Abstract
The gut microbiota is a complex and dynamic ecosystem essential for the proper functioning of the organism, affecting the health and disease status of the individuals. There is continuous and bidirectional communication between gut microbiota and the host, conforming to a unique entity known as “holobiont”. Among these crosstalk mechanisms, the gut microbiota synthesizes a broad spectrum of bioactive compounds or metabolites which exert pleiotropic effects on the human organism. Many of these microbial metabolites can cross the blood–brain barrier (BBB) or have significant effects on the brain, playing a key role in the so-called microbiota-gut-brain axis. An altered microbiota-gut-brain (MGB) axis is a major characteristic of many neuropsychiatric disorders, including major depressive disorder (MDD). Significative differences between gut eubiosis and dysbiosis in mental disorders like MDD with their different metabolite composition and concentrations are being discussed. In the present review, the main microbial metabolites (short-chain fatty acids -SCFAs-, bile acids, amino acids, tryptophan -trp- derivatives, and more), their signaling pathways and functions will be summarized to explain part of MDD pathophysiology. Conclusions from promising translational approaches related to microbial metabolome will be addressed in more depth to discuss their possible clinical value in the management of MDD patients.
1. Introduction
Microorganisms belonging to the human body are acquiring more relevance nowadays as they establish mutualistic relationships with our cells modulating our health status. Since the early 1990s, Lynn Margulis stated the basis of endosymbiotic theories and coined the term “holobiont”, explaining the coevolution of symbionts and host [1], microbes inhabiting the human being have gained more attention in health research and clinical practice. Although today we also know that nearly 50 years before, Adolf Meyer-Abich had already started talking about his forgotten and not spread theory of “holobiosis” concept defending that the assemblage of independent organisms could create a strong whole “holobiome” [2]. There are different ecosystems of bacteria, protozoa, archaea, and viruses distributed along the whole body (skin, mouth, intestine, vagina…) playing a key role in the homeostasis by communicating bidirectionally with host immune cells and functions. In this context, the Human Microbiome Project (HMP) was born in 2007 collecting biological and clinical data for the research of microbiome populations and behavior in health and disease [3]. The scientific bibliography alleges that the correct proportion of microbes with respect to human cells is approximately 1:1, being bacteria the most representative kingdom [4].
Gut microbiota is considered as a dynamic organ, as some say, “the last human organ under active research” [5]. Its microbial composition varies across the digestive tract, being the colon, the place containing the largest population of bacteria in the organism (3.8 × 1013) with the dominant phyla: Firmicutes and Bacteroidetes in a proportion of 90%, and a 10% of Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia [6]. Diversity and relative abundances of different species of bacteria also vary through the individual’s life stages, being some beneficial genera declined with associated age progression [7]. The dynamism of gut microbes is heavily shaped by environmental factors, being the diet the greatest but not the only one. All these vital factors that alter gut microbiota homeostasis are also the essential pillars of health: diet, physical activity, stress, and sleep hygiene, all these affected by chronobiology.
Microbial dysbiosis can contribute to alterations in the physiological response [8], setting the progression of inflammatory and neurodegenerative diseases [9]. The impaired gut microbiome operation modulating the disrupted immunostasis makes the host being more susceptible to infections, hypersensitivity reactions, autoimmunity, chronic inflammation, and even cancer [10]. The dysbiosis condition is known to have a significant correlation with immunological and neuroimmunological status in physical and psychological pathologies. This imbalance leads to a disruption in the already known microbiota-gut-brain axis (MGB) and explains part of the altered microbial and human production of neurotransmitters like serotonin, dopamine, gamma-aminobutyric acid (GABA), and noradrenaline [11], and the distorted crosstalk dialogue of other microbial metabolites with neuroimmune functions. All in all, the health of the ecosystem that harbors the intestine, signifies one more key element in the complex network of multifactorial non-communicable diseases, many of them being comorbid metabolic diseases and mental disorders like the greater and greater emerging Major Depressive Disorder (MDD).
MDD is approximately affecting 350 million people in the world, which implies nearly 5% of the entire population and supposes the third leading cause of disability. The latest numbers collected by the Global Health Data Exchange (GHDx) and the World Health Organization (WHO) show that in its most severe manifestation, this incapacitating condition leads to 700,000 suicide commitments every year, ranking the fourth cause of death among individuals in the 15–19 age group; and it is also estimated that more than 3 out of 4 patients do not ask for help and not receive treatments in low and middle-income countries, where perhaps social stigma is greater [12,13]. There are differences of prevalence regarding sex too: more women suffer from depression than men [14] and more men die due to suicide than women [15].
Healthcare costs and economic burden derived from the loss of productivity reach more than 200 billion $. The quantity is expected to keep rising these days due to the impact of COVID-19 pandemic times, which has exacerbated more social stressors like isolation and unemployment besides the illness [16]. At the same time, this public health emergency, considered also as a global psychological pandemic [17], has promoted to invest in finding mental wellbeing in most countries [18], which hopefully has shed more light to mitigate the barriers of self and public’s beliefs and stigma related to mental health disorders.
Every time, it is becoming more evident that the rising prevalence of depression, especially in developed countries, is linked to changes in dietary and sleep patterns derived from high levels of stress related to occupations, studies, and social relationships, among others. The research suggests that the base medical treatment frequently tends to fail when those aspects are not addressed [19] as psychiatric drug therapies alone, although may stabilize the patient, are not enough to cure the disorder. For this reason, nowadays holistic approaches are kept in mind for improving mental health care. This management may include nutritional intervention, peer supports, self-control of stress with psychotherapy and guided mindfulness techniques; and, encouragement for practicing physical exercise and re-introducing good habits to ameliorate the individual’s chronobiology, inflammatory status, and symptoms.
In the present work, we will focus on the evidence from adjuvant therapies primarily based on nutritional intervention with supplementation or advising certain foods containing prebiotics that boost the metabolome function in the so appealing MGB axis. After brief statements about the biological basis of MDD and microbial metabolites motion in the pathophysiology of this condition; this descriptive review mainly aims to gather the most recent updates related to translational applications of bioactive compounds equivalent to microbial products and other feasible ways to target gut microbiota functioning, from research to clinical practice, all in the MDD framework.
2. The Role of Gut Microbiota in Major Depression: The Microbiota-Gut-Brain Axis
Once upon a time, the brain was thought to be an organ with relative independence from the rest of the body. Nowadays it is widely accepted that there is an intricate, bidirectional, and integrative brain-body interplay which may be summarized by the famous quote “Mens sana in corpore sano” [20,21,22]. In this sense, different systems have been recognized in this relationship, like the psychoneuroimmunoendocrinology [23] or the interaction between the psychology, nervous, endocrine, and immune system-, a heart–brain communication [24] or how the brain affects and is affected by the heart- and of course a muscle–brain crosstalk [25] the regulation of muscle contraction from the nervous system and their derived effects on the proper brain. One structure should perhaps be noticed as a critical modulator of proper brain functioning and well-being, the gut. Indeed, several authors frequently considered the gut as the “second brain” [26]. As the gut microbiota is a major representative element located in this organ with pleiotropic and vital functions, not only at local but also at systemic levels, the authors have designed an MGB axis to the complex relationship between these elements [27].
There are three main mechanisms by which the MGB axis works. The first is related to the presence of a prominent innervation in the gut, represented by an intrinsic or enteric nervous system (ENS) and an extrinsic or central nervous system (CNS). The former is essential for gastrointestinal functioning and the second is responsible for a thorough monitoring and regulation of this system through three different pathways: (1) The vagus nerve, projected from the brainstem; (2) The thoracolumbar spinal cord and (3) Lumbosacral spinal cord, all of them presenting afferent (sensory) innervation and efferent (motor innervation) [28]. It is of note that both the ENS and CNS fulfill mutual actions to control the gut activity and the microbiota. In turn, the microbiota and intestinal cells may also influence the neural networks of the ENS and CNS. For instance, the release of some neurotransmitters by the efferent neurons from the CNS in the gut may be collected by certain types of bacteria, determining the growth of those microbial populations and simultaneously, the presence of some microorganism may produce or modulate the release of some neurotransmitters and metabolites from the gut to the afferent pathways to the CNS [29]. Thus, through this mechanism, the brain may influence the gut and microbiota function while the microbiota and the gut may orchestrate the CNS and brain activity. The second great mechanism by which the MGB axis works is the immune system. There are several mechanisms implicated in the gut microbiota-immune systems interplay, including microbial products, inflammatory cytokines, and the proper bacterial metabolites [30]. An adequate microbial composition (eubiosis) is related to a proper and regulated immune response. Conversely, an altered microbiota (dysbiosis) is accompanied by an exacerbated immune response and damage in the intestinal barrier. Eventually, this may lead to bacterial translocation and endotoxemia, enhancing a condition of systemic inflammation, negatively affecting the brain [31,32,33]. Finally, the third pathway involved in the MGB axis is the systemic widespread to the bloodstream of several elements, including hormones, metabolites, or neurotransmitters [34].
As summarized in Figure 1, the gut microbiota is a major source of several metabolites with critical functions in the MGB axis by several pathways, including the stimulation of the vagus nerve, modulating the gut, systemic and neuroinflammation, central neurotransmission, and the integrity of the blood–brain barrier (BBB), also binding to host receptors in the brain [35,36]. Several studies have reported the role of abnormal metabolite production by gut microbiota in different neuropsychiatric disorders [37,38,39]. Thus, in this study, we will explore the role of microbial metabolites in the brain with a special focus on their role in the pathophysiology of MDD and the main translational opportunities derived from this knowledge.
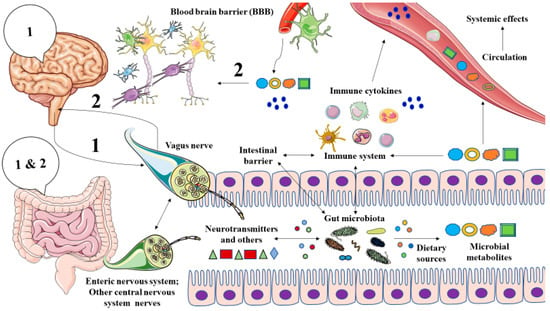
Figure 1.
A general picture of the microbiota-gut-brain axis. As represented, there is bidirectional communication between the brain and the gut through different mechanisms. The vagus nerve may send signals (“1”) from the brain to the gut, although there may be other indirect mechanisms like endocrine factors that could reach the gut. The gut, and prominently, the microbiota integrates these signals and in turn, they respond by different pathways to the brain. Those mechanisms include (1) Vagal activation, due to the direct action of the gut microbiota or through the interactions with other nervous fibers from the enteric nervous system or central nervous system; (2) By influencing the local and systemic immune system and intestinal barrier, leading to the production of a set of signaling molecules -mainly cytokines- that are distributed via systemic circulation and (3) Thanks to the production of different microbial metabolites that are either transported by systemic circulation or exert immunomodulatory effects, or stimulate the vagus nerve or signaling in different tissues within the body. Both cytokines and microbial metabolites can cross the blood–brain barrier, therefore providing an effect on the brain. Overall, these 3 mechanisms send a signal to the brain (“2”), which integrates the information received from the gut.
3. Microbial Metabolites in MDD
3.1. Biological Basis of MDD
MDD is a multifactorial and entangling concern with many psychosocial and biological changes implicated. What causes MDD in an individual is not fully understood and several factors appear to be involved in the origin of this complex disorder. For instance, particular polymorphisms in some genes affecting the functioning of various critical areas in the brain appear to be associated with a higher risk of suffering from MDD [40,41]. However, no single gene has been identified as a clear causative agent of MDD and genetic susceptibility may be accompanied by other environmental factors to result in MDD. Early-life stress (ELS) is perhaps one of the most plausible causative agents related to the development of MDD and previous studies have demonstrated an increased risk of suffering from depression and many psychiatric disorders in individuals exposed to ELS, even in childhood and adolescence [42,43]. The biological explanation of this association is that ELS triggers an epigenetic reprogramming in the brain leading to the onset of several pathophysiological mechanisms involved in the genesis of MDD [44]. Similar effects may be attributed to the exposure to chronic stress, driving to the development of MDD [45]. Another pivotal determinant related to the increased risk of MDD that should be noticed here includes sex. Prior epidemiological studies have found that the risk of MDD is approximately twofold higher in women than men, although this difference may be even higher in younger ages [14]. Different reasons have been given here, including genetic vulnerability, hormonal fluctuations related to reproductive function, and a plethora of psychosocial factors [46]. This means that men and women often display different symptoms and behaviors in response to MDD [15]. Thus, sex should be considered as an important variable related to the development of MDD although many social and cultural determinants may also contribute to the pathophysiology of MDD [47].
The biological basis of the MDD could be classified at three great levels: (1) Molecular and cellular alterations affecting the neurons and glia; (2) Structural and functional impairment in key brain regions and (3) Systemic factors that hit the central nervous system. The molecular and cellular basis of MDD involves a set of alterations in the neuronal and glial behavior, function, communication, and even on their viability that are observed in depressed subjects. These changes often occur in certain vital regions of the brain which may increase or reduce their size as well as their connectivity and functionality [48]. Besides, systemic signals from the rest of the body may impact the brain directly or indirectly. Thus, a broad spectrum of mechanisms is working in the pathobiology of MDD as it will be summarized hereunder.
Within the brain, neurons are strongly dysregulated in depressed individuals. There are plenty of molecular changes in these cells, all related to a dysregulation of neuroprotective and neurotrophic signals. In this sense, it has been elucidated the central role of brain-derived neurotrophic factor (BDNF), a critical molecule downregulated in MDD with important effects in neurogenesis, neural growth, maturation, survival, and neuroplasticity [49]. Similar consequences are also observed for the glial cells, which are notably reduced in frontal cortical and limbic brain regions [50]. An important consequence of this glial dysregulation is a synaptic dysfunction in these areas that are considered as one of the major pathophysiological mechanisms of MDD [51]. The monoamine hypothesis was one of the first discoveries of disrupted neurotransmission in MDD. This theory supports that the monoamines (serotonin, dopamine, and noradrenaline) are importantly dysregulated in the brain of depressed patients and the evidence shows that many of the antidepressants target these components [52]. However, the role of monoamines in the pathophysiology of MDD is incomplete, as a representative group of the patients may only exert a partial or absent response to these therapies received thereby supporting that the monoamines are a part but not the entire picture of MDD [53,54]. Other important neurotransmitters dysregulated in MDD are GABA, acetylcholine, and glutamate.
Likewise, different neuropeptides are also implicated in the pathophysiology of MDD. Vasopressin (AVP) and oxytocin (OXT), two main nonapeptides prominently involved in a plethora of social and behavioral functions seem to be significantly affected in depressed subjects. It is thought that an imbalance between AVP and OXT in favor of the former may be related to the development of MDD [55]. AVP and OXT are also related to an abnormal functioning of the Hypothalamic Pituitary Adrenal (HPA) axis, a central mechanism in many psychiatric disorders like MDD [56]. The altered HPA axis is strongly related to the body’s response to stress. This system is frequently hyperactivated in depressed subjects, characterized by an augmented corticotropin release factor (CRF), reduced negative feedback probably due to a resistance of glucocorticoid receptors (GR) and hypercortisolism [57]. Melatonin, a major neuropeptide involved in circadian rhythms is equally an important point of study in many psychiatric disorders, as depressed patients show an altered melatonin action and chronobiology that may be considered in the development of effective therapies [58].
Oxidative stress (OS) is an important local and systemic marker of damage observed in patients with MDD [59]. OS is the result of an imbalance of oxidants (free radicals) production with regard to antioxidants, leading to local damage in the brain with systemic consequences [60]. Aberrant neuroinflammation is a major source of OS in the brain and turn, the OS is a key driver of the neuroinflammatory process [61]. Besides, compelling evidence has demonstrated that neuroinflammation is a key factor involved in the abnormal neurotransmission, HPA axis, and neurogenesis, with a pivotal role of the microglia in all these events [62]. The origin of the neuroinflammation may be either primary due to an impaired vascular function in the brain and/or underlying damage in this structure or secondary being related to any inflammatory condition related to an augmented cytokine production that is able to cross the BBB [63].
As above mentioned, a disrupted MGB axis is implicated in the development of several neuropsychiatric diseases including MDD, which has been proven to influence several pathophysiological mechanisms implicated in this mental disorder [64]. In the following section, we will summarize the role of microbial metabolites, a crucial way of communication in the MGB axis in the pathophysiology of MDD, with a special emphasis on the bidirectional implications of the brain and gut disturbances.
