Cord Blood Metabolite Profiles and Their Association with Autistic Traits in Childhood
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Metabolomics Measurements
2.3. Autistic Traits
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Neonatal Cord Blood Metabolomics and Autistic Traits at the Ages of 6 and 13
3.3. Sensitivity Analysis
4. Discussion
4.1. Interpretation of Main Findings
4.2. Methodological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Amino acids |
| AAA | Aromatic amino acids |
| BCAA | Branched-chain amino acids |
| Carn | Carnitines |
| Carn.a | Acyl-carnitines |
| CV | Coefficient of variation |
| EAA | Essential amino acids |
| FDR | False discovery rate |
| HPLC | High-performance liquid chromatography |
| IUPAC-IUB | International Union of Pure and Applied Chemistry—International Union of Biochemistry |
| Lyso.PC.a | Acyl-lysophosphatidylcholines |
| Lyso.PC.e | Alkyl-lysophosphatidylcholines |
| LC/MS-MS | Liquid chromatography–mass spectrometry |
| NEFA | Non-esterified fatty acids |
| NEAA | Non-essential amino acids |
| NMR | Nuclear Magnetic Resonance |
| PC | Phosphatidylcholines |
| PC.aa | Diacyl-phosphatidylcholines |
| PC.ae | Acyl-alkyl-phosphatidylcholines |
| PL | Phospholipids |
| QC | Quality control |
| SD | Standard deviation |
| SDS | Standard deviation scores |
| SGA | Small size for gestational age |
| SM | Sphingomyelines |
| SRS | Social Responsiveness Scale |
References
- First, M.B. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J. Nerv. Ment. Dis. 2013, 201, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Constantino, J.N.; Todd, R.D. Autistic traits in the general population: A twin study. Arch. Gen. Psychiatry 2003, 60, 524–530. [Google Scholar]
- Lundström, S.; Chang, Z.; Råstam, M.; Gillberg, C.; Larsson, H.; Anckarsäter, H.; Lichtenstein, P. Autism spectrum disorders and autistic like traits: Similar etiology in the extreme end and the normal variation. Arch. Gen. Psychiatry 2012, 69, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [PubMed]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [PubMed]
- Mesleh, A.G.; Abdulla, S.A.; El-Agnaf, O. Paving the Way toward Personalized Medicine: Current Advances and Challenges in Multi-OMICS Approach in Autism Spectrum Disorder for Biomarkers Discovery and Patient Stratification. J. Pers. Med. 2021, 11, 41. [Google Scholar] [PubMed]
- Shen, L.; Liu, X.; Zhang, H.; Lin, J.; Feng, C.; Iqbal, J. Biomarkers in autism spectrum disorders: Current progress. Clin. Chim. Acta 2020, 502, 41–54. [Google Scholar]
- Glinton, K.E.; Elsea, S.H. Untargeted Metabolomics for Autism Spectrum Disorders: Current Status and Future Directions. Front. Psychiatry 2019, 10, 647. [Google Scholar] [PubMed]
- Likhitweerawong, N.; Thonusin, C.; Boonchooduang, N.; Louthrenoo, O.; Nookaew, I.; Chattipakorn, N.; Chattipakorn, S.C. Profiles of urine and blood metabolomics in autism spectrum disorders. Metab. Brain Dis. 2021, 36, 1641–1671. [Google Scholar]
