/10.3390/metabo12111153
Available online: https://www.mdpi.com/2218-1989/12/11/1153
5. “Measurement of the Effect of Accelerated Aging on the Aromatic Compounds of Gewürztraminer and Teroldego Wines, Using a SPE-GC-MS/MS Protocol”
by Silvia Carlin, Cesare Lotti, Ludovica Correggi, Fulvio Mattivi, Panagiotis Arapitsas and Urška Vrhovšek
Metabolites 2022, 12(2), 180; https://doi.org/10.3390/metabo12020180
Available online: https://www.mdpi.com/2218-1989/12/2/180
6. “Comprehensive Metabolomic Comparison of Five Cereal Vinegars Using Non-Targeted and Chemical Isotope Labeling LC-MS Analysis”
by Zhihua Li, Chi Zhao, Ling Dong, Yu Huan, Miwa Yoshimoto, Yongqing Zhu, Ipputa Tada, Xiaohang Wang, Shuang Zhao, Fengju Zhang et al.
Metabolites 2022, 12(5), 427; https://doi.org/10.3390/metabo12050427
Available online: https://www.mdpi.com/2218-1989/12/5/427
7. “Stimulation of GLUT4 Glucose Uptake by Anthocyanin-Rich Extract from Black Rice (Oryza sativa L.) via PI3K/Akt and AMPK/p38 MAPK Signaling in C2C12 Cells”
by Shui-Yuan Feng, Shu-Jing Wu, Yun-Ching Chang, Lean-Teik Ng and Sue-Joan Chang
Metabolites 2022, 12(9), 856; https://doi.org/10.3390/metabo12090856
Available online: https://www.mdpi.com/2218-1989/12/9/856
8. “Unlike Glycerophosphocholine or Choline Chloride, Dietary Phosphatidylcholine Does Not Increase Plasma Trimethylamine-N-Oxide Levels in Sprague-Dawley Rats”
by Bungo Shirouchi, Ayano Fukuda and Taiki Akasaka
Metabolites 2022, 12(1), 64; https://doi.org/10.3390/metabo12010064
Available online: https://www.mdpi.com/2218-1989/12/1/64