3.2. Microbial Metabolites Involved in the Pathophysiology of MDD
By using the different nutrients and components ingested in the diet, the gut microbiota produces different metabolites targeting the proper microbiota and host cells [65]. Technological advances in the field of metagenomics, metatranscriptomics, and metabolomics have facilitated a better comprehension of the gut microbiota functionality, having brought the discovery of plenty microbial metabolites with prominent consequences for health and disease [66]. Hereunder, we will describe some of the most relevant microbial metabolites involved in the development of MDD.
3.2.1. Short-Chain Fatty Acids
Short-chain fatty acids (SCFAs) are perhaps one of the most deeply studied metabolites derived from gut microbiota. SCFAs are a subtype of fatty acids notably abundant in the proximal colon due to the fermentation of partial and non-digestible polysaccharides like dietary fiber or resistant starch [67]. There are three main types of SCFAs: Butyrate, propionate, and acetate. Eubacterium rectale, Roseburia faecis, Eubacterium hallii, and Faecalibacterium prausnitzii appear to be the major producers of butyrate in the gut [68]. Veilonella spp., Lactobacillus spp., Bacteroides spp., and Propionibacterium spp. are some of the most representative bacteria involved in propionate production [69]. Conversely, acetate production is commonly spread among numerous bacterial classes [70]. Virtually all cell types present at least one type of SCFA receptors, which include free fatty acid receptor-2 and -3 (FFAR2 and FFAR3), G-Protein-Coupled receptor (GPCR) like GPR43, GPR41, GPR109a, and Olfr78 [71,72].
The local actions of SCFAs in the gut are multiple, aiding to keep the intestinal integrity by regulating the luminal pH, mucus production, epithelial cells activity, and the immune system, being also crucial for microbial communication, affecting quorum sensing and limiting the invasion or growth of different microorganisms and pathogens [73,74]. Moreover, the action of SCFAs extends far beyond the gut, exerting pleiotropic functions in the entire organism and influencing several host processes, with a remarkable role in the host metabolism and epigenomic modifications [75]. Besides, SCFAs are shown to be a central mechanism of gut-brain communication. SCFAs may interact with the enteroendocrine cells and promote indirect signaling to the brain by the systemic circulation or vagal pathways, inducing the production of several hormones and neurotransmitters like GABA and serotonin in the gut. SCFAs are also related to a systemic regulation of inflammation and cytokine profile, being able to cross the BBB positively influencing its integrity and activating several mechanisms in the brain, modulating the levels of neurotrophic factors, neurotransmitters, neurogenesis, and reducing the neuroinflammation and glial dysfunction [76]. Thus, SCFAs may mediate a set of mechanisms potentially involved in the pathobiology of MDD.
Jiang et al. [77] compared the fecal microbiota composition in patients with MDD and healthy individuals. Despite a wide variety of phyla and specific bacteria being significantly altered in depressed versus non-depressed subjects, reduced levels of Faecalibacterium, an important butyrate producer was observed in patients with MDD, with a negative correlation with depression severity. In this line, Valles-Colomer et al. [78] also found that butyrate-producing Coprococcus spp. were also depleted in depression, being both Faecalibacterium and Coprococcus abundance directly associated with a higher quality of life indicators. Likewise, Wu et al. [79] compared the gut microbiome of depressed and non-depressed mice to explore the associations between these differences with SCFAs and neurotransmitters changes. They found that 29 different bacterial taxa can be found between depressed and non-depressed animals, remarking the abundance of the Allobaculum genus and Ruminococcaceae family. Significant differences in acetate, propionate norepinephrine and serotonin were reported, with a positive association between Allobaculum, acetate, and serotonin levels. Many studies have described the central action of SCFAs in serotonin production mainly due to their interaction with enteroendocrine cells [80]. In this sense, different mechanisms and specific microbial populations have been demonstrated. For instance, Engevik et al. [81] have recently studied the relevance of Bifidobacterium dentium and acetate in the release of serotonin both in rodent and human enteroids, which is also associated with an enhanced expression of the serotonin receptor 2a in the hippocampus in comparison to non-treated mice. Besides, spore-forming bacteria also appear to be critical modulators of serotonin production by enteroendocrine cells, prominently due to their effects in SCFAs production [82]. Biochemically, SCFAs appear to induce tryptophan hydroxylase 1 (tph1) in vitro, a rate-limiting enzyme implicated in serotonin production and the marker of neuroendocrine secretion chromogranin A by enteroendocrine cells [83].
A significant reduction of fecal SCFAs between depressed and non-depressed women was also proposed to be a potential contributor of suffering from MDD with a possible inverse association with depression severity [84]. Fecal measures of SCFAs also found a significant association between acetate, propionate, and butyrate levels with both depressive and gastrointestinal symptoms in young adults, therefore supporting that SCFAs are importantly related to the development of MDD [85]. SCFAs and particularly propionate production are associated with a decreased anticipatory response of the reward system, related to the monoamine dopamine [86].
As previously reviewed, altered dopaminergic neurotransmission is a major feature underpinning MDD, which is prominently related to the clinical manifestation of anhedonia one of the two main criteria of MDD [87]. Prior studies have proven the antidepressant effect of butyrate by reversing behavioral alterations in mouse models like anhedonia [88], low energy, cognitive and social abilities [76]. Similarly, the use of rifaximin was related to a decreased depressive-like behavior due to its favorable effects on Ruminococcaceae and Lachnospiraceae, positively correlated with butyrate production [89].
Two central mechanisms should be highlighted to understand the anti-depressant effect of SCFAs: (1) epigenetic regulation and (2) immunomodulatory action. Depressed patients show a prominent epigenetic footprint in comparison to non-depressed subjects, including critical alterations in DNA methylation, histone modifications, and non-coding RNAs (i.e., microRNAs) [90,91,92]. SCFAs act as histone deacetylases (HDAC) inhibitors, playing a key role in the DNA methylation, histone modification, and chromatin restructuring to alter gene expression [93]. For instance, SCFA-mediated inhibition of HDAC may lead to the hyperacetylation of histones H3/H4 and hence increase BDNF expression, showing antidepressant effects in mice [94]. This epigenetic modulation is mostly responsible for the immunomodulatory action of SCFAs. In the CNS, SCFAs are major mediators of microglial maturation and function whereas, in the gut, SCFAs induce the differentiation of Treg cells [93].
Overall, the epigenetic and immunomodulatory effects of acetate, and more prominently propionate and butyrate exert promising antidepressant effects, as they are mostly correlated with favorable microbial populations. Conversely, another type of SCFA, isovaleric acid has been shown to be correlated with certain bacterial populations related to MDD and augmented cortisol levels. A proposed mechanism of this pro-depressive effect of isovaleric acid is through interfering with synaptic neurotransmitter release [95]. Future studies should be destinated to deepen the different mechanisms of SCFAs in the pathology of MDD with a possible focus on some unexplored mechanisms like microRNAs.
3.2.2. Lactate
Lactate is a crucial metabolic product of the anaerobic glycolysis involved in multiple physiologic and pathologic conditions due to its role as a relevant signaling molecule [96]. Despite not being considered as an SCFA, lactate is an important metabolite produced by some microorganisms including lactic acid bacteria, bifidobacteria, or proteobacteria and frequently it is converted into SCFA by several types of microorganisms [97]. Because of that, lactate is not abundantly found in the colon; however, it has been demonstrated that under physiological conditions, this metabolite can cross the BBB to match the energetic needs of the brain, influencing many neuronal functions such as excitability, plasticity, and memory consolidation [98]. The gut microbiota is not the only source of lactate, as this is also produced by the astrocytes in the brain, which is a local reservoir of lactate that transfers this metabolite to neurons and more prominently, by muscle cells during exercise [99].
The role of lactate in different brain disorders is undoubtedly complex. Bradley et al. [100] found that adolescents with MDD showed increased ventricular lactate, suggesting that mitochondrial dysfunction may be responsible for the augmentation of this component. Patients with bipolar depression (BD) also show an increased brain lactate detection in the brain that is ameliorated after treatment [101]. On the other hand, patients with severe depression show a significant detection of lactate in the urine in comparison to moderate and non-depressed subjects [102], therefore supporting the possible role of lactate in the etiopathogenesis of MDD. Conversely, lactate may also exert potential antidepressant effects in the brain by several mechanisms. One of them is related to the ability of lactate to revert the effects of stress on inducing depressive-like behavior, mainly supporting the hippocampal neurogenesis [103]. Lactate also promoted resilience to stress and rescued social avoidance and anxiety by restoring hippocampal HDAC levels and activity [104]. Similarly, the peripheral administration of lactate exerts antidepressant effects that were correlated with raised hippocampal lactate levels and changes in serotonin receptor trafficking, astrocyte functions, neurogenesis, nitric oxide synthesis, and cAMP signaling [105].
Overall, the role of lactate in the etiopathogenesis of MDD is poorly understood. However, a plausible explanation based on the available literature is that an accumulation of lactate in the brain due to an impaired mitochondrial function -a major feature of different psychiatric diseases- [106] or due to unknown mechanisms may be a cause or a consequence of MDD. Oppositely, increased levels of lactate in some critical brain regions like the hippocampus, possibly obtained from systemic sources like the microbiota or the muscles may be related to antidepressant effects. Physical exercise may be a powerful link between lactate and antidepressant effects influencing not only the muscle production of lactate but also the MGB axis [107].
3.2.3. Tryptophan Metabolites
Tryptophan (Trp) is an essential and the least abundant amino acid with many bioactive effects in the body, especially through its multiple metabolites [108]. Gut microbiota is a key mediator of the actions of Trp. Despite some microbial species like Escherichia coli can synthesize Trp, there is not enough evidence supporting the relevance of gut microbiota. Conversely, the gut microbiota is critical for Trp metabolism, determining the benefits or harms [109]. The gut microbiota can convert Trp into a large list of products including small molecules like the antifungal pyrrolnitrin to long peptides with antibacterial activity as cyclomarin [110]. However, the most relevant metabolites derived from Trp with important actions for the MGB axis are (a) The transformation of Trp into the neurotransmitter serotonin, with benefits in the brain and gut function, and (b) the metabolism of Trp into kynurenine, tryptamine, and indole with neuroendocrine and immune-modulatory actions [111]. 5 bacterial phyla have been related to Trp metabolism, including Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Fusobacteria. Of them, Clostridium, Burkholderia, Streptomyces, Pseudomonas, and Bacillus appear to be the most relevant genera involved in these events, according to an in silico study [112].
Because of the direct influence of Trp in serotonin synthesis, this amino acid may be pivotal for mood and cognitive modulation, hence supporting a possible role of Trp and its metabolism in the onset of MDD [113]. In this context, some studies demonstrate the benefits of certain types of bacteria involved in Trp metabolism. Tian et al. [114] reported how the presence of Bifidobacterium longum subsp. infantis E41 and Bifidobacterium breve M2CF22M7 appears to be related to an augmented level of 5-hydroxytryptophan, tph1, serotonin, and BDNF in the brain, significatively reducing depressive behavior. However, despite these effects, scientific evidence has failed to find promising and global benefits from increasing dietary Trp or by supplementation, which may benefit only some specific groups of patients who have a personal or family history of depression [115]. A potential explanation of this fact is that the gut dysbiosis found in patients with MDD may interfere with Trp metabolism and serotoninergic activity, as has been shown in some animal models [116]. In this line, it is widely accepted that an imbalance between the kynurenine pathway mediated by the enzyme indoleamine 2,3-dioxygenase (IDO) and serotonin synthesis may be a critical event of MDD. The pro-inflammatory environment in the gut leads to an increase in the activity of IDO, leading to enhanced production of some neurotoxic metabolites like 3-hydroxykynurenine and quinolinic acid and a decrease in serotonin synthesis [117]. Likewise, it seems that enhanced IDO activity may be a prominent pathophysiological mechanism related to the inflammation-induced depressive-like behavior by endotoxemia [118].
Interestingly, these alterations may also be responsible for the hyperactivation of the HPA axis [117]. These results are in consonance with some systematic reviews and meta-analysis that showed that patients with MDD displayed reduced levels of Trp, kynurenine, and kynurenic acid -two components of this pathway with neuroprotective effects-; low kynurenine to Trp ratio-supporting the imbalance between kynurenine route and serotonin synthesis- and an augmented detection of different neurotoxic substances from this pathway like quinolinic acid [119,120]. Therefore, dysregulation of the Kynurenine pathway with an increased neurotoxic/neuroprotective ratio appears to be a critical pathophysiological mechanism involved in MDD.
Apart from serotonin depletion, the aberrant kynurenine pathway is also related to abnormal glutamatergic neurotransmission [121] and more worryingly, with neuroinflammation. Tryptophan, kynurenine, 3-hydroxykynurenine, and cytokines may cross the BBB. In the brain, cytokines, and glucocorticoids enhance the kynurenine pathway, driving to an increased formation of the neurotoxic quinolinic acid in the microglia and infiltrated macrophages, favoring the neuroinflammatory response [122]. Many of the kynurenine metabolites exert their immunomodulatory action through the activation of aryl hydrocarbon receptors (AHR) and that single nucleotide polymorphisms (SNPs) affecting this gene may be involved in the development of different immune and inflammatory diseases [123]. In patients with MDD, SNPs affecting AHRs were associated with interindividual variations in the plasma levels of kynurenine that in turn was associated with MDD severity [124].
Moreover, the kynurenine pathway may also interfere with the tryptamine activity in the brain [121]. The function of tryptamine in the brain is not fully understood yet, although it is hypothesized that it may play a possible neurotransmitter or neuromodulatory role [125]. In the gut, different microorganisms are able to synthesize tryptamine through the enzyme tryptophan decarboxylase. An increased tryptamine production may be related to reduced Trp bioavailability and serotonin synthesis in the brain [126]. Finally, Trp may also be converted into indole, indican, and skatole as well as indole acid derivatives by the gut microbiota. These metabolites as well as tryptamine are also important ligands of AhRs [127]. Jaglin et al. [128] demonstrated that chronic overproduction of indole by gut microbiota was related to anxiety- and depressive-like behaviors in rats, possibly by augmenting brain levels of oxindole and through the activation of the vagus nerve. Conversely, it seems that certain types of indoles produced by the gut microbiota may exert neuroprotective effects like indole propionic acid [129]. Because of that, the analysis of indole and indolic derivates as blood biomarkers in different disorders like MDD is receiving growing attention [130].
Overall, compelling evidence is supporting the relevance of gut microbiota-derived Trp metabolites in the etiopathogenesis of MDD. However, further efforts to unravel the role of Trp metabolites in depression are still needed, especially regarding tryptamine, indole, and their derivates.
3.2.4. Microbial Influence in Other Amino Acids
Not only Trp but also other amino acid metabolism appears to be tightly linked to the gut microbiota, with noteworthy implications for patients with MDD. Glutamate and phenylalanine are some amino acids significantly altered in body fluids of depressed subjects [131,132,133]. Gut microbiota plays a pivotal role in the regulation of both amino acids. Regarding glutamate, many bacteria consume this amino acid to generate energy or to form their structural components. Simultaneously, other bacteria can produce glutamate and some species may synthesize GABA from glutamate [134]. Among the main glutamate producers, it should be highlighted the role of coryneform and lactic acid bacteria whereas some gram-positive and gram-negativee bacteria expressions transform glutamate into GABA through the enzyme glutamate decarboxylase (GAD) [135]. Lactobacillus and Bifidobacterium are two central genera involved in the production of glutamate and GABA. Altaib et al. [136] found an interesting association between the fecal abundance of the Bifidobacteriaceae family and GABA levels in healthy individuals. Bifidobacterium dentium appears to be a pivotal microorganism involved in GABA production from glutamate, glutamine, and succinate, which was also related to an augmented tyrosine production [137]. Bifidobacterium adolescentis, and particularly the strains PRL2019 and HD17T2H also seem to be major producers of GABA in the gut [138] whereas Bravo et al. [139] studied that the administration of Lactobacillus rhamnosus (JB-1) induced through vagal pathway noteworthy alterations of GABA receptor expression in different brain regions related to MDD, including cortical structures like the cingulate and prelimbic regions, the hippocampus, amygdala, and locus coeruleus. Besides, Bacteroides Parabacteroides and Escherichia species are also importantly involved in the synthesis of GABA, with a negative correlation between fecal Bacteroides and brain signatures associated with depression [140]. In this line, a recent study conducted by Otaru et al. [141] has shown that Bacteroides spp. is a critical agent involved in the GABA production due to the activation of the GAD enzyme in a pH-dependent manner, acting as a protective mechanism against acid stress in these microorganisms. Collectively, gut microorganisms are key mediators of glutamate and GABA production, with important neurotransmitter activity in the brain. Similarly, phenylalanine, another product also synthesized by some microorganisms, is the precursor of the catecholamines epinephrine, norepinephrine, and dopamine, which are involved in the pathogenesis of MDD and therapy resistance [142].