- Qureshi, F.; Adams, J.B.; Audhya, T.; Hahn, J. Multivariate Analysis of Metabolomic and Nutritional Profiles among Children with Autism Spectrum Disorder. J. Pers. Med. 2022, 12, 923. [Google Scholar]
- Needham, B.D.; Adame, M.D.; Serena, G.; Rose, D.R.; Preston, G.M.; Conrad, M.C.; Campbell, A.S.; Donabedian, D.H.; Fasano, A.; Ashwood, P.; et al. Plasma and Fecal Metabolite Profiles in Autism Spectrum Disorder. Biol. Psychiatry 2021, 89, 451–462. [Google Scholar] [PubMed]
- Smith, A.M.; King, J.J.; West, P.R.; Ludwig, M.A.; Donley, E.L.R.; Burrier, R.E.; Amaral, D.G. Amino Acid Dysregulation Metabotypes: Potential Biomarkers for Diagnosis and Individualized Treatment for Subtypes of Autism Spectrum Disorder. Biol. Psychiatry 2019, 85, 345–354. [Google Scholar] [PubMed]
- Ritz, B.; Yan, Q.; Uppal, K.; Liew, Z.; Cui, X.; Ling, C.; Inoue, K.; von Ehrenstein, O.; Walker, D.I.; Jones, D.P. Untargeted Metabolomics Screen of Mid-pregnancy Maternal Serum and Autism in Offspring. Autism Res. 2020, 13, 1258–1269. [Google Scholar] [CrossRef]
- Panjwani, A.A.; Ji, Y.; Fahey, J.W.; Palmer, A.; Wang, G.; Hong, X.; Zuckerman, B.; Wang, X. Maternal Dyslipidemia, Plasma Branched-Chain Amino Acids, and the Risk of Child Autism Spectrum Disorder: Evidence of Sex Difference. J. Autism Dev. Disord. 2020, 50, 540–550. [Google Scholar]
- Smith, A.M.; Natowicz, M.R.; Braas, D.; Ludwig, M.A.; Ney, D.M.; Donley, E.L.R.; Burrier, R.E.; Amaral, D.G. A Metabolomics Approach to Screening for Autism Risk in the Children’s Autism Metabolome Project. Autism Res. 2020, 13, 1270–1285. [Google Scholar] [PubMed]
- Orozco, J.S.; Hertz-Picciotto, I.; Abbeduto, L.; Slupsky, C.M. Metabolomics analysis of children with autism, idiopathic-developmental delays, and Down syndrome. Transl. Psychiatry 2019, 9, 243. [Google Scholar]
- Kelly, R.S.; Boulin, A.; Laranjo, N.; Lee-Sarwar, K.; Chu, S.H.; Yadama, A.P.; Carey, V.; Litonjua, A.A.; Lasky-Su, J.; Weiss, S.T. Metabolomics and Communication Skills Development in Children; Evidence from the Ages and Stages Questionnaire. Metabolites 2019, 9, 42. [Google Scholar] [CrossRef]
- Ristori, M.V.; Mortera, S.L.; Marzano, V.; Guerrera, S.; Vernocchi, P.; Ianiro, G.; Gardini, S.; Torre, G.; Valeri, G.; Vicari, S.; et al. Proteomics and Metabolomics Approaches towards a Functional Insight onto AUTISM Spectrum Disorders: Phenotype Stratification and Biomarker Discovery. Int. J. Mol. Sci. 2020, 21, 6274. [Google Scholar] [CrossRef]
- Courraud, J.; Ernst, M.; Svane Laursen, S.; Hougaard, D.M.; Cohen, A.S. Studying Autism Using Untargeted Metabolomics in Newborn Screening Samples. J. Mol. Neurosci. 2021, 71, 1378–1393. [Google Scholar] [PubMed]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar]
- El-Ansary, A.; Chirumbolo, S.; Bhat, R.S.; Dadar, M.; Ibrahim, E.M.; Bjørklund, G. The Role of Lipidomics in Autism Spectrum Disorder. Mol. Diagn. Ther. 2020, 24, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Brister, D.; Rose, S.; Delhey, L.; Tippett, M.; Jin, Y.; Gu, H.; Frye, R.E. Metabolomic Signatures of Autism Spectrum Disorder. J. Pers. Med. 2022, 12, 1727. [Google Scholar] [CrossRef]