Studies of the fecal metabolome and microbiota in chronic unpredictable mild stress (CUMS) rats, an animal model of depression reported consistent changes in the levels of many amino acids like threonine, isoleucine, alanine, serine, tyrosine, and oxidized proline, thereby suggesting that alterations in the gut microbiota are linked to changes in circulating amino acids and depressive behavior [143]. In this line, Kawase et al. [144] compared the serum levels of different amino acids in specific pathogen-free (SPF) and germ-free (GF) mice. Interestingly, they claimed that the concentration of different amino acids in the host brain seems to be dependent on the presence of the gut microbiota, concluding that amino acid metabolism in the host brain could be modified only by manipulating gut microbial communities. Moreover, Steffens et al. [145] reduced levels of phenylalanine, aspartate, glutamate, and serine were found in depressed individuals with heart failure in comparison to patients without MDD, although the role of heart failure in these differences was unclear.
Collectively, almost all the amino acid levels were altered in animal models and human patients of MDD [134]. Gut microbiota is likely a central mediator of these changes, participating in the synthesis and metabolism of many amino acids. Future studies are warranted to understand the precise actions of gut microbiota in these components and how it could be managed.
3.2.5. Secondary Bile Acids
Bile acids (BA) are crucial physiological agents with prominent functions in digestion, nutrient absorption, and the elimination of lipids, toxic metabolites, and xenobiotics. BA are key regulators of the gut microbiota composition and in turn, gut microbiota metabolizes BA, determining the bile acid pool size [146]. Primary BA is produced by the liver and secreted into the small intestine whereas secondary BA corresponds to the metabolism of these products by the gut microbiota. Cholic acid (CA) and chenodeoxycholic acid (CDCA) are the two major primary BA and they are frequently conjugated with taurine or glycine for secretion into bile [147].
On the other hand, secondary BA is formed by different reactions including the biotransformation and hydrolysis of conjugated BA to free BA and taurine or glycine thanks to the action of the bile salt hydrolase (BSH); 7α-dehydroxylation of the CA or CDCA leading to the conversion of deoxycholic acid (DCA) and lithocholic acid (LCA), the epimerization of CDCA into ursodeoxycholic acid (UDCA) and subsequently the 7β-dehydroxylation of UDCA yielding LCA [148]. Most BSH bacteria are Gram-positive gut bacteria including Clostridium, Enterococcus, Bifidobacterium, and Lactobacillus. The genus Bacteroides is the only Gram-negative bacteria with BSH, and some archaea like Methanobrevibacter smithii and Methanosphera stadmanae may also have BSH activity [149].
The dehydroxylation of BA is also conducted by many bacteria from the Bacteroides and Clostridia genus, and in E. coli, C. testosteroni, and Ruminococcus spp. [150]. Approximately, 95% of the BA is reabsorbed in the terminal ileum and returned to the liver via enterohepatic circulation, including secondary BA [151]. However, BA also exerts multiple metabolic functions in the body as well as acting as signaling molecules due to their binding to two main receptors: Farnesoid-X-Receptor (FXR) and the G-Protein-Coupled Bile Acid receptor-1 (GPBAR-1, also known as TGR5) [152]. Therefore, secondary BA influences a plethora of modulatory functions not only at local but also at systemic levels.
In this sense, previous studies have supported that secondary BA is a critical molecule involved in the MGB axis. The brain may also present its BA pool, and despite the brain may synthesizing its BA, it is mostly suspected that BA acts as a communication bridge between the gut microbiota and the brain [153]. This statement is supported by growing evidence that describes a worrying association between an aberrant BA communication and the development of neurodegenerative diseases [154,155,156]. There are two main pathways by which BA may influence the brain: (1) BA may cross the BBB and bind to FXR and TGR5 receptors in the brain and (2) Activation of the intestinal FXR and TGR5 receptors can result in the release of fibroblast growth factor 19 (FGF19) and glucagon-like peptide 1 (GLP-1), leading to indirect signaling of BA to the brain [157].
Physiologically, the effects of BA in the brain are mainly mediated through GLP-1. However, under pathological conditions, the activation of the FXR may play a central role in the onset of different psychiatric disorders like MDD. In this sense, an overexpression of hippocampal FXR seems to cause depression-like symptoms and reduced BDNF levels in rats [158]. Conversely, the activation of TGR5 by the secondary bile acid tauroursodeoxycholic acid (TUDCA) and other receptors in the brain of BA like vitamin D receptor (VDR) and pregnane X receptor (PXR) activated by LCA appears to present antidepressant effects, thereby suggesting that the prodepressant or antidepressant effects of secondary BA in the brain may be dependent of the receptor in which they act, although this hypothesis must be confirmed [159].
Nowadays, the action of secondary BA on the pathogenesis of MDD remains to be fully elucidated. Simultaneously, the use of some antidepressant drugs such as paroxetine -a selective serotonin reuptake inhibitor (SSRI)- seems to alter gut microbiota and BA production in mice and it is unknown if these changes may be counterproductive for patients with MDD [160]. Further efforts are needed to unravel the relevance of secondary BA in MDD.
3.2.6. Vitamins
Vitamin deficiencies are frequently observed in patients with MDD, especially vitamin D and those from the B complex [161,162]. Vitamin D regulates gut microbiota composition through several mechanisms whereas gut microbiota may synthesize a wide variety of B vitamins and vitamin K [163,164]. Thus, despite vitamin D supplementation being an interesting approach to improve gut microbiota in patients with MDD, in this section we will explore the importance of B vitamins as a metabolite produced by intestinal bacteria.
Low levels of vitamin B1 (thiamine), B2 (riboflavin) B3 (niacin), B6 (pyridoxine) B7/8 (biotin), B9 (folate) and B12 (cyanocobalamin) are pivotal B vitamins associated with MDD [165,166]. However, despite B vitamin supplementation having provided some benefits for healthy individuals and at-risk populations for stress, the evidence has failed to support the use of these vitamins for ameliorating depressive symptoms [167]. A potential explanation may rely on the fact that gut dysbiosis impairs the synthesis of these vitamins [168]. Thus, the metabolic production of B vitamins from the gut microbiota is crucial for the proper functioning of the brain. Indeed, several studies are confirming the benefits of using certain vitamins in combination with probiotics, frequently represented by different species and strains of Bifidobacterium and Lactobacillus [166]. For instance, Reininghaus et al. [169] proved the effects of combining probiotic plus biotin supplementation for four weeks in patients with MDD. They reported that in comparison to the groups that only received vitamin B7 plus placebo or probiotics plus placebo, patients supplemented with both probiotics and vitamin showed an increased synthesis of vitamin B6 and B7 and improved clinical outcomes. Thus, the gut microbiota is crucial for determining the body levels of B vitamins, but an adequate dietary context is needed to obtain the benefits from those bacteria involved in vitamin synthesis, with noteworthy positive outcomes for the brain.
3.2.7. Choline-Derived Metabolites
Choline metabolites are an example of the importance of gut microbiota and their metabolites in mental health. Choline is a nutrient especially found in meat, milk, grain, eggs, and their derived products and fish [170]. Choline may be transformed into a wide variety of relevant metabolites like trimethylamine (TMA), betaine, phosphocholine, and acetylcholine. The gut microbiota plays a critical role in choline metabolism, is importantly involved in TMA production and then it is transformed into trimethylamine-N-oxide (TMAO) in the liver [171]. Moreover, there are some bacteria capable of using betaine obtained from dietary sources (i.e., dark green leafy vegetables, whole grains, or seafood) like Akkermansia muciniphila leading to some favorable effects due to the increase of SCFAs production and restoring gut microbial populations [172,173]. Both choline and betaine have been recognized as central biomarkers of cognitive performance [174,175]. Likewise, altered levels of choline in different brain regions have been associated with MDD [176,177], and betaine has been identified as a novel adjunctive therapy for improving the antidepressant effects of ketamine while preventing some its main adverse effects [178]. However, there is some evidence supporting that the gut microbiota status and the TMA/TMAO levels rather than choline or betaine may be better indicators of the favorable or detrimental effects of these metabolites [179]. Main bacteria involved in TMA production are included in the Firmicutes phyla, especially the Clostridium cluster XIVa and Eubacterium strains, but also some Actinobacteria and Proteobacteria [180]. Gut dysbiosis is harmfully associated with increased TMA and TMAO production, which may be accumulated in the different tissues and affect different organs and systems in the body, particularly the cardiovascular system and the liver [181]. Indeed, previous studies have found a positive relationship between TMAO levels and the severity of depressive symptoms in females and males [182]. In addition, Romano et al. [183] denoted that raised levels of choline-consuming bacteria may have detrimental effects on the global DNA methylation patterns in mice, as choline betaine are involved in regulating the host metabolism epigenetics and behavior.
All in all, future studies are needed to unravel the role of the gut microbiota in the mediation of choline, betaine, and TMA/TMAO effects in the brain of patients with MDD.
3.2.8. Estrobolome
The last point explored in this section is a novel but interesting approach that may serve to understand the pathogenesis of MDD in women: The estrobolome. This term refers to a set of bacteria that are able of metabolizing and modulate the estrogen levels in the body. Estrogen is transported in the blood through the sex hormone-binding globulin (SHBG), which is produced by the liver and determines the proportion of estrogen-free (Biologically active) or bound (Inactive) [184]. Free estrogen activates two distinct nuclear receptors, estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ), virtually regulating all biological processes [185].
The gut microbiota is a major determinant of the circulating levels of estrogens through different mechanisms. Firstly, they produce and secrete the enzyme β-glucuronidase, which deconjugates estrogen and increases the bioavailability of its active form [186]. Furthermore, some microorganisms break down indigestible dietary polyphenols to synthesize estrogen-like compounds or estrogen mimics that exhibit varied estrogenic potency [187]. In men and postmenopausal women, gut microbiota diversity and functions influence the levels of non-ovarian estrogens via enterohepatic circulation, where Clostridia taxa and genera from the Ruminococcaceae family could be key microbial populations involved in this regulation [188]. Likewise, estrogen levels influence the growth of certain types of bacteria as well as the consumption of the above-mentioned polyphenols, thus indicating the bidirectional role of the gut microbiota and estrogens [189]. On the other hand, gut dysbiosis may significantly impair the action of gut microbiota in the estrogen metabolism, driving the reduction of circulating estrogens, which is associated with a plethora of adverse outcomes ranging from obesity, metabolic syndrome, malignancies, endometrial hyperplasia, endometriosis, polycystic ovary syndrome, fertility, cardiovascular disease, and cognitive dysfunction [190].
The evidence supporting the role of altered sexual hormones in the pathogenesis of MDD in women could be found in the existence of three different types of depression related to the menstrual cycle: Premenstrual dysphoric disorder (PMDD), postpartum depression, and perimenopausal depression. The use of estradiol, but not progesterone appears to be effective for PMDD, postpartum, and moderate/severe perimenopausal depression [191,192]. These benefits may arise from the neuroprotective effects of estrogens mediating some neuroplastic actions in the hippocampus and regulating serotonin receptors levels. However, the use of estradiol as the first-line treatment is not supported by current scientific evidence [193]. Instead, targeting the estrobolome in the prevention and treatment of these specific disorders could be an effective interesting approach, as estrogen levels may be directly influenced by the gut microbiota and women may benefit from the actions of these bacteria. It is also of note that some studies have reported that lower levels of testosterone but not estrogen were associated with more severe depressive symptoms in a group of 23 women with MDD [194]. In this context, Markle et al. [195] reported that gut microbiota transplantation from male to female mice led to an augmentation of testosterone levels and metabolomic changes in females and future studies may consider the benefits from using testosterone-producer microorganisms to raise testosterone levels.
Considering the current evidence, as gut microbial diversity is directly related to the levels of non-ovaric estrogen levels and the intake of certain dietary components like polyphenols may aid in the modulation of beneficial microbial communities and ER activation, nutritional and lifestyle interventions may be a potential strategy to consider for boosting the estrobolome. Some examples of this strategy will be later discussed in the following section.
4. Translational Applications for the Depressed Patient
4.1. The Importance of Boosting Individual’s Microbial Metabolome
It is undeniable that every person harbors a unique gut microbiota that results from their genetic factors and history of epigenetics throughout life, implying seasonal and temporal variations as well [196,197]. Moreover, the development of certain body systems, especially the brain, coincide with the periods of major change in the microbiota, denoting the importance of microbiota establishment in the influence on epigenetic modifications shaping CNS, which could be interesting as “windows of opportunity” for disease prevention [29,198].
For these reasons, each patient’s approach must be personalized. Each individual’s alterations in microbiome explain the different individual’s responses to pharmacologic agents, hence talking not only about pharmacogenetics and pharmacogenomics; but also about “pharmacomicrobiomics”, as the different drug action and effect due to microbiome variations [199,200]. To standardize human biome types is also not an easy task due to the wide variety of across the different geographical areas and the different socioeconomic standards now that even two people from the same place with the same job and lifestyle may have many differences in their microbiomes and both may be perfectly healthy [201].
In all this context, it has been crucial to rely on systems biology, especially to study the interactions of microbial and host metabolomics determining the physiological characterization in health and disease based on plasticity and diversity of microbes and specific metabolites [202,203]. The challenge here resides in understanding how microbially produced metabolites subjected to the influence of diet and drugs can modulate epigenetic mechanisms and link them to disease risk [204,205]. Despite individual variations in health status, certain related-pathology patterns are repeatedly observed, and thus, the “Microbiome Wide Association Studies” (MWAS), mapping the microbiome, are providing new biomarkers of disease. These analyses allow clinical medicine to keep on the way towards integrating traditional clinical epidemiology with microbial one [206]. Nevertheless, there is still so much effort to put into the data analysis at this level, and on introducing algorithms in health systems to assess patients this way.
Furthermore, although colon microbiota has some differences with fecal, the latter keeps being useful for sampling methods, being non-invasive, and seems helpful in the discovery of pathology biomarkers. New sampling technologies are trying to find non-invasive solutions to palliate these differences [207]. Lessons from animal models have allowed the study of metabolites produced by representative gut microbiota in MDD. To target gut dysbiosis in depressed patients, the strategy to follow can be stimulating the diminished beneficial bacteria metabolism over not desired ones, being both diagnosis and treatment biomarkers. For instance, we already mentioned that in fecal microbiota from patients with MDD, Enterobacteriaceae and Alistipes are found over-represented whereas butyrate producer Faecalibacterium is underrepresented and negatively correlated with the severity of depressive symptoms [77].
But to reconstruct the depressed patient’s eubiosis, integrative therapies are required. Preferred counseling for targeting gut microbiota are probiotics and prebiotics, and/or food containing these [11]. Today, there are also potential pharmaceutical products grouped under the name of “pharmabiotics”, that join several probiotics, prebiotics, and nutraceutical compounds in a single food supplement [208]. New advances into holistic treatments for mental disease are being estimated due to the multidimensional system in which the gut microbiome is involved (immune, endocrine, CNS, HPA, Trp metabolism, neurotransmission…) [209]. In the following sections, we discuss complementary therapies including not only oral adjuvant treatments but also recommendations in lifestyle factors like diet, exercise, or sleep, that profoundly shape microbiota mechanisms and hence, host epigenetics pathways.
4.2. Emulation of Microbial Metabolism
4.2.1. Adjuvant Therapy
Supplementation with Probiotics
Moreover, evidence reports probiotics have denoted effectiveness in reducing depressive symptoms when combined with antidepressants and ameliorating the coexisting metabolic disorders, but there are no standardized recommendations for MDD [210]. Popularly known, Lactobacillus and Bifidobacterium have been the most extensively used genera for the restraint of undesired bacteria (i.e., overgrowth of Escherichia coli or the opportunistic infection of Citrobacter rodentium) and the strengthening of intestinal epithelia in gastrointestinal disorders. But more specifically, Skonieczna-żydecka et al. [211] reviewed beneficial bacteria “psychobiotics” that had reunited documented efficacy for the treatment of MGB and HPA axis disorders, concretely for neutralizing anxiety and depressive symptoms: Lactobacillus fermentum NS8 and NS9, Lactobacillus casei Shirota, Lactobacillus gasseri OLL2809, Lactobacillus rhamnosus JB-1, Lactobacillus helveticus Rosell-52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactococcus lactis W19 and W58, Bifidobacterium longum Rosell-175, Bifidobacterium longum NCC3001, Bifidobacterium longum 1714, Bifidobacterium bifidum W23, Bifidobacterium lactis W52, and Lactobacillus plantarum 299v.
These neurobiotics entail bacteria that exert neuroactive metabolite synthesis and modulate neurotrophic factors against MGB axis stress [211]. These would be SCFAs-producing bacteria and neuroactive compounds producing bacteria. Lessons from animal models show the biotechnological advances in genetic engineering of these new strains. The example of Bifidobacterium breve CCFM1025 resulted in increased expression of BDNF, restoration of chronic stress-induced gut microbiota, and the increase in SCFAs and serotonin production [212]. Chronic treatment with new strains of Lactobacillus plantarum, like Lp 286, also exposed anti-depressant- and anti-anxiolytic-like effects in mice but needed further investigation about GABA mediation [213].
Commercial formulas, i.e., Ecologic® barrier (B. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, Lc. lactis W19, Lc. lactis W58) (NCT04951687) including multispecies have been proved on memory performance and cognitive reactivity to sad mood using the Leiden Index of depression sensitivity scale, tested in randomized human trials, denoting significant reduced depressive-like behavior in mild to moderately depressed patients.
More evidence from clinical trials with the multispecies combination is needed to standardize methods for clinical practice guidelines of MDD. One controlled trial with multispecies supplementation (Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, and Lactococcus lactis (W19 and W58)) focused on prevention, administered the formula to non-depressed individuals; then, evaluating their behavior, 4 weeks after the intervention they showed significantly reduced rumination, aggressive thoughts and balanced cognitive reactivity to a sad mood [214]. These effects may be due to their association with OXT and AVP, two critical neuropeptides involved in the pathogenesis of MDD. Previous studies have described that gut microbiota may regulate OXT levels in the brain, and as OXT may bind to the AVP receptor, the gut microbiota can influence the actions of both neuropeptides [215]. Lactobacillus reuteri has been shown to be one of the most important regulators of OXT levels in the hypothalamus, with favorable effects on social behaviors [216]. Besides, a recent study shows that this effect was accomplished even by using a non-viable lysate of this microorganism [217]. Thus, it is possible that some undiscovered peptides or metabolites produced by Lactobacillus reuteri and other bacteria may directly regulate the levels and actions of OXT and AVP.
In Clinical Trials website we can already find the trials that are being accomplished for MDD treatment, some of them combined with prebiotics and some with common antidepressants: Ganeden BC30 (Bacillus coagulans GBI-30) (NCT01337609); multi-strain probiotic BioKult (Bacillus subtilis, Bifidobacterium bifidum, Bifidobacterium breve, Bifidobacterium infantis, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus helveticus, Lactobacillus salivarius, Lactococcus lactis ssp. lactis, Streptococcus thermophilus) (NCT03893162); Lactobacillus Plantarum PS128 (NCT03237078); Probio’Stick (Lactobacillus helveticus + Bifidobacterium longum) (NCT02838043); Lactobacillus Plantarum 299v + sertraline + escitalopram (NCT02469545) among others. Their objective is also testing symptoms besides metabolites (tryptophan, kynurenic pathway), cortisol concentration, and pro- and anti-inflammatory cytokines.
In depressed patients, there are repeatedly observed shifts in the relative abundance of more bacterial genera like Akkermansia, Ruminococcus, or Prevotella spp. These appear decreased and correlate with behavioral metrics of depression and anxiety [218]. Also, the under-representation of Faecalibacterium and over-representation of Actinobacteria and Enterobacteriaceae, are related to clinical characteristics and metabolic markers in MDD and bipolar disorder [219]. Specific strains like Akkermansia muciniphila and Faecalibacterium prausnitzii, have been studied in vitro with human epithelial colorectal cells, showing an impact on the expression of serotonin system-related genes [220] like the up-regulation of SERT gene (serotonin transporters) [221]. So, these two are promising probiotics also as potential modulators of the homeostasis at the serotonin system level.
Interestingly to mention is that certain antidepressant drugs like fluoxetine, escitalopram, venlafaxine, duloxetine, or desipramine can alter relative abundances of different strains in gut bacteria, so the approach here is to optimize these antidepressants to not affect the microbial composition or the suggestion of supplementation with those bacteria that become diminished by the drug. For example, Ruminococcus flavefaciens in preclinical models as a potential probiotic seems to depressive-like behavior by ameliorating mitochondrial oxidative phosphorylation in brain networks, boosting antidepressant efficiency as well [222].
Psychobiotics are the new field to explore with large marketing behind. Safety and efficacy studies are still needed, just as probiotics’ effectiveness has not been widely supported as they do not have the immediate effects medicines may offer [223]. However, Yong et al. [224] allege that the advantages presented for probiotics over standard antidepressants include the absence of secondary effects and the stigma involved Many effects of probiotic strains are also summarized in their review [224]. Apparently, in the era of precision medicine in which microbiota-directed therapy has been included, more new terms related to probiotics are arising, like “postbiotics”, alluding to the molecular signature of probiotics [225]. Furthermore, “pharmabiotics” consist of a new concept of biologically active therapeutics, comprising dead organisms, their components, and bioproducts [208].
Although this line of research is not directly included as “probiotics”, the basis of fecal microbiota transplantation (FMT) is quite similar. Simply, FMT consists of transferring stool from a healthy donor into the colon of a patient with an established pathology related to an altered microbiota with the aim to restore the normal microbiota and cure or ameliorate the disease [226]. In general views, FMT could be considered a safe therapeutic method with few adverse effects, although there are two main limitations to address before being recommended for patients with MDD. The first is related to the fact that there is still some limited knowledge about the long-term outcomes of FMT. It is still needed great efforts to personalize FMT for different patients and conditions according to varied hosts and diseases [227]. The second issue is related to the donor. Compelling evidence is supporting that FMT has the potential not only for ameliorating depressive or anxiety-like symptoms when healthy gut microbiota is transferred but also for inducing or aggravating the symptoms if the donor shows an aberrant gut microbiota and depressive behavior [228]. It is essential to fulfilling a thorough examination of the donor with a questionnaire, interview, blood tests, and stool analysis before allowing the transplantation [227]. However, because every individual exerts a unique gut microbiota footprint and the current limitations above described, we think that there is still a long road to cover before considering the application of FMT for the treatment of MDD.
Supplementation with Prebiotics
Following the International Scientific Association for Probiotics and Prebiotics (ISAPP), a prebiotic is a substrate that is selectively utilized by host microorganisms, that stimulate the growth and/or activity of these in the digestive system, while conferring a health benefit and reducing the risk of diseases mediated by microbiota aberrations [229]. In general, prebiotics is non-digestible carbohydrates (fructans, galactooligosaccharides, starch, and others), although the current definition considers the use of other non-carbohydrates compounds as prebiotics [230]. Some of the mechanisms proposed of the prebiotics in the microorganisms are that they may aid in the production of energy, metabolites, and micronutrients in the microorganism which may be relevant for the host, also allowing the growth of certain groups of beneficial bacteria [230,231].
A recent systematic review and meta-analysis showed that contrary to probiotics, prebiotics alone did not exert any significant differences in depressive or anxiety symptoms when compared to placebo [232]. However, this fact does not implicate prebiotics are completely unusefulness for MDD. Prebiotics are bioactive compounds that ease helpful bacteria in the gut and from probiotics to bloom. They might be a good option to fortify formulas with certain psychobiotic/probiotic strains. The term “symbiotic” refers to that combination of pre and probiotic. Noonan et al. reviewed patients with co-morbidities like Intestinal Bowel Syndrome can get better benefits from pre/probiotic treatments but that there is still a lack of studies with larger sample sizes and duration to support their effects on anxiety and depression [233]. Some of the proposed mechanisms of the antidepressant effects of prebiotics are the following: (1) Enhanced SCFAs and Treg in the gut; (2) Decreased plasma corticosterone and (3) Augmented activity of the prefrontal cortex, BDNF, and serotonin accumulation while decreasing amygdala activity, reduced IDO expression, cytokines detection in the brain and microglial activation [234]. However, there is still little evidence supporting the real benefits of these actions, and as has been above-mentioned, to achieve a significant antidepressant effect, prebiotics must be combined with probiotics.
Nowadays, prebiotics/probiotics and symbiotics are starting to be employed, not only in form of a specific formula but also in foods, beverages, and even in topical products like toilet paper [235,236]. Following the scientific evidence here, it should be importantly noticed that a food or beverage does not present any benefit itself by presenting any prebiotic/probiotic/symbiotic. Instead, the whole composition of the food/beverage, the interactions between those components (both facts can be grouped under the term “food matrix”) and the degree of processing are the most relevant factors to determine the healthy or unhealthy effects of the food [237]. In this sense, fermented foods may be one of the most interesting approaches from the use of prebiotics/probiotics/symbiotics in the food, although the results of the real efficacy of fermented foods/beverages are quite heterogeneous [238,239]. Preclinical studies show promising, but still inconsistent results of the antidepressant effects of fermented foods, due to their probiotic, prebiotic, and biogenic actions [240].
Future studies are warranted first to unravel the antidepressant mechanisms of prebiotics and symbiotics in patients with MDD, to find an adequate combination of both, and to explore the most appropriated vehicle (in formula or fortified non-ultra-processed foods and drinks).
Supplementation with SCFAs and Derivatives
SCFAs oral administration has proved evidence as a potential microbiota-targeted therapy for stress-related disorders. In a mice model, a mixture of acetate, propionate, and butyrate could promote anxiolytic and antidepressant effects, with ameliorated corticosterone levels, although changes in caecal microbiota composition were not registered, as well as colonic gene receptors for those fatty acids were not upregulated [88]. In a study conducted by Müller et al. [85], in fecal samples from depressive people, the correlation of shifts in SCFAs ratio with depressive symptoms was positive with acetate levels and negative with butyrate and propionate. SCFAs as nutrabiotics may therefore seem interesting for treatment.
Preclinical models have tested the effects of epigenetic drugs like sodium butyrate (NaBut), which is a histone deacetylase inhibitor and has chemical similarities with SCFA butyric acid. NaBut denoted antidepressant-like effects by promoting a decrease of methylation in BDNF, therefore supporting its upregulation, and then having effects in neurogenesis and neuroplasticity [241]. The observation is that NaBut can ameliorate chronic stress and cognitive related proteins at the hippocampus [242], improving memory function [243,244], and also has been observed increased activity in tricarboxylic acid cycle enzymes from MDD-associated mitochondrial dysfunction [245].
For its part, acetate supplementation in mice promotes antidepressant-like effects also at epigenetic levels by increasing histone acetylation with consequent down-regulation of BDNF, enhanced dendritic branches, and spine density of hippocampal pyramidal neurons, hence improving synaptic plasticity in the hippocampus [246]. Similarly, daily vinegar (acetic acid) ingestion was clinically tested in healthy adults, showing improvements in depression scores and amino acids metabolisms (increased metabolism of glycine, serine, threonine) [247].
Eventually, there is also evidence from preclinical studies with other derivative salts from propionate. In a mice model, sodium propionate effects are dose-dependent, being an antidepressant when administered at a low dose and pro-depressant when administered at a very high dose (reduction of norepinephrine, dopamine, and Trp; and increase of GABA, Kynurenine, and 3-hydroxykynurenine). Due to the observed disruption in the homeostasis of neurotransmitters, in the same study, Hao et al. warned about the potential danger of propionate as a commonly used food additive [248].
Supplementation with Nutraceuticals
Nutraceuticals are foods or part of them with multiple physiological actions in the body, aiding to prevent the onset of different pathologies and showing a promising role as adjunctive therapy in established diseases [249]. Scientific evidence gives a central role of vitamin D, S-adenosylmethionine (SAMe), omega 3 polyunsaturated fatty acid (ω-3 PUFA), and methylfolate is the most relevant antidepressant nutraceuticals [250]; although it is being currently explored the antidepressant actions of other nutraceuticals like creatine, amino acids, micronutrients, and some plant-derived bioactive compounds [251]. Most of these nutraceuticals have significant effects on the gut microbiota, and simultaneously, gut microbiota may be responsible for some of the benefits reported by nutraceuticals.
Vitamin D is an example of the synergic actions between gut microbiota and nutraceuticals. On the one hand, vitamin D has direct actions in the gut microbiota. Singh et al. [164] recently discovered that vitamin D supplementation leads to an increase in microbial diversity, with an increase of Bacteroidetes phyla, reduced Firmicutes/Bacteroidetes ratio, and enhancing some microbial populations with potential health benefits like Akkermansia muciniphilla and Bifidobacterium spp. among other changes. These changes in the microbiota composition may lead to an augmented production of some microbial metabolites like SCFAs which exert similar and synergic immunomodulatory actions like vitamin D, enhancing their benefits [252].
SAMe is a metabolic product synthesized during the cycle of methionine, is considered the universal methyl donor in living organisms [253]. In other words, SAMe has considered one of the major epigenetic regulators discovered through its actions in the one-carbon cycle [254] and the gut microbiota may also play a crucial role in these favorable actions. Jabs et al. [255] described that gut microbiota composition may affect the metabolism of SAMe. More detailed, Akkermansia muciniphila and Lactobacillus plantarum were able to lead epitranscriptomic modifications in the host, hence regulating the role of SAMe in many epigenetic reactions. In turn, mice fed with high levels of methionine, the precursor of SAMe lead to an impairment in gut microbiota composition, causing small bowel bacterial colonization and increased the abundance of pathogenic bacterial species Burkholderiales [256].
5-methyltetrahydrofolate (5-MTHF) is the active form of vitamin B9 (folate). This component is essential for restoring SAMe levels in the one-carbon cycle [257]. The gut microbiota is a pivotal source of folate production in the gut and some patients may benefit from the actions of microbial folate, as some patients fail to transform the folate into their activities. Particularly, those with single-nucleotide polymorphisms (SNPs) in dihydrofolate reductase (DHFR), which is required to convert folic acid to THF are not able to use folate supplements and target the gut microbiota (i.e., with probiotics) and their folate production could be essential to ensure the actions of 5-MTHF [258].
Omega 3 PUFA is a major structural and functional component of the brain, with many antidepressant mechanisms, described [251]. Among them, omega 3 PUFA exerts important prebiotic actions in the gut, is associated with a decrease in Faecalibacterium, an increase in the Bacteroidetes phyla, and butyrate-producing bacteria belonging to the Lachnospiraceae family, has been observed [259].
Among the most interesting plant-based compounds with antidepressant effects related to the gut microbiota, we must consider dietary polyphenols. Indeed, currently, the symbiotic use of probiotics with polyphenols is being widely explored in the management of MDD, as both combined seem to ameliorate neuroinflammation, microglial and inflammasome activation, reduce OS and balance serotonin metabolism [260]. In this sense, anthocyanins, a specific group of polyphenols participate in the regulation of the MGB axis by regulating host tryptophan metabolism, increasing the production of the neuroprotective metabolite kynurenic acid [261]. Likewise, a recent interventional randomized clinical trial has proven the benefits of using flavonoids-enriched orange juice in the alleviation of depressive symptoms, due to an increase in BDNF levels and Lachnospiraceae family in the gut [262]. The combination of polyphenols and other bioactive compounds in coffee, tea, and chocolate also show a promising role in the modulation of gut microbiota and MGB axis [260], thereby supporting the promising role of increasing polyphenols consumption as powerful antidepressant substances.
4.2.2. Lifestyle Intervention
Diet
In the above section of prebiotics, we already alluded to advances in the food industry with fortified food in prebiotics and probiotics, especially in fermented food. Here we focus on non-processed and natural sources to stimulate host bacteria. First of all, it should be noted that diet is the greatest gut microbiota shaping factor, therefore improper dietary habits for the individual will determine the dysbiosis status. Unsuitable use of dietary supplements, uncontrolled intermittent fasting, or high fat, animal proteins, and high caloric carbohydrate are some dietary ways to settle the dysbiosis [263].
SCFAs, in their common definition, are metabolic byproducts primarily derived from colon microbial carbohydrate fermentation of dietary fibers that escape digestion in the upper gastrointestinal tract [264]. Therefore, dietary fibers or microbiota-accessible carbohydrates, positively shape the microbial metabolism and the composition of gut microbiota [265,266].
What growing evidence asserts is that well-balanced diets like the Mediterranean Diet, with richness of plant-based ingredients (fruits, vegetables, legumes, olive oil…) fiber, and omega 3 PUFAs, establish a diverse microbiome and produces adequate ratios of imperative microbial metabolites [30]. Here we could mention the vast number of bioactive compounds (polyphenols, carotenoids, phytosterols) that are easy to find in its culinary aromatic spices and herbs (oregano, rosemary, thyme…) conferring health benefits on intestinal barrier and gut microbiota [267,268]. These positive findings have been also associated with the protection against depression and psychosocial maladjustment [265].