- Taniguchi, M.; Okazaki, T. The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochim. Biophys. Acta 2014, 1841, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Voerman, E.; Jaddoe, V.W.V.; Uhl, O.; Shokry, E.; Horak, J.; Felix, J.F.; Koletzko, B.; Gaillard, R. A population-based resource for intergenerational metabolomics analyses in pregnant women and their children: The Generation R Study. Metabolomics 2020, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Harder, U.; Koletzko, B.; Peissner, W. Quantification of 22 plasma amino acids combining derivatization and ion-pair LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 495–504. [Google Scholar] [CrossRef]
- IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature and symbolism for amino acids and peptides. Recommendations. Biochem. J. 1984, 219, 345–373. [Google Scholar]
- Hellmuth, C.; Weber, M.; Koletzko, B.; Peissner, W. Nonesterified fatty acid determination for functional lipidomics: Comprehensive ultrahigh performance liquid chromatography-tandem mass spectrometry quantitation, qualification, and parameter prediction. Anal. Chem. 2012, 84, 1483–1490. [Google Scholar] [CrossRef]
- Uhl, O.; Fleddermann, M.; Hellmuth, C.; Demmelmair, H.; Koletzko, B. Phospholipid Species in Newborn and 4 Month Old Infants after Consumption of Different Formulas or Breast Milk. PLoS ONE 2016, 11, e0162040. [Google Scholar] [CrossRef]
- Rauschert, S.; Mori, T.A.; Beilin, L.J.; Jacoby, P.; Uhl, O.; Koletzko, B.; Oddy, W.H.; Hellmuth, C. Early Life Factors, Obesity Risk, and the Metabolome of Young Adults. Obesity 2017, 25, 1549–1555. [Google Scholar] [CrossRef]
- Hellmuth, C.; Uhl, O.; Standl, M.; Demmelmair, H.; Heinrich, J.; Koletzko, B.; Thiering, E. Cord Blood Metabolome Is Highly Associated with Birth Weight, but Less Predictive for Later Weight Development. Obes. Facts 2017, 10, 85–100. [Google Scholar] [CrossRef]
- Shokry, E.; Marchioro, L.; Uhl, O.; Bermúdez, M.G.; García-Santos, J.A.; Segura, M.T.; Campoy, C.; Koletzko, B. Impact of maternal BMI and gestational diabetes mellitus on maternal and cord blood metabolome: Results from the PREOBE cohort study. Acta Diabetol. 2019, 56, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wang, J.; Su, M.; Jia, E.; Chen, S.; Chen, T.; Ni, Y. Missing Value Imputation Approach for Mass Spectrometry-based Metabolomics Data. Sci. Rep. 2018, 8, 663. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Ntambi, J. Role of stearoyl-CoA desaturase in human metabolic disease. Future Lipidol. 2008, 3, 163–173. [Google Scholar] [CrossRef]
- Squeri, A. Coronary Artery Disease—New Insights and Novel Approaches; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Kirchberg, F.F.; Brandt, S.; Moß, A.; Peissner, W.; Koenig, W.; Rothenbacher, D.; Brenner, H.; Koletzko, B.; Hellmuth, C.; Wabitsch, M. Metabolomics reveals an entanglement of fasting leptin concentrations with fatty acid oxidation and gluconeogenesis in healthy children. PLoS ONE 2017, 12, e0183185. [Google Scholar] [CrossRef] [PubMed]