With high-fiber diets, Eubacterium, Lactobacillus, and Bifidobacterium species, which are known MAC degraders, are likely to flourish. Short-term (2-week) dietary intervention with MAC showed shifts in bacterial composition, increasing levels of these genera, and upregulated genes involved in inositol degradation [269]. Other studies have demonstrated that, however, not all fermentable fibers can stimulate SCFA production, and they warn about the importance of an individual’s microbial composition when advising certain foods. In this case, Ruminococcus bromii and Clostridium chartatabidum can contribute to enhanced butyrogenic effects [270]. Recent studies with functional cereals rich in fiber, which can be considered also part of the Mediterranean Diet, test their effectiveness in fecal microbiota composition. For instance, the prebiotic effect of fiber-rich barley increased the population of butyric acid-producing bacteria [271].
Precup and Vodnar [272] revised that due to its metabolite signature, dietary fiber fermenter Prevotella is considered a potential biomarker for homeostasis or disease state, nevertheless, in other cases it has been found to be an opportunistic intestinal pathobiont [272] promoting chronic inflammation, mucosal Th17 response and IL-8 and IL-6 production [273]. For this reason, this genus might not be a common probiotic option, because over-representation could lead to low-grade inflammation, so diet intervention with fiber should be oriented at this point to keep the optimal ratio of this genus that contributes to polysaccharide breakdown.
Conversely, many flavonoids from dark chocolate and cocoa powder cause increased neurogenesis and changes in neuronal regions implicated in learning and memory. Their consumption is well known to trigger rapid positive effects on mood even under emotional stress conditions [274]. Theobromine and cacao diets in animal models have shown that SCFA content is higher after intakes, predominantly butyrate production [275]. Similarly, regular tea consumption, due to its richness in teasaponin, catechins, and L-theanine, up-regulates BDNF signaling pathway. These compounds modulate dopaminergic activity and the MGB axis by promoting the growth of Bifidobacterium spp. and Lactobacillus/Enterococcus groups, increasing serotonin and GABA [276].
The dietary pattern may also be a major determinant of gut microbial composition and the metabolome. For instance, vegans often present a higher intake of fiber and lower fat in comparison to omnivore individuals that in turn is associated with a reduced level of fecal primary and secondary BAs [277]. It is of note that higher consumption of some animal products (meat, fish, margarine) and fat like fried potatoes were more prominently related to higher BA levels, as well as coffee consumption. Besides, many of these food groups like meat, fish, animal products, or some vegetables like potatoes are also associated with increased choline consumption and hence, upregulated TMA/TMAO levels [181]. Although the detection of both secondary BA and TMA/TMAO levels may be related to depressive symptoms, reducing the consumption of some of these foods may be counterproductive, as the evidence supports the relevance of some products like fish or coffee as potentially neuroprotective and antidepressant agents [278,279]. Instead, for proper microbiota composition and functioning, independently from a vegan or omnivore dietary pattern, it is by far more important to focus on the quality and the origin of the foods, ensuring a high fiber intake, polyphenols, or high-quality fats while limiting overconsumption of red and processed meats, low-quality fats intake or ultra-processed foods) [30].
In general, the depressed patient often adjusts to a profile of malnutrition with a lack of micronutrients we have been mentioning vitamin B, tryptophan. Those are at the same time microbial metabolites, therefore diet ought to supply those deficiencies that are common for gut microbiota and host [280]. Dietary nutrients act as modulators of brain function and behavior, at the same time microbiome metabolism modulates brain function by altering nutrient content from the diet, as precursors for producing its metabolites with psychoactive properties [281]. For the treatment of patients with MDD, the miscellaneous multidimensional microbiota-diet-depression linkage should be addressed [282]. So, we shall not forget Hippocrates’ quote “let food be thy medicine and medicine be thy food”.
Exercise
Recent studies allege that exercise can enrich microbiota diversity and potentiate beneficial species, which refines microbiota endocrine functions. SCFAs producers may expand by exercise benefits and the MGB axis also becomes modulated [283,284]. Evidence suggests that aerobic exercise triggers diversity and abundance of genera from the Firmicutes phylum, with possible linkage with effects of exercise on the gut and brain [107]. What is undeniable is that synergic effects of microbiota with exercise on cognition and emotion play crucial roles in serotonin signaling and metabolism in general [285].
The antidepressant-like effects of exercise have been compared to drug therapy with fluoxetine in depressed rats, demonstrating that regular exercise treatment could displace bacterial groups: increase Bacteroides, Actinomycetes, and Proteobacteria [286].
The effects of acute moderate-intensity physical activities in amateur athletes display changes in serum and fecal metabolome besides gut microbiota, concrete changes at phenylalanine, tyrosine, and Trp pathways, and a greater abundance of Rombustia and Ruminococcocaceae genera, which are Trp producers [287].
The already mentioned lactate molecule is another microbial and host metabolite, an important fuel for many cells including neurons and exercise supposes a vital source. The subjacent mechanisms involving this molecule in neuroprotection and memory formation remain unclear [288] but what is known about this compound is to be responsible for mediating exercise-induced adaptations as an ergogenic aid and even being implicated in norepinephrine release and brain plasticity [289]. Additionally, lactate produced during exercise can be utilized by the Veillonella genus and trigger its growth. The conversion of lactate to propionate by Veillonella atypica has been also positively correlated with run time athletic performance [290].
In athletes’ studies, it is no less important to mention that excess exercise can lead to gastrointestinal problems and psychological conditions like depression, but this training goes frequently hand in hand with improper and troubling dietary recommendations characterized by low consumption of plant polysaccharides, which is associated with reduced microbiota diversity and functionality, less synthesis of SCFAs and neurotransmitters [291].
The already mentioned NaBut can boost their effects thanks to exercising. In a mice model, there were found increased BDNF upregulation and so cognitive benefits of NaBut when administered post-training. Both exercise and NaBut could exert similar epigenetic control mechanisms [292].
The inclusion of exercise, recommendations are encouraging for the patient in terms of stress management besides being a safe, reliable, cost-effective, and pleiotropic effective factor implying multi-organ systemic adaptations [293].
Sleep
Currently, it is known that gut microbiota and its metabolites have oscillatory nature, diurnal rhythmicity that responds to the feeding/fasting cycle and that controls sleep duration in the host. The awareness of sleep hygiene is being now more appreciated as interruptions or short sleep durations are associated with hyperactivation of the HPA axis and dysbiosis [294]. Expanding research is demonstrating the nexus between the high incidence of insomnia and MDD with biological rhythms, immune function, and nutrient metabolism. In other words, host sleep and their emotions will depend on circadian rhythms affecting the microbiome [295].
Sleep efficiency has recently been positively correlated with richness in Bacteroidetes and Firmicutes phyla; and these parameters have been linked to the host immune system and cognition [296]. In depressed patients, there are also repeatedly observed shifts in the relative abundance of Dorea spp. [218]. This genus has been observed to have the particularity of being related to the quality of sleep simultaneously to depression severity [297]. Then Dorea (Dorea longicatena and Dorea formicigenerans) may be another pathological biomarker closely related to circadian disruption and associated gut microbiota alteration [298] we frequently find in patients with MDD [299,300]. Some advice for restoration of chronobiology in depressed patients could be encouraging them to practice some physical activity or trying to set schedules of eating, working, and then sleeping, aiding to ameliorate the bacterial activity that depends on sleep/wake cycle circadian rhythms. This is also worthy for prevention, as epidemiological studies allege that insomnia in nondepressed individuals is a risk factor for later MDD onset [299].
5. Conclusions
A deep understanding of metabolic dynamics in gut microbiota is providing another branch in the complex network of MDD modeling (Figure 2). All this knowledge we have witnessed along the last two decades is shedding light on optimizing clinical practice guidelines for diagnosis, treatment, and prevention by integrating more elements to the equation of depressed patient management. Holistic approaches are needed for a successful response to treatment, and this may entail the addition of psychotherapy, antidepressant drugs, and adjuvant food supplements plus the intervention in environmental factors like diet, physical activity, and sleep. Collectively, the influence of these factors would modulate microbially produced metabolites and consequently, promote a more desired activation of host gene expression in homeostatic mechanisms. For greater coverage, the adjuvant strategies that accompany the main treatment, are pursuing to rely on new probiotics (psychobiotics) or pharmabiotics with SCFAs and neuroactive metabolites- producing bacteria that antagonize characteristic depressive stress in the whole MGB axis. Further research and evidence with a critical focus on clinical trials regarding standardized combinations of therapeutic agents with dietary and exercise intervention remains required to support these integrative therapies that exert synergic effects that can better benefit the patient. The main results reviewed in the present work are summarized in Table 1.
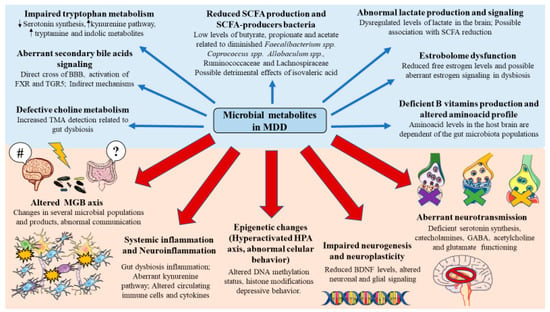
Figure 2.
Dysregulation of microbial metabolites in MDD and its consequences. As summarized, a noteworthy dysregulation of SCFAs, lactate, secondary bile acids, choline metabolites, vitamins, amino acids, estrogen, and tryptophan metabolites are potentially involved in the pathogenesis of MDD. Some of the mechanisms reviewed in this article included an altered MGB axis, systemic and local neuroinflammation, epigenetic changes affecting multiple brain regions, impaired neurogenesis and neuroplasticity as well as leading to aberrant neurotransmission.

Table 1.
Summarized results reviewed in this work.
Author Contributions
Conceptualization, M.A.O., M.A.A.-M., C.G.-M., O.F.-M. and M.Á.-M.; methodology, M.A.O., M.A.A.-M., C.G.-M., O.F.-M. and M.Á.-M.; validation, M.Á.-M.; formal analysis, M.A.O., M.A.A.-M., C.G.-M., O.F.-M. and M.Á.-M.; investigation, M.A.O., M.A.A.-M., C.G.-M., O.F.-M., L.G.G., G.L., J.M., P.V., F.M., R.R.-J., J.Q. and M.Á.-M.; data curation, M.A.O., M.A.A.-M., C.G.-M., O.F.-M. and M.Á.-M.; writ-ing—original draft preparation, M.A.O., M.A.A.-M., C.G.-M., O.F.-M., L.G.G., G.L., J.M., P.V., F.M., R.R.-J., J.Q. and M.Á.-M.; writing—review and editing, M.A.O., M.A.A.-M., C.G.-M., O.F.-M., L.G.G., G.L., J.M., P.V., F.M., R.R.-J., J.Q. and M.Á.-M.; supervision, M.A.O. and M.Á.-M.; project administration, M.A.O. and M.Á.-M.; funding acquisition, M.Á.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by grants from the Fondo de Investigación de la Seguridad Social, Instituto de Salud Carlos III (PI18/01726, PI19/00766), Spain, Programa de Actividades de I+D de la Comunidad de Madrid en Biomedicina (B2017/BMD3804, B2020/MITICAD-CM), and HALEKULANI S.L.
Acknowledgments
Oscar Fraile-Martinez had a predoctoral fellowship from the University of Alcalá during the course of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- O’Malley, M.A. From Endosymbiosis to Holobionts: Evaluating a Conceptual Legacy. J. Theor. Biol. 2017, 434, 34–41. [Google Scholar] [CrossRef]
- Baedke, J.; Fábregas-Tejeda, A.; Nieves Delgado, A. The Holobiont Concept before Margulis. J. Exp. Zool. Part B Mol. Dev. Evol. 2020, 334, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Baquero, F.; Nombela, C. The Microbiome as a Human Organ. Clin. Microbiol. Infect. 2012, 18 (Suppl. 4), 2–4. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, H.; Qiu, P. Aging Progression of Human Gut Microbiota. BMC Microbiol. 2019, 19, 236. [Google Scholar] [CrossRef]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M.; Mancini, C.; Cicerone, C.; Corazziari, E.; Pantanella, F.; et al. Eubiosis and Dysbiosis: The Two Sides of the Microbiota-PubMed. New Microbiol. 2016, 39, 1–12. [Google Scholar]
- Vamanu, E.; Rai, S.N.; Plaza-Díaz, J.; Battino, M. The Link between Obesity, Microbiota Dysbiosis, and Neurodegenerative Pathogenesis. Diseases 2021, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Ditu, L.M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1. [Google Scholar] [CrossRef]
- Giulio, P. The Intestinal Microbiota: Towards a Multifactorial Integrative Model. Eubiosis and Dysbiosis in Morbid Physical and Psychological Conditions. Arch. Clin. Gastroenterol. 2021, 7, 24–35. [Google Scholar] [CrossRef]
- GBD Results Tool|GHDx. Available online: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b (accessed on 7 December 2021).
- Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 7 December 2021).