- Molnos, S.; Wahl, S.; Haid, M.; Eekhoff, E.M.W.; Pool, R.; Floegel, A.; Deelen, J.; Much, D.; Prehn, C.; Breier, M.; et al. Metabolite ratios as potential biomarkers for type 2 diabetes: A DIRECT study. Diabetologia 2018, 61, 117–129. [Google Scholar] [CrossRef]
- Pickens, C.A.; Vazquez, A.I.; Jones, A.D.; Fenton, J.I. Obesity, adipokines, and C-peptide are associated with distinct plasma phospholipid profiles in adult males, an untargeted lipidomic approach. Sci. Rep. 2017, 7, 6335. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, G.; Aitken, D.; Likhodii, S.; Liu, M.; Martin, G.; Furey, A.; Randell, E.; Rahman, P.; Jones, G.; et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology 2016, 55, 1566–1574. [Google Scholar] [CrossRef]
- Constantino, J.N.; Davis, S.A.; Todd, R.D.; Schindler, M.K.; Gross, M.M.; Brophy, S.L.; Metzger, L.M.; Shoushtari, C.S.; Splinter, R.; Reich, W. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. J. Autism Dev. Disord. 2003, 33, 427–433. [Google Scholar] [CrossRef]
- Van den Dries, M.A.; Guxens, M.; Pronk, A.; Spaan, S.; El Marroun, H.; Jusko, T.A.; Longnecker, M.P.; Ferguson, K.K.; Tiemeier, H. Organophosphate pesticide metabolite concentrations in urine during pregnancy and offspring attention-deficit hyperactivity disorder and autistic traits. Environ. Int. 2019, 131, 105002. [Google Scholar] [CrossRef]
- Lyall, K.; Hosseini, M.; Ladd-Acosta, C.; Ning, X.; Catellier, D.; Constantino, J.N.; Croen, L.A.; Kaat, A.J.; Botteron, K.; Bush, N.R.; et al. Distributional Properties and Criterion Validity of a Shortened Version of the Social Responsiveness Scale: Results from the ECHO Program and Implications for Social Communication Research. J. Autism Dev. Disord. 2021, 51, 2241–2253. [Google Scholar] [CrossRef]
- Niklasson, A.; Albertsson-Wikland, K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Hollowood-Jones, K.; Adams, J.B.; Coleman, D.M.; Ramamoorthy, S.; Melnyk, S.; James, S.J.; Woodruff, B.K.; Pollard, E.L.; Snozek, C.L.; Kruger, U.; et al. Altered metabolism of mothers of young children with Autism Spectrum Disorder: A case control study. BMC Pediatr. 2020, 20, 557. [Google Scholar] [CrossRef] [PubMed]
- Yap, I.K.; Angley, M.; Veselkov, K.A.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J. Proteome Res. 2010, 9, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.M.M.; Bolker, B.; Walker, S. Linear Mixed-Effects Models Using ‘Eigen’ and S4; CRAN—R Project: Vienna, Austria, 2023. [Google Scholar]
- Van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 2007, 16, 219–242. [Google Scholar] [CrossRef]
- Liang, L.; Rasmussen, M.H.; Piening, B.; Shen, X.; Chen, S.; Röst, H.; Snyder, J.K.; Tibshirani, R.; Skotte, L.; Lee, N.C.; et al. Metabolic Dynamics and Prediction of Gestational Age and Time to Delivery in Pregnant Women. Cell 2020, 181, 1680–1692.e1615. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, R.; Xu, Y.; Zhou, Z.; Guan, P.; Wu, Y.; Zhou, J.; Cheng, Z.; Zhang, L. Altered Metabolic Characteristics in Plasma of Young Boys with Autism Spectrum Disorder. J. Autism Dev. Disord. 2022, 52, 4897–4907. [Google Scholar] [CrossRef]