- Albert, P.R. Why Is Depression More Prevalent in Women? J. Psychiatry Neurosci. 2015, 40, 219. [Google Scholar] [CrossRef] [PubMed]
- Ogrodniczuk, J.S.; Oliffe, J.L. Men and Depression. Can. Fam. Physician 2011, 57, 153. [Google Scholar]
- The Lancet Global Health. Mental Health Matters. Lancet Glob. Health 2020, 8, e1352. [Google Scholar] [CrossRef]
- Thakur, V.; Jain, A. COVID 2019-Suicides: A Global Psychological Pandemic. Brain Behav. Immun. 2020, 88, 952. [Google Scholar] [CrossRef]
- Santomauro, D.F.; Mantilla Herrera, A.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global Prevalence and Burden of Depressive and Anxiety Disorders in 204 Countries and Territories in 2020 Due to the COVID-19 Pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Sibley, S.; Sauers, L.; Daltry, R. Humanity and Resilience Project: The Development of a New Outreach Program for Counseling Centers at Colleges and Universities. J. Coll. Stud. Psychother. 2018, 33, 67–74. [Google Scholar] [CrossRef]
- Rao, G. Mens Sana in Corpore Sano. PDA J. Pharm. Sci. Technol. 2013, 67, 568. [Google Scholar] [CrossRef] [PubMed]
- Gianaros, P.J.; Wager, T.D. Brain-Body Pathways Linking Psychological Stress and Physical Health. Curr. Dir. Psychol. Sci. 2015, 24, 313–321. [Google Scholar] [CrossRef]
- Renoir, T.; Hasebe, K.; Gray, L. Mind and Body: How the Health of the Body Impacts on Neuropsychiatry. Front. Pharmacol. 2013, 4, 158. [Google Scholar] [CrossRef]
- Nemeroff, C.B. Psychoneuroimmunoendocrinology: The Biological Basis of Mind-Body Physiology and Pathophysiology. Depress. Anxiety 2013, 30, 285–287. [Google Scholar] [CrossRef]
- Daemen, M.J.A.P. The Heart and the Brain: An Intimate and Underestimated Relation. Neth. Heart J. 2013, 21, 53. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical Activity and Muscle–Brain Crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Ridaura, V.; Belkaid, Y. Gut Microbiota: The Link to Your Second Brain. Cell 2015, 161, 193–194. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [CrossRef] [PubMed]
- Louwies, T.; Johnson, A.C.; Orock, A.; Yuan, T.; Greenwood-Van Meerveld, B. The Microbiota-Gut-Brain Axis: An Emerging Role for the Epigenome. Exp. Biol. Med. 2020, 245, 138–145. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Alvarez-Mon, M.A.; Gomez-Lahoz, A.M.; Orozco, A.; Lahera, G.; Sosa-Reina, M.D.; Diaz, D.; Albillos, A.; Quintero, J.; Molero, P.; Monserrat, J.; et al. Blunted Expansion of Regulatory T Lymphocytes Is Associated With Increased Bacterial Translocation in Patients With Major Depressive Disorder. Front. Psychiatry 2021, 11, 1530. [Google Scholar] [CrossRef]
- Álvarez-Mon, M.A.; Gómez-Lahoz, A.M.; Orozco, A.; Lahera, G.; Diaz, D.; Ortega, M.A.; Albillos, A.; Quintero, J.; Aubá, E.; Monserrat, J.; et al. Expansion of CD4 T Lymphocytes Expressing Interleukin 17 and Tumor Necrosis Factor in Patients with Major Depressive Disorder. J. Pers. Med. 2021, 11, 220. [Google Scholar] [CrossRef]
- Alvarez-Mon, M.A.; Gómez, A.M.; Orozco, A.; Lahera, G.; Sosa, M.D.; Diaz, D.; Auba, E.; Albillos, A.; Monserrat, J.; Alvarez-Mon, M. Abnormal Distribution and Function of Circulating Monocytes and Enhanced Bacterial Translocation in Major Depressive Disorder. Front. Psychiatry 2019, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Eguilaz, M.; Ramon-Traperom, J.L.; Perez-Martinez, L.; Blanco, J.R. El Eje Microbiota-Intestino-Cerebro y Sus Grandes Proyecciones [The Microbiota-Gut-Brain Axis and Its Great Projections]. Rev. Neurol. 2019, 68, 111–117. [Google Scholar]
- Caspani, G.; Swann, J. Small Talk: Microbial Metabolites Involved in the Signaling from Microbiota to Brain. Curr. Opin. Pharmacol. 2019, 48, 99–106. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut Microbes and Metabolites as Modulators of Blood-Brain Barrier Integrity and Brain Health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Generoso, J.S.; Giridharan, V.V.; Lee, J.; Macedo, D.; Barichello, T. The Role of the Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders. Rev. Bras. Psiquiatr. 2021, 43, 293–305. [Google Scholar] [CrossRef]
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation. Clin. Ther. 2015, 37, 984–995. [Google Scholar] [CrossRef]
- Levey, D.F.; Stein, M.B.; Wendt, F.R.; Pathak, G.A.; Zhou, H.; Aslan, M.; Quaden, R.; Harrington, K.M.; Nuñez, Y.Z.; Overstreet, C.; et al. Bi-Ancestral Depression GWAS in the Million Veteran Program and Meta-Analysis in >1.2 Million Individuals Highlight New Therapeutic Directions. Nat. Neurosci. 2021, 24, 954–963. [Google Scholar] [CrossRef]
- Flint, J.; Kendler, K.S. The Genetics of Major Depression. Neuron 2014, 81, 484–503. [Google Scholar] [CrossRef]
- Syed, S.A.; Nemeroff, C.B. Early Life Stress, Mood, and Anxiety Disorders. Chronic Stress 2017, 1, 2470547017694461. [Google Scholar] [CrossRef] [PubMed]
- LeMoult, J.; Humphreys, K.L.; Tracy, A.; Hoffmeister, J.A.; Ip, E.; Gotlib, I.H. Meta-Analysis: Exposure to Early Life Stress and Risk for Depression in Childhood and Adolescence. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 842–855. [Google Scholar] [CrossRef]
- Li, M.; Fu, X.; Xie, W.; Guo, W.; Li, B.; Cui, R.; Yang, W. Effect of Early Life Stress on the Epigenetic Profiles in Depression. Front. Cell Dev. Biol. 2020, 8, 867. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Y.; Wang, Y.; Liu, L.; Zhang, X.; Li, B.; Cui, R. The Effects of Psychological Stress on Depression. Curr. Neuropharmacol. 2015, 13, 494. [Google Scholar] [CrossRef]
- Noble, R.E. Depression in Women. Metab. Clin. Exp. 2005, 54, 49–52. [Google Scholar] [CrossRef]
- Alegría, M.; NeMoyer, A.; Falgàs Bagué, I.; Wang, Y.; Alvarez, K. Social Determinants of Mental Health: Where We Are and Where We Need to Go. Curr. Psychiatry Rep. 2018, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Peng, W.; Sweeney, J.A.; Jia, Z.Y.; Gong, Q.Y. Brain Structure Alterations in Depression: Psychoradiological Evidence. CNS Neurosci. Ther. 2018, 24, 994. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, Y.K. The Roles of BDNF in the Pathophysiology of Major Depression and in Antidepressant Treatment. Psychiatry Investig. 2010, 7, 231. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J.J. Gliogenesis and Glial Pathology in Depression. CNS Neurol. Disord. Drug Targets 2007, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Rial, D.; Lemos, C.; Pinheiro, H.; Duarte, J.M.; Gonçalves, F.Q.; Real, J.I.; Prediger, R.D.; Gonçalves, N.; Gomes, C.A.; Canas, P.M.; et al. Depression as a Glial-Based Synaptic Dysfunction. Front. Cell. Neurosci. 2015, 9, 521. [Google Scholar] [CrossRef]
- Owens, M.J. Selectivity of Antidepressants: From the Monoamine Hypothesis of Depression to the SSRI Revolution and beyond-PubMed. J. Clin. Psychiatry 2004, 65, 5–10. [Google Scholar]
- Hirschfeld, R.M.A. History and Evolution of the Monoamine Hypothesis of Depression. J. Clin. Psychiatry 2000, 61, 4–6. [Google Scholar] [PubMed]
- Heninger, G.R.; Delgado, P.L.; Charney, D.S. The Revised Monoamine Theory of Depression: A Modulatory Role for Monoamines, Based on New Findings from Monoamine Depletion Experiments in Humans. Pharmacopsychiatry 1996, 29, 2–11. [Google Scholar] [CrossRef]
- Neumann, I.D.; Landgraf, R. Balance of Brain Oxytocin and Vasopressin: Implications for Anxiety, Depression, and Social Behaviors. Trends Neurosci. 2012, 35, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M.; Schatzberg, A.F. HPA Axis in Major Depression: Cortisol, Clinical Symptomatology, and Genetic Variation Predict Cognition. Mol. Psychiatry 2017, 22, 527. [Google Scholar] [CrossRef] [PubMed]
- Van Den Eede, F.; Claes, S.J. Mechanisms of Depression: Role of the HPA Axis. Drug Discov. Today Dis. Mech. 2004, 1, 413–418. [Google Scholar] [CrossRef]
- Quera Salva, M.A.; Hartley, S.; Barbot, F.; Alvarez, J.C.; Lofaso, F.; Guilleminault, C. Circadian Rhythms, Melatonin and Depression. Curr. Pharm. Des. 2011, 17, 1459–1470. [Google Scholar] [CrossRef]
- Maria Michel, T.; Pulschen, D.; Thome, J. The Role of Oxidative Stress in Depressive Disorders. Curr. Pharm. Des. 2012, 18, 5890–5899. [Google Scholar] [CrossRef]
- Bajpai, A.; Verma, A.K.; Srivastava, M.; Srivastava, R. Oxidative Stress and Major Depression. J. Clin. Diagn. Res. 2014, 8, CC04. [Google Scholar] [CrossRef]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune Mechanisms Linked to Depression via Oxidative Stress and Neuroprogression. Immunology 2015, 144, 365. [Google Scholar] [CrossRef]
- Brites, D.; Fernandes, A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and MicroRNA Dysregulation. Front. Cell. Neurosci. 2015, 9, 476. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and Depression: A Review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Du, Y.; Gao, X.R.; Peng, L.; Ge, J.F. Crosstalk between the Microbiota-Gut-Brain Axis and Depression. Heliyon 2020, 6, e04097. [Google Scholar] [CrossRef]
- Sittipo, P.; Shim, J.W.; Lee, Y.K. Microbial Metabolites Determine Host Health and the Status of Some Diseases. Int. J. Mol. Sci. 2019, 20, 5296. [Google Scholar] [CrossRef] [PubMed]
- Martinez, K.B.; Leone, V.; Chang, E.B. Microbial Metabolites in Health and Disease: Navigating the Unknown in Search of Function. J. Biol. Chem. 2017, 292, 8553. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189. [Google Scholar] [CrossRef]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of Human Colonic Butyrate-Producing Bacteria Revealed by Analysis of the Butyryl-CoA:Acetate CoA-Transferase Gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Propionate as a Health-Promoting Microbial Metabolite in the Human Gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short Chain Fatty Acids and Its Producing Organisms: An Overlooked Therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef] [PubMed]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L. Short Chain Fatty Acids and Their Receptors: New Metabolic Targets. Transl. Res. J. Lab. Clin. Med. 2013, 161, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Wang, Q.; Wu, Q.; Mao-Draayer, Y.; Kim, C.H. Bidirectional Regulatory Potentials of Short-Chain Fatty Acids and Their G-Protein-Coupled Receptors in Autoimmune Neuroinflammation. Sci. Rep. 2019, 9, 8837. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Deng, Z.; Luo, X.M.; Liu, J.; Wang, H. Quorum Sensing, Biofilm, and Intestinal Mucosal Barrier: Involvement the Role of Probiotic. Front. Cell. Infect. Microbiol. 2020, 10, 504. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-Chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The Neuroactive Potential of the Human Gut Microbiota in Quality of Life and Depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.J.; Xie, J.; Chen, J.J. Associations between Disordered Gut Microbiota and Changes of Neurotransmitters and Short-Chain Fatty Acids in Depressed Mice. Transl. Psychiatry 2020, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, T.; Saito, S.; Osaki, Y.; Hamada, A.; Aoki-Yoshida, A.; Sonoyama, K. Organoids as an Ex Vivo Model for Studying the Serotonin System in the Murine Small Intestine and Colon Epithelium. Biochem. Biophys. Res. Commun. 2016, 474, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Engevik, M.A.; Luck, B.; Visuthranukul, C.; Ihekweazu, F.D.; Engevik, A.C.; Shi, Z.; Danhof, H.A.; Chang-Graham, A.L.; Hall, A.; Endres, B.T.; et al. Human-Derived Bifidobacterium Dentium Modulates the Mammalian Serotonergic System and Gut-Brain Axis. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 221–248. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut Microbes Promote Colonic Serotonin Production through an Effect of Short-Chain Fatty Acids on Enterochromaffin Cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Skonieczna-żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal Short Chain Fatty Acids Profile Is Changed in Polish Depressive Women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef]
- Müller, B.; Rasmusson, A.J.; Just, D.; Jayarathna, S.; Moazzami, A.; Novicic, Z.K.; Cunningham, J.L. Fecal Short-Chain Fatty Acid Ratios as Related to Gastrointestinal and Depressive Symptoms in Young Adults. Psychosom. Med. 2021, 83, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Alhabeeb, H.; Chhina, N.; Morrison, D.J.; Preston, T.; Tedford, C.; Fitzpatrick, J.; Irani, C.; Busza, A.; et al. Increased Colonic Propionate Reduces Anticipatory Reward Responses in the Human Striatum to High-Energy Foods. Am. J. Clin. Nutr. 2016, 104, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Gorwood, P. Neurobiological Mechanisms of Anhedonia. Dialogues Clin. Neurosci. 2008, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain Fatty Acids: Microbial Metabolites That Alleviate Stress-induced Brain-Gut Axis Alterations. J. Physiol. 2018, 596, 4923. [Google Scholar] [CrossRef]
- Li, H.; Xiang, Y.; Zhu, Z.; Wang, W.; Jiang, Z.; Zhao, M.; Cheng, S.; Pan, F.; Liu, D.; Ho, R.C.M.; et al. Rifaximin-Mediated Gut Microbiota Regulation Modulates the Function of Microglia and Protects against CUMS-Induced Depression-like Behaviors in Adolescent Rat. J. Neuroinflamm. 2021, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Tsai, S.J. Epigenetics and Depression: An Update. Psychiatry Investig. 2019, 16, 654. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Alvarez-Mon, M.A.; García-Montero, C.; Fraile-Martinez, O.; Lahera, G.; Monserrat, J.; Muñoz-Merida, L.; Mora, F.; Rodríguez-Jiménez, R.; Fernandez-Rojo, S.; et al. MicroRNAs as Critical Biomarkers of Major Depressive Disorder: A Comprehensive Perspective. Biomedicines 2021, 9, 1659. [Google Scholar] [CrossRef]
- Sun, H.; Kennedy, P.J.; Nestler, E.J. Epigenetics of the Depressed Brain: Role of Histone Acetylation and Methylation. Neuropsychopharmacology 2012, 38, 124–137. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, Y.; Singh, S.; Singh, R.B. Gut Microbiome-Mediated Epigenetic Regulation of Brain Disorder and Application of Machine Learning for Multi-Omics Data Analysis. Genome 2021, 64, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Zalar, B.; Haslberger, A.; Peterlin, B. The Role of Microbiota in Depression-a Brief Review. Psychiatr. Danub. 2018, 30, 136–141. [Google Scholar] [CrossRef]
- Szczesniak, O.; Hestad, K.A.; Hanssen, J.F.; Rudi, K. Isovaleric Acid in Stool Correlates with Human Depression. Nutr. Neurosci. 2016, 19, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez-Seijas, J. Comprehensive Review on Lactate Metabolism in Human Health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and Their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the Brain: From Metabolic End-Product to Signalling Molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Riske, L.; Thomas, R.K.; Baker, G.B.; Dursun, S.M. Lactate in the Brain: An Update on Its Relevance to Brain Energy, Neurons, Glia and Panic Disorder. Ther. Adv. Psychopharmacol. 2017, 7, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.A.L.; Mao, X.; Case, J.A.C.; Kang, G.; Shungu, D.C.; Gabbay, V. Increased Ventricular Cerebrospinal Fluid Lactate in Depressed Adolescents. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2016, 32, 1–8. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Zanetti, M.V.; Otaduy, M.C.; De Sousa, R.T.; Soeiro-De-Souza, M.G.; Costa, A.C.; Carvalho, A.F.; Leite, C.C.; Busatto, G.F.; Zarate, C.A.; et al. Increased Brain Lactate during Depressive Episodes and Reversal Effects by Lithium Monotherapy in Drug-Naive Bipolar Disorder: A 3T 1H-MRS Study. J. Clin. Psychopharmacol. 2017, 37, 40. [Google Scholar] [CrossRef]
- Chen, J.-J.; Zhou, C.-J.; Zheng, P.; Cheng, K.; Wang, H.-Y.; Li, J.; Zeng, L.; Xie, P. Differential Urinary Metabolites Related with the Severity of Major Depressive Disorder. Behav. Brain Res. 2017, 332, 280–287. [Google Scholar] [CrossRef]
- Carrard, A.; Cassé, F.; Carron, C.; Burlet-Godinot, S.; Toni, N.; Magistretti, P.J.; Martin, J.L. Role of Adult Hippocampal Neurogenesis in the Antidepressant Actions of Lactate. Mol. Psychiatry 2021, 76. [Google Scholar] [CrossRef]
- Karnib, N.; El-Ghandour, R.; El Hayek, L.; Nasrallah, P.; Khalifeh, M.; Barmo, N.; Jabre, V.; Ibrahim, P.; Bilen, M.; Stephan, J.S.; et al. Lactate Is an Antidepressant That Mediates Resilience to Stress by Modulating the Hippocampal Levels and Activity of Histone Deacetylases. Neuropsychopharmacology 2019, 44, 1152–1162. [Google Scholar] [CrossRef]
- Carrard, A.; Elsayed, M.; Margineanu, M.; Boury-Jamot, B.; Fragnière, L.; Meylan, E.M.; Petit, J.M.; Fiumelli, H.; Magistretti, P.J.; Martin, J.L. Peripheral Administration of Lactate Produces Antidepressant-like Effects. Mol. Psychiatry 2018, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Scaglia, F. The Role of Mitochondrial Dysfunction in Psychiatric Disease. Dev. Disabil. Res. Rev. 2010, 16, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise Influence on the Microbiome–Gut–Brain Axis. Gut Microbes 2019, 10, 555. [Google Scholar] [CrossRef]
- Modoux, M.; Rolhion, N.; Mani, S.; Sokol, H. Tryptophan Metabolism as a Pharmacological Target. Trends Pharmacol. Sci. 2021, 42, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Alkhalaf, L.M.; Ryan, K.S. Biosynthetic Manipulation of Tryptophan in Bacteria: Pathways and Mechanisms. Chem. Biol. 2015, 22, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in Silico Analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the Role of 5-Hydroxytryptophan Synthesis Regulation Alleviates the Symptom of Depression and Related Microbiota Dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef]
- Soh, N.L.; Walter, G. Tryptophan and Depression: Can Diet Alone Be the Answer? Acta Neuropsychiatr. 2011, 23, 3–11. [Google Scholar] [CrossRef]
- Lukić, I.; Getselter, D.; Koren, O.; Elliott, E. Role of Tryptophan in Microbiota-Induced Depressive-like Behavior: Evidence from Tryptophan Depletion Study. Front. Behav. Neurosci. 2019, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A Link between Stress and Depression: Shifts in the Balance between the Kynurenine and Serotonin Pathways of Tryptophan Metabolism and the Etiology and Pathophysiology of Depression. Stress 2008, 11, 198–209. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-Induced Depressive-like Behavior Is Mediated by Indoleamine 2,3-Dioxygenase Activation in Mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The Kynurenine Pathway in Major Depressive Disorder, Bipolar Disorder, and Schizophrenia: A Meta-Analysis of 101 Studies. Mol. Psychiatry 2020, 26, 4158–4178. [Google Scholar] [CrossRef] [PubMed]
- Ogyu, K.; Kubo, K.; Noda, Y.; Iwata, Y.; Tsugawa, S.; Omura, Y.; Wada, M.; Tarumi, R.; Plitman, E.; Moriguchi, S.; et al. Kynurenine Pathway in Depression: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2018, 90, 16–25. [Google Scholar] [CrossRef]
- Dantzer, R. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression–Preclinical Approaches. Curr. Top. Behav. Neurosci. 2017, 31, 117. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Huang, X.F.; Newell, K.A. The Kynurenine Pathway in Major Depression: What We Know and Where to Next. Neurosci. Biobehav. Rev. 2021, 127, 917–927. [Google Scholar] [CrossRef]
- Neavin, D.R.; Liu, D.; Ray, B.; Weinshilboum, R.M. The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases. Int. J. Mol. Sci. 2018, 19, 3851. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ray, B.; Neavin, D.R.; Zhang, J.; Athreya, A.P.; Biernacka, J.M.; Bobo, W.V.; Hall-Flavin, D.K.; Skime, M.K.; Zhu, H.; et al. Beta-Defensin 1, Aryl Hydrocarbon Receptor and Plasma Kynurenine in Major Depressive Disorder: Metabolomics-Informed Genomics. Transl. Psychiatry 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, D.D. Tryptamine: A Metabolite of Tryptophan Implicated in Various Neuropsychiatric Disorders. Metab. Brain Dis. 1993, 8, 1–44. [Google Scholar] [CrossRef]
- Williams, B.B.; Van Benschoten, A.H.; Cimermancic, P.; Donia, M.S.; Zimmermann, M.; Taketani, M.; Ishihara, A.; Kashyap, P.C.; Fraser, J.S.; Fischbach, M.A. Discovery and Characterization of Gut Microbiota Decarboxylases That Can Produce the Neurotransmitter Tryptamine. Cell Host Microbe 2014, 16, 495. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Jaglin, M.; Rhimi, M.; Philippe, C.; Pons, N.; Bruneau, A.; Goustard, B.; Daugé, V.; Maguin, E.; Naudon, L.; Rabot, S. Indole, a Signaling Molecule Produced by the Gut Microbiota, Negatively Impacts Emotional Behaviors in Rats. Front. Neurosci. 2018, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.A.; Perry, G.; Fang, X.; Zagorski, M.; Sambamurti, K.; Poeggeler, B. Indoles as Essential Mediators in the Gut-Brain Axis. Their Role in Alzheimer’s Disease. Neurobiol. Dis. 2021, 156, 105403. [Google Scholar] [CrossRef]
- Beloborodova, N.V.; Chernevskaya, E.A.; Getsina, M.L. Indolic Structure Metabolites as Potential Biomarkers of Non-Infectious Diseases. Curr. Pharm. Des. 2021, 27, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Roiser, J.P.; McLean, A.; Ogilvie, A.D.; Blackwell, A.D.; Bamber, D.J.; Goodyer, I.; Jones, P.B.; Sahakian, B.J. The Subjective and Cognitive Effects of Acute Phenylalanine and Tyrosine Depletion in Patients Recovered from Depression. Neuropsychopharmacology 2005, 30, 775. [Google Scholar] [CrossRef]
- Zheng, S.; Yu, M.; Lu, X.; Huo, T.; Ge, L.; Yang, J.; Wu, C.; Li, F. Urinary Metabonomic Study on Biochemical Changes in Chronic Unpredictable Mild Stress Model of Depression. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 204–209. [Google Scholar] [CrossRef]
- Inoshita, M.; Umehara, H.; Watanabe, S.Y.; Nakataki, M.; Kinoshita, M.; Tomioka, Y.; Tajima, A.; Numata, S.; Ohmori, T. Elevated Peripheral Blood Glutamate Levels in Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 945. [Google Scholar] [CrossRef]
- Averina, O.V.; Zorkina, Y.A.; Yunes, R.A.; Kovtun, A.S.; Ushakova, V.M.; Morozova, A.Y.; Kostyuk, G.P.; Danilenko, V.N.; Chekhonin, V.P. Bacterial Metabolites of Human Gut Microbiota Correlating with Depression. Int. J. Mol. Sci. 2020, 21, 9234. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, R.; Pessione, E. The Neuro-Endocrinological Role of Microbial Glutamate and GABA Signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef]
- Altaib, H.; Nakamura, K.; Abe, M.; Badr, Y.; Yanase, E.; Nomura, I.; Suzuki, T. Differences in the Concentration of the Fecal Neurotransmitters GABA and Glutamate Are Associated with Microbial Composition among Healthy Human Subjects. Microorganisms 2021, 9, 378. [Google Scholar] [CrossRef]
- Luck, B.; Horvath, T.D.; Engevik, K.A.; Ruan, W.; Haidacher, S.J.; Hoch, K.M.; Oezguen, N.; Spinler, J.K.; Haag, A.M.; Versalovic, J.; et al. Neurotransmitter Profiles Are Altered in the Gut and Brain of Mice Mono-Associated with Bifidobacterium Dentium. Biomolecules 2021, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium Adolescentis as a Key Member of the Human Gut Microbiota in the Production of GABA. Sci. Rep. 2020, 10, 14112. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-Modulating Bacteria of the Human Gut Microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Otaru, N.; Ye, K.; Mujezinovic, D.; Berchtold, L.; Constancias, F.; Cornejo, F.A.; Krzystek, A.; de Wouters, T.; Braegger, C.; Lacroix, C.; et al. GABA Production by Human Intestinal Bacteroides spp.: Prevalence, Regulation, and Role in Acid Stress Tolerance. Front. Microbiol. 2021, 12, 860. [Google Scholar] [CrossRef]
- Martins-de-Souza, D. Proteomics, Metabolomics, and Protein Interactomics in the Characterization of the Molecular Features of Major Depressive Disorder. Dialogues Clin. Neurosci. 2014, 16, 63–73. [Google Scholar] [CrossRef]
- Li, J.; Jia, X.; Wang, C.; Wu, C.; Qin, X. Altered Gut Metabolome Contributes to Depression-like Behaviors in Rats Exposed to Chronic Unpredictable Mild Stress. Transl. Psychiatry 2019, 9, 40. [Google Scholar] [CrossRef]
- Kawase, T.; Nagasawa, M.; Ikeda, H.; Yasuo, S.; Koga, Y.; Furuse, M. Gut Microbiota of Mice Putatively Modifies Amino Acid Metabolism in the Host Brain. Br. J. Nutr. 2017, 117, 775–783. [Google Scholar] [CrossRef]
- Steffens, D.C.; Jiang, W.; Krishnan, K.R.R.; Karoly, E.D.; Mitchell, M.W.; O’Connor, C.M.; Kaddurah-Daouk, R. Metabolomic Differences in Heart Failure Patients with and without Major Depression. J. Geriatr. Psychiatry Neurol. 2010, 23, 138–146. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile Acids and the Gut Microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile Acid Metabolism and Signaling. Compr. Physiol. 2013, 3, 1191. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of Bile Salt Biotransformations by Intestinal Bacteria. Gut Microbes 2016, 7, 22. [Google Scholar] [CrossRef]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef] [PubMed]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of Gut Microbiota with Bile Acid Metabolism and Its Influence on Disease States. Appl. Microbiol. Biotechnol. 2017, 101, 47. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Cassaro, S. Physiology, Bile Acids; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Martinot, E.; Sèdes, L.; Baptissart, M.; Lobaccaro, J.M.; Caira, F.; Beaudoin, C.; Volle, D.H. Bile Acids and Their Receptors. Mol. Asp. Med. 2017, 56, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Cardoso, V.F.; Corlianò, M.; Singaraja, R.R. Bile Acids: A Communication Channel in the Gut-Brain Axis. Neuromolecular Med. 2021, 23, 99–117. [Google Scholar] [CrossRef]
- Mulak, A. Bile Acids as Key Modulators of the Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 84, 461–477. [Google Scholar] [CrossRef]
- Jia, W.; Rajani, C.; Kaddurah-Daouk, R.; Li, H. Expert Insights: The Potential Role of the Gut Microbiome-Bile Acid-Brain Axis in the Development and Progression of Alzheimer’s Disease and Hepatic Encephalopathy. Med. Res. Rev. 2020, 40, 1496–1507. [Google Scholar] [CrossRef]
- Li, P.; Killinger, B.A.; Ensink, E.; Beddows, I.; Yilmaz, A.; Lubben, N.; Lamp, J.; Schilthuis, M.; Vega, I.E.; Woltjer, R.; et al. Gut Microbiota Dysbiosis Is Associated with Elevated Bile Acids in Parkinson’s Disease. Metabolites 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Mertens, K.L.; Kalsbeek, A.; Soeters, M.R.; Eggink, H.M. Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front. Neurosci. 2017, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, P.; Herbet, M. Role of the Intestinal Microbiome, Intestinal Barrier and Psychobiotics in Depression. Nutrients 2021, 13, 927. [Google Scholar] [CrossRef]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut Microbial Metabolites in Depression: Understanding the Biochemical Mechanisms. Microb. Cell 2019, 6, 454. [Google Scholar] [CrossRef] [PubMed]
- Dethloff, F.; Vargas, F.; Elijah, E.; Quinn, R.; Park, D.I.; Herzog, D.P.; Müller, M.B.; Gentry, E.C.; Knight, R.; Gonzalez, A.; et al. Paroxetine Administration Affects Microbiota and Bile Acid Levels in Mice. Front. Psychiatry 2020, 11, 518. [Google Scholar] [CrossRef]
- Anglin, R.E.S.; Samaan, Z.; Walter, S.D.; Sarah, D.M. Vitamin D Deficiency and Depression in Adults: Systematic Review and Meta-Analysis. Br. J. Psychiatry J. Ment. Sci. 2013, 202, 100–107. [Google Scholar] [CrossRef]
- Rao, T.S.S.; Asha, M.R.; Ramesh, B.N.; Rao, K.S.J. Understanding Nutrition, Depression and Mental Illnesses. Indian J. Psychiatry 2008, 50, 77. [Google Scholar] [CrossRef]
- Ramakrishna, B.S. Role of the Gut Microbiota in Human Nutrition and Metabolism. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 4), 9–17. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The Potential Role of Vitamin D Supplementation as a Gut Microbiota Modifier in Healthy Individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Prakash, M.; Apostolopoulos, V. The Effects of Vitamin B on the Immune/Cytokine Network and Their Involvement in Depression. Maturitas 2017, 96, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Young, L.M.; Pipingas, A.; White, D.J.; Gauci, S.; Scholey, A. A Systematic Review and Meta-Analysis of B Vitamin Supplementation on Depressive Symptoms, Anxiety, and Stress: Effects on Healthy and ‘At-Risk’ Individuals. Nutrients 2019, 11, 2232. [Google Scholar] [CrossRef] [PubMed]
- Uebanso, T.; Shimohata, T.; Mawatari, K.; Takahashi, A. Functional Roles of B-Vitamins in the Gut and Gut Microbiome. Mol. Nutr. Food Res. 2020, 64, 2000426. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H.; et al. PROVIT: Supplementary Probiotic Treatment and Vitamin B7 in Depression-A Randomized Controlled Trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef]
- Vennemann, F.B.C.; Ioannidou, S.; Valsta, L.M.; Dumas, C.; Ocké, M.C.; Mensink, G.B.M.; Lindtner, O.; Virtanen, S.M.; Tlustos, C.; D’Addezio, L.; et al. Dietary Intake and Food Sources of Choline in European Populations. Br. J. Nutr. 2015, 114, 2046–2055. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-Oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, P.; Luo, J.; Shen, L.; Zhang, S.; Gu, H.; He, J.; Wang, L.; Zhao, X.; Gan, M.; et al. Dietary Betaine Prevents Obesity through Gut Microbiota-Drived MicroRNA-378a Family. Gut Microbes 2021, 13, 1862612. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Jiao, F.; Shi, C.; Pei, M.; Wang, L.; Gong, Z. Betaine Inhibits Toll-like Receptor 4 Responses and Restores Intestinal Microbiota in Acute Liver Failure Mice. Sci. Rep. 2020, 10, 21850. [Google Scholar] [CrossRef]
- Zhong, C.; Lu, Z.; Che, B.; Qian, S.; Zheng, X.; Wang, A.; Bu, X.; Zhang, J.; Ju, Z.; Xu, T.; et al. Choline Pathway Nutrients and Metabolites and Cognitive Impairment after Acute Ischemic Stroke. Stroke 2021, 52, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Nurk, E.; Refsum, H.; Bjelland, I.; Drevon, C.A.; Tell, G.S.; Ueland, P.M.; Vollset, S.E.; Engedal, K.; Nygaard, H.A.; Smith, D.A. Plasma Free Choline, Betaine and Cognitive Performance: The Hordaland Health Study. Br. J. Nutr. 2013, 109, 511–519. [Google Scholar] [CrossRef]
- Riley, C.A.; Renshaw, P.F. Brain Choline in Major Depression: A Review of the Literature. Psychiatry Res. Neuroimaging 2018, 271, 142–153. [Google Scholar] [CrossRef]
- Skonieczna-żydecka, K.; Jakubczyk, K.; Maciejewska-Markiewicz, D.; Janda, K.; Kaźmierczak-Siedlecka, K.; Kaczmarczyk, M.; Łoniewski, I.; Marlicz, W. Gut Biofactory-Neurocompetent Metabolites within the Gastrointestinal Tract. A Scoping Review. Nutrients 2020, 12, 3369. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-T.; Hsieh, C.-P.; Lee, M.-Y.; Chen, L.-C.; Huang, C.-M.; Chen, H.-H.; Chan, M.-H. Betaine Prevents and Reverses the Behavioral Deficits and Synaptic Dysfunction Induced by Repeated Ketamine Exposure in Mice. Biomed. Pharmacother. 2021, 144, 112369. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, W.H.W.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic Value of Choline and Betaine Depends on Intestinal Microbiota-Generated Metabolite Trimethylamine-N-Oxide. Eur. Heart J. 2014, 35, 904–910. [Google Scholar] [CrossRef]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the Trimethylamine-Producing Bacteria of the Human Gut Microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Arboleya, S.; Allison, J.; Kaliszewska, A.; Higarza, S.G.; Gueimonde, M.; Arias, J.L. The Relationship between Choline Bioavailability from Diet, Intestinal Microbiota Composition, and Its Modulation of Human Diseases. Nutrients 2020, 12, 2340. [Google Scholar] [CrossRef]
- Meinitzer, S.; Baranyi, A.; Holasek, S.; Schnedl, W.J.; Zelzer, S.; Mangge, H.; Herrmann, M.; Meinitzer, A.; Enko, D. Sex-Specific Associations of Trimethylamine-N-Oxide and Zonulin with Signs of Depression in Carbohydrate Malabsorbers and Nonmalabsorbers. Dis. Markers 2020, 2020, 7897240. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Martinez-del Campo, A.; Kasahara, K.; Chittim, C.L.; Vivas, E.I.; Amador-Noguez, D.; Balskus, E.P.; Rey, F.E. Metabolic, Epigenetic, and Transgenerational Effects of Gut Bacterial Choline Consumption. Cell Host Microbe 2017, 22, 279–290.e7. [Google Scholar] [CrossRef]