- Wang, H.; Liang, S.; Wang, M.; Gao, J.; Sun, C.; Wang, J.; Xia, W.; Wu, S.; Sumner, S.J.; Zhang, F.; et al. Potential serum biomarkers from a metabolomics study of autism. J. Psychiatry Neurosci. 2016, 41, 27–37. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar]
- Schneider, N.; Hauser, J.; Oliveira, M.; Cazaubon, E.; Mottaz, S.C.; O’Neill, B.V.; Steiner, P.; Deoni, S.C.L. Sphingomyelin in Brain and Cognitive Development: Preliminary Data. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013, 35, 45–52. [Google Scholar] [CrossRef]
- Bent, S.; Lawton, B.; Warren, T.; Widjaja, F.; Dang, K.; Fahey, J.W.; Cornblatt, B.; Kinchen, J.M.; Delucchi, K.; Hendren, R.L. Identification of urinary metabolites that correlate with clinical improvements in children with autism treated with sulforaphane from broccoli. Mol. Autism 2018, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Staellberg-Stenhagen, S.; Svennerholm, L. Fatty Acid Composition of Human Brain Sphingomyelins: Normal Variation with Age and Changes during Myelin Disorders. J. Lipid Res. 1965, 6, 146–155. [Google Scholar] [CrossRef]
- Lv, Q.Q.; You, C.; Zou, X.B.; Deng, H.Z. Acyl-carnitine, C5DC, and C26 as potential biomarkers for diagnosis of autism spectrum disorder in children. Psychiatry Res. 2018, 267, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Desoky, T.; Sakhr, H.M.; Gabra, R.H.; Bakri, A.H. Possible Metabolic Alterations among Autistic Male Children: Clinical and Biochemical Approaches. J. Mol. Neurosci. 2019, 67, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Alaimo, S.; Messina, M.; Pulvirenti, A.; Bastin, J.; Group, M.I.-A.; Ferro, A.; Frye, R.E.; Rizzo, R. A Subset of Patients with Autism Spectrum Disorders Show a Distinctive Metabolic Profile by Dried Blood Spot Analyses. Front. Psychiatry 2018, 9, 636. [Google Scholar] [CrossRef]
- McGill, M.R.; Li, F.; Sharpe, M.R.; Williams, C.D.; Curry, S.C.; Ma, X.; Jaeschke, H. Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch. Toxicol. 2014, 88, 391–401. [Google Scholar] [PubMed]
- Liu, X.; Strable, M.S.; Ntambi, J.M. Stearoyl CoA desaturase 1: Role in cellular inflammation and stress. Adv. Nutr. 2011, 2, 15–22. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front. Physiol. 2014, 5, 150. [Google Scholar] [CrossRef]
- Uhl, O.; Glaser, C.; Demmelmair, H.; Koletzko, B. Reversed phase LC/MS/MS method for targeted quantification of glycerophospholipid molecular species in plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 3556–3564. [Google Scholar] [CrossRef]

| Characteristics | Total Sample n = 783 |
|---|---|
| Maternal characteristics | |
| Age at enrolment mean (±SD), years | 31.8 (3.9) |
| Education level, high, n (%) | 510 (65.1%) |
| Pre-pregnancy body mass index, median (95% range), kg/m2 | 22.4 (18.5, 34.0) |