- Hammond, G.L. Plasma Steroid-Binding Proteins: Primary Gatekeepers of Steroid Hormone Action. J. Endocrinol. 2016, 230, R13. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-R.; Kim, T.-H.; Choi, K.-C. Functions and Physiological Roles of Two Types of Estrogen Receptors, ERα and ERβ, Identified by Estrogen Receptor Knockout Mouse. Lab. Anim. Res. 2012, 28, 71. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Wu, J.; Chen, J. The Role of Gut Microbial β-Glucuronidase in Estrogen Reactivation and Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 2067. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Sharma, D. The Microbiome–Estrogen Connection and Breast Cancer Risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal Microbial Determinants of Fecal and Systemic Estrogens and Estrogen Metabolites: A Cross-Sectional Study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Kalhoro, D.H.; Kalhoro, M.S.; Metwally, E.; Chughtai, M.I.; Mazhar, M.U.; Khan, S.A. Relationship between Gut Microbiota and Host-Metabolism: Emphasis on Hormones Related to Reproductive Function. Anim. Nutr. 2021, 7, 1. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–Gut Microbiome Axis: Physiological and Clinical Implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Freeman, E.W. Treatment of Depression Associated with the Menstrual Cycle: Premenstrual Dysphoria, Postpartum Depression, and the Perimenopause. Dialogues Clin. Neurosci. 2002, 4, 177–191. [Google Scholar] [CrossRef]
- Hantsoo, L.; Epperson, C.N. Premenstrual Dysphoric Disorder: Epidemiology and Treatment. Curr. Psychiatry Rep. 2015, 17, 87. [Google Scholar] [CrossRef]
- Cutter, W.J.; Norbury, R.; Murphy, D.G.M. Oestrogen, Brain Function, and Neuropsychiatric Disorders. J. Neurol. Neurosurg. Psychiatry 2003, 74, 837–840. [Google Scholar] [CrossRef][Green Version]
- Flores-Ramos, M.; Burrola-Suárez, M.A.; Guiza-Zayas, R.; Enciso-Araujo, J.M.; Islas-Preciado, D.; Camarena, E.E. Evaluation of Hormonal and Metabolic Factors Related to Depression in Reproductive Age Women. Salud Ment. 2020, 43, 35–41. [Google Scholar] [CrossRef]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; Von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Franzosa, E.A.; Huang, K.; Meadow, J.F.; Gevers, D.; Lemon, K.P.; Bohannan, B.J.M.; Huttenhower, C. Identifying Personal Microbiomes Using Metagenomic Codes. Proc. Natl. Acad. Sci. USA 2015, 112, E2930–E2938. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220. [Google Scholar] [CrossRef]
- Cowan, C.S.M.; Dinan, T.G.; Cryan, J.F. Annual Research Review: Critical Windows-the Microbiota-Gut-Brain Axis in Neurocognitive Development. J. Child Psychol. Psychiatry Allied Discip. 2020, 61, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Elrakaiby, M.; Dutilh, B.E.; Rizkallah, M.R.; Boleij, A.; Cole, J.N.; Aziz, R.K. Pharmacomicrobiomics: The Impact of Human Microbiome Variations on Systems Pharmacology and Personalized Therapeutics. J. Integr. Biol. 2014, 18, 402. [Google Scholar] [CrossRef] [PubMed]
- Rizkallah, M.; Saad, R.; Aziz, R. The Human Microbiome Project, Personalized Medicine and the Birth of Pharmacomicrobiomics. Curr. Pharm. Pers. Med. 2012, 8, 182–193. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Haiser, H.J.; Van Treuren, W.; Garg, N.; Reddivari, L.; Vanamala, J.; Dorrestein, P.C.; Turnbaugh, P.J.; Knight, R. The Intestinal Metabolome: An Intersection between Microbiota and Host. Gastroenterology 2014, 146, 1470–1476. [Google Scholar] [CrossRef]
- Kosmides, A.K.; Kamisoglu, K.; Calvano, S.E.; Corbett, S.A.; Androulakis, I.P. Metabolomic Fingerprinting: Challenges and Opportunities. Crit. Rev. Biomed. Eng. 2013, 41, 205. [Google Scholar] [CrossRef]
- Riscuta, G.; Xi, D.; Pierre-Victor, D.; Starke-Reed, P.; Khalsa, J.; Duffy, L. Diet, Microbiome, and Epigenetics in the Era of Precision Medicine. Methods Mol. Biol. 2018, 1856, 141–156. [Google Scholar] [CrossRef]
- Hullar, M.A.J.; Fu, B.C. Diet, the Gut Microbiome, and Epigenetics. Cancer J. 2014, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current Understanding of the Human Microbiome. Nat. Med. 2018, 24, 392. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Jin, G.; Wang, G.; Liu, T.; Liu, X.; Wang, B.; Cao, H. Current Sampling Methods for Gut Microbiota: A Call for More Precise Devices. Front. Cell. Infect. Microbiol. 2020, 10, 151. [Google Scholar] [CrossRef]
- María Remes Troche, J.; Coss Adame, E.; Ángel Valdovinos Díaz, M.; Gómez Escudero, O.; Eugenia Icaza Chávez, M.; Antonio Chávez-Barrera, J.; Zárate Mondragón, F.; Antonio Ruíz Velarde Velasco, J.; Rafael Aceves Tavares, G.; Antonio Lira Pedrín, M.; et al. Lactobacillus Acidophilus LB: A Useful Pharmabiotic for the Treatment of Digestive Disorders. Ther. Adv. Gastroenterol. 2020, 13, 1756284820971201. [Google Scholar] [CrossRef]
- Flux, M.C.; Lowry, C.A. Finding Intestinal Fortitude: Integrating the Microbiome into a Holistic View of Depression Mechanisms, Treatment, and Resilience. Neurobiol. Dis. 2020, 135, 104578. [Google Scholar] [CrossRef]
- Gawlik-Kotelnicka, O.; Strzelecki, D. Probiotics as a Treatment for “Metabolic Depression”? A Rationale for Future Studies. Pharmaceuticals 2021, 14, 384. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-żydecka, K.; Marlicz, W.; Misera, A.; Koulaouzidis, A.; Łoniewski, I. Microbiome—The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. J. Clin. Med. 2018, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; O’Riordan, K.J.; Lee, Y.-K.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a Psychobiotic Therapy for Depression: Bifidobacterium Breve CCFM1025 Reverses Chronic Stress-Induced Depressive Symptoms and Gut Microbial Abnormalities in Mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef]
- Barros-Santos, T.; Silva, K.S.O.; Libarino-Santos, M.; Cata-Preta, E.G.; Reis, H.S.; Tamura, E.K.; De Oliveira-Lima, A.J.; Berro, L.F.; Uetanabaro, A.P.T.; Marinho, E.A.V. Effects of Chronic Treatment with New Strains of Lactobacillus Plantarum on Cognitive, Anxiety- and Depressive-like Behaviors in Male Mice. PLoS ONE 2020, 15, e0234037. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A Randomized Controlled Trial to Test the Effect of Multispecies Probiotics on Cognitive Reactivity to Sad Mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef]
- Varian, B.J.; Poutahidis, T.; DiBenedictis, B.T.; Levkovich, T.; Ibrahim, Y.; Didyk, E.; Shikhman, L.; Cheung, H.K.; Hardas, A.; Ricciardi, C.E.; et al. Microbial Lysate Upregulates Host Oxytocin. Brain Behav. Immun. 2017, 61, 36. [Google Scholar] [CrossRef] [PubMed]
- McGaughey, K.D.; Yilmaz-Swenson, T.; Elsayed, N.M.; Cruz, D.A.; Rodriguiz, R.M.; Kritzer, M.D.; Peterchev, A.V.; Roach, J.; Wetsel, W.C.; Williamson, D.E. Relative Abundance of Akkermansia spp. and Other Bacterial Phylotypes Correlates with Anxiety- and Depressive-like Behavior Following Social Defeat in Mice. Sci. Rep. 2019, 9, 3281. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Lai, J.B.; Du, Y.L.; Xu, Y.; Ruan, L.M.; Hu, S.H. Current Understanding of Gut Microbiota in Mood Disorders: An Update of Human Studies. Front. Genet. 2019, 10, 98. [Google Scholar] [CrossRef]
- Yaghoubfar, R.; Behrouzi, A.; Zare Banadkoki, E.; Ashrafian, F.; Lari, A.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; Khatami, S.; Siadat, S.D. Effect of Akkermansia Muciniphila, Faecalibacterium Prausnitzii, and Their Extracellular Vesicles on the Serotonin System in Intestinal Epithelial Cells. Probiotics Antimicrob. Proteins 2021, 13, 1546–1556. [Google Scholar] [CrossRef]
- Yaghoubfar, R.; Behrouzi, A.; Fateh, A.; Nojoumi, S.A.; Vaziri, F.; Khatami, S.; Siadat, S.D. Effects of Akkermansia Muciniphila and Faecalibacterium Prausnitzii on Serotonin Transporter Expression in Intestinal Epithelial Cells. J. Diabetes Metab. Disord. 2021, 20, 1–5. [Google Scholar] [CrossRef]
- Lukić, I.; Getselter, D.; Ziv, O.; Oron, O.; Reuveni, E.; Koren, O.; Elliott, E. Antidepressants Affect Gut Microbiota and Ruminococcus Flavefaciens Is Able to Abolish Their Effects on Depressive-like Behavior. Transl. Psychiatry 2019, 9, 133. [Google Scholar] [CrossRef]
- Zou, R.; Tian, P.; Xu, M.; Zhu, H.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Psychobiotics as a Novel Strategy for Alleviating Anxiety and Depression. J. Funct. Foods 2021, 86, 1756–4646. [Google Scholar] [CrossRef]
- Yong, S.J.; Tong, T.; Chew, J.; Lim, W.L. Antidepressive Mechanisms of Probiotics and Their Therapeutic Potential. Front. Neurosci. 2019, 13, 1361. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Bhati, A.; Priya, K.; Sharma, K.K.; Singhal, B. Precision Postbiotics and Mental Health: The Management of Post-COVID-19 Complications. Probiotics Antimicrob. Proteins 2021, 1, 1. [Google Scholar] [CrossRef]
- Vindigni, S.M.; Surawicz, C.M. Fecal Microbiota Transplantation. Gastroenterol. Clin. N. Am. 2017, 46, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal Microbiota Transplantation: Review and Update. J. Formos. Med. Assoc. 2019, 118 (Suppl. 1), S23–S31. [Google Scholar] [CrossRef]
- Chinna Meyyappan, A.; Forth, E.; Wallace, C.J.K.; Milev, R. Effect of Fecal Microbiota Transplant on Symptoms of Psychiatric Disorders: A Systematic Review. BMC Psychiatry 2020, 20, 299. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Corzo, N.; Alonso, J.L.; Azpiroz, F.; Calvo, M.A.; Cirici, M.; Leis, R.; Lombó, F.; Mateos-Aparicio, I.; Plou, F.J.; Ruas-Madiedo, P.; et al. Prebiotics: Concept, Properties and Beneficial Effects. Nutr. Hosp. 2015, 31 (Suppl. 1), 99–118. [Google Scholar] [CrossRef]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and Probiotics for Depression and Anxiety: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Noonan, S.; Zaveri, M.; Macaninch, E.; Martyn, K.; Sanjay, M. Food & Mood: A Review of Supplementary Prebiotic and Probiotic Interventions in the Treatment of Anxiety and Depression in Adults. BMJ Nutr. Prev. Health 2020, 3, 351. [Google Scholar] [CrossRef] [PubMed]
- Paiva, I.H.R.; Duarte-Silva, E.; Peixoto, C.A. The Role of Prebiotics in Cognition, Anxiety, and Depression. Eur. Neuropsychopharmacology 2020, 34, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Varzakas, T.; Kandylis, P.; Dimitrellou, D.; Salamoura, C.; Zakynthinos, G.; Proestos, C. Innovative and Fortified Food: Probiotics, Prebiotics, GMOs, and Superfood. In Preparation and Processing of Religious and Cultural Foods; Woodhead Publishing: Sawston, UK, 2018; pp. 67–129. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J.M. The Food Matrix: Implications in Processing, Nutrition and Health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef] [PubMed]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health Benefits of Fermented Foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Stiemsma, L.T.; Nakamura, R.E.; Nguyen, J.G.; Michels, K.B. Does Consumption of Fermented Foods Modify the Human Gut Microbiota? J. Nutr. 2020, 150, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Green, J.; Jacka, F.N.; Collier, F.; Berk, M.; Pasco, J.; Dawson, S.L. Fermented Foods, the Gut and Mental Health: A Mechanistic Overview with Implications for Depression and Anxiety. Nutr. Neurosci. 2020, 23, 659–671. [Google Scholar] [CrossRef]
- Wei, Y.B.; Melas, P.A.; Wegener, G.; Mathe, A.A.; Lavebratt, C. Antidepressant-like Effect of Sodium Butyrate Is Associated with an Increase in TET1 and in 5-Hydroxymethylation Levels in the Bdnf Gene. Int. J. Neuropsychopharmacol. 2014, 18, pyv026. [Google Scholar] [CrossRef]
- Han, A.; Sung, Y.B.; Chung, S.Y.; Kwon, M.S. Possible Additional Antidepressant-like Mechanism of Sodium Butyrate: Targeting the Hippocampus. Neuropharmacology 2014, 81, 292–302. [Google Scholar] [CrossRef]
- Peng, J. Effects of Sodium Butyrate on Spatial Learning and Memory Ability in Chronic Stress Model Rats. China Pharm. 2016, 27, 2233–2235. [Google Scholar] [CrossRef]
- Valvassori, S.; Varela, R.; Arent, C.; Dal-Pont, G.; Bobsin, T.; Budni, J.; Reus, G.; Quevedo, J. Sodium Butyrate Functions as an Antidepressant and Improves Cognition with Enhanced Neurotrophic Expression in Models of Maternal Deprivation and Chronic Mild Stress. Curr. Neurovascular Res. 2014, 11, 359–366. [Google Scholar] [CrossRef]
- Valvassori, S.; Resende, W.; Budni, J.; Dal-Pont, G.; Bavaresco, D.; Reus, G.; Carvalho, A.; Goncalves, C.; Furlanetto, C.; Streck, E.; et al. Sodium Butyrate, a Histone Deacetylase Inhibitor, Reverses Behavioral and Mitochondrial Alterations in Animal Models of Depression Induced by Early- or Late-Life Stress. Curr. Neurovascular Res. 2015, 12, 312–320. [Google Scholar] [CrossRef]
- Huang, W.; Hu, W.; Cai, L.; Zeng, G.; Fang, W.; Dai, X.; Ye, Q.; Chen, X.; Zhang, J. Acetate Supplementation Produces Antidepressant-like Effect via Enhanced Histone Acetylation. J. Affect. Disord. 2021, 281, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Jasbi, P.; Jin, Y.; Bauer, S.; Williams, S.; Fessler, S.N.; Gu, H. Daily Vinegar Ingestion Improves Depression Scores and Alters the Metabolome in Healthy Adults: A Randomized Controlled Trial. Nutrients 2021, 13, 4020. [Google Scholar] [CrossRef]
- Hao, C.; Gao, Z.; Liu, X.J.; Rong, Z.; Jia, J.; Kang, K.; Guo, W.; Li, J. Intravenous Administration of Sodium Propionate Induces Antidepressant or Prodepressant Effect in a Dose Dependent Manner. Sci. Rep. 2020, 10, 19917. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of Nutraceuticals in Human Health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am. J. Psychiatry 2016, 173, 575–587. [Google Scholar] [CrossRef]
- Alvarez-Mon, M.A.; Ortega, M.A.; García-Montero, C.; Fraile-Martinez, O.; Monserrat, J.; Lahera, G.; Mora, F.; Rodriguez-Quiroga, A.; Fernandez-Rojo, S.; Quintero, J.; et al. Exploring the Role of Nutraceuticals in Major Depressive Disorder (MDD): Rationale, State of the Art and Future Prospects. Pharmaceuticals 2021, 14, 821. [Google Scholar] [CrossRef]
- Malaguarnera, L. Vitamin D and Microbiota: Two Sides of the Same Coin in the Immunomodulatory Aspects. Int. Immunopharmacol. 2020, 79, 106112. [Google Scholar] [CrossRef] [PubMed]
- Lauinger, L.; Kaiser, P. Sensing and Signaling of Methionine Metabolism. Metabolites 2021, 11, 83. [Google Scholar] [CrossRef]
- Mentch, S.J.; Locasale, J.W. One-Carbon Metabolism and Epigenetics: Understanding the Specificity. Ann. N. Y. Acad. Sci. 2016, 1363, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Jabs, S.; Biton, A.; Bécavin, C.; Nahori, M.A.; Ghozlane, A.; Pagliuso, A.; Spanò, G.; Guérineau, V.; Touboul, D.; Giai Gianetto, Q.; et al. Impact of the Gut Microbiota on the M6A Epitranscriptome of Mouse Cecum and Liver. Nat. Commun. 2020, 11, 1344. [Google Scholar] [CrossRef]
- Miousse, I.R.; Pathak, R.; Garg, S.; Skinner, C.M.; Melnyk, S.; Pavliv, O.; Hendrickson, H.; Landes, R.D.; Lumen, A.; Tackett, A.J.; et al. Short-Term Dietary Methionine Supplementation Affects One-Carbon Metabolism and DNA Methylation in the Mouse Gut and Leads to Altered Microbiome Profiles, Barrier Function, Gene Expression and Histomorphology. Genes Nutr. 2017, 12, 22. [Google Scholar] [CrossRef]
- Zheng, Y.; Cantley, L.C. Toward a Better Understanding of Folate Metabolism in Health and Disease. J. Exp. Med. 2019, 216, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Engevik, M.A.; Morra, C.N.; Röth, D.; Engevik, K.; Spinler, J.K.; Devaraj, S.; Crawford, S.E.; Estes, M.K.; Kalkum, M.; Versalovic, J. Microbial Metabolic Capacity for Intestinal Folate Production and Modulation of Host Folate Receptors. Front. Microbiol. 2019, 10, 2305. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Pasinetti, G.M. The Gut Microbiota Links Dietary Polyphenols With Management of Psychiatric Mood Disorders. Front. Neurosci. 2019, 13, 1196. [Google Scholar] [CrossRef]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.E.; Mateus, N.; Calhau, C. Gut Microbiota Modulation Accounts for the Neuroprotective Properties of Anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef]
- Park, M.; Choi, J.; Lee, H.J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef]
- Bajinka, O.; Tan, Y.; Abdelhalim, K.A.; Özdemir