| Smoking, n (%) | |
| Never smoked during pregnancy | 555 (79.1%) |
| Smoked until pregnancy was known | 63 (9.0%) |
| Continued smoking during pregnancy | 84 (11.9%) |
| Alcohol use, n (%) | |
| No alcohol consumption during pregnancy | 207 (29.6%) |
| Alcohol consumption until pregnancy was known | 103 (14.7%) |
| Alcohol consumption continued during pregnancy | 389 (55.7%) |
| Psychopathology, median (95% range) | 0.1 (0, 1) |
| Folic acid supplements use, yes, n (%) * | 600 (92.9%) |
| Vitamin D deficiency, yes, n (%) ** | 221 (30.2%) |
| Fetal characteristics | |
| Fetal sex, female, n (%) | 372 (47.5%) |
| Gestational age at birth in weeks, median (95% range) | 40.3 (36.9, 42.4) |
| Birthweight in grams, mean (±SD) | 3541 (494.0) |
| Birthweight < 2500 g, n (%) | 16 (2.0%) |
| Birthweight 2500 to 4500 g, n (%) | 744 (95.0%) |
| Birthweight > 4500 g, n (%) | 23 (3.0%) |
| Child characteristics | |
| 6 years visit | |
| Age at visit in years, median (95% range) | 5.9 (5.7, 6.8) |
| SRS autistic traits score, median (95% range) | 3.0 (0, 12) |
| 13 years visit | |
| Age at visit in years, median (95% range) | 13.5 (13, 14.4) |
| SRS autistic traits score, median (95% range) | 4.0 (0, 15) |
| SRS Age 6 | SRS Age 13 | |||||||
|---|---|---|---|---|---|---|---|---|
| Basic Model | Main Model | Basic Model | Main Model | |||||
| Beta | p-Value * | Beta | p-Value * | Beta | p-Value * | Beta | p-Value * | |
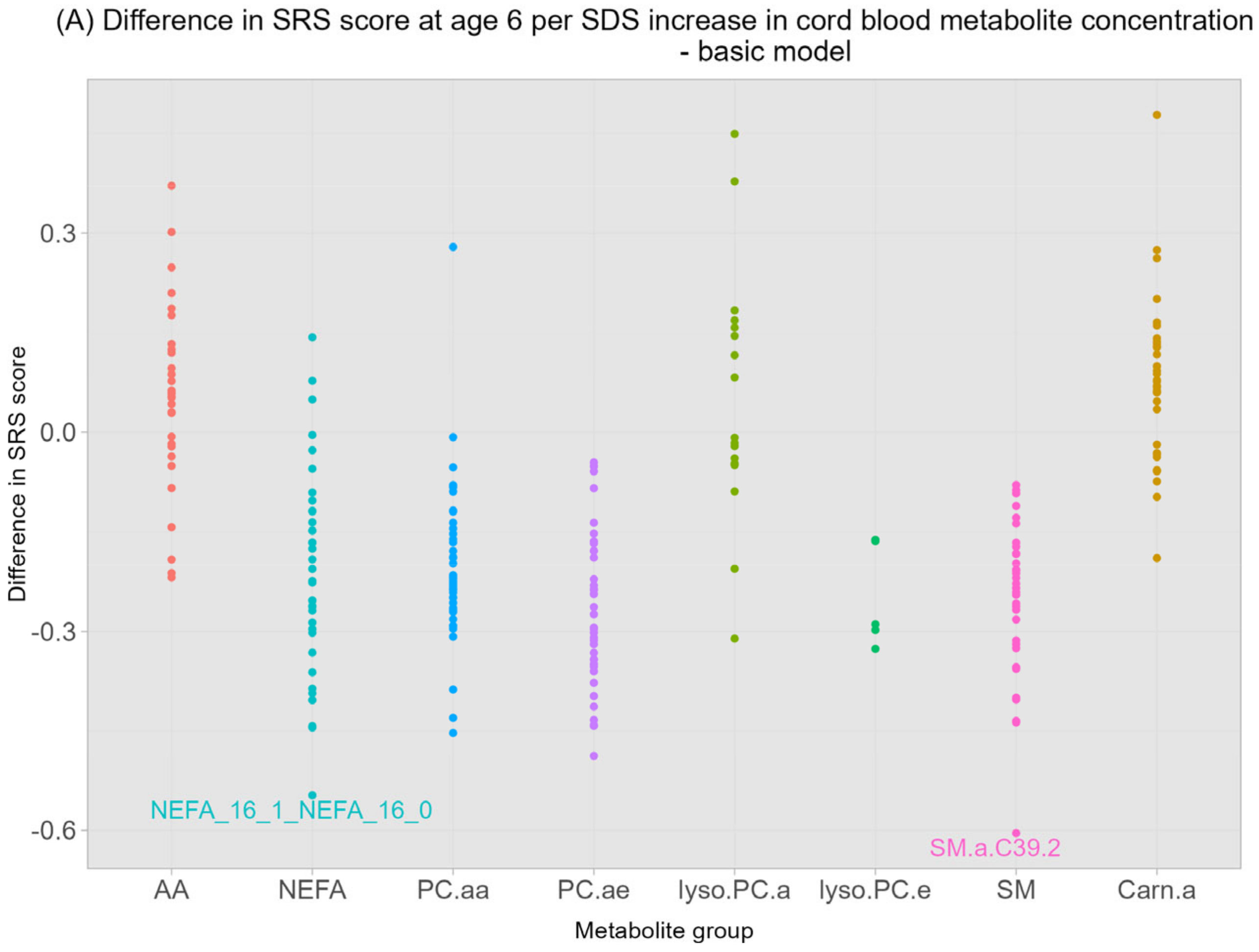
| Sphingomyelines SM.a.C.39.2 | −0.60 | 0.01 | −0.54 | 0.08 | −0.05 | 0.88 | 0.02 | 0.99 |
| Carnitines Carn.a.C.18.2 | 0.48 | 0.06 | 0.51 | 0.08 | 0.28 | 0.51 | 0.31 | 0.69 |
| NEFA Ratio NEFA 16:1/16:0 | −0.55 | 0.02 | −0.48 | 0.08 | −0.38 | 0.40 | −0.32 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaupper, C.S.; Blaauwendraad, S.M.; Cecil, C.A.M.; Mulder, R.H.; Gaillard, R.; Goncalves, R.; Borggraefe, I.; Koletzko, B.; Jaddoe, V.W.V. Cord Blood Metabolite Profiles and Their Association with Autistic Traits in Childhood. Metabolites 2023, 13, 1140. https://doi.org/10.3390/metabo13111140
Kaupper CS, Blaauwendraad SM, Cecil CAM, Mulder RH, Gaillard R, Goncalves R, Borggraefe I, Koletzko B, Jaddoe VWV. Cord Blood Metabolite Profiles and Their Association with Autistic Traits in Childhood. Metabolites. 2023; 13(11):1140. https://doi.org/10.3390/metabo13111140
Chicago/Turabian StyleKaupper, Christin S., Sophia M. Blaauwendraad, Charlotte A. M. Cecil, Rosa H. Mulder, Romy Gaillard, Romy Goncalves, Ingo Borggraefe, Berthold Koletzko, and Vincent W. V. Jaddoe. 2023. "Cord Blood Metabolite Profiles and Their Association with Autistic Traits in Childhood" Metabolites 13, no. 11: 1140. https://doi.org/10.3390/metabo13111140
APA StyleKaupper, C. S., Blaauwendraad, S. M., Cecil, C. A. M., Mulder, R. H., Gaillard, R., Goncalves, R., Borggraefe, I., Koletzko, B., & Jaddoe, V. W. V. (2023). Cord Blood Metabolite Profiles and Their Association with Autistic Traits in Childhood. Metabolites, 13(11), 1140. https://doi.org/10.3390/metabo13111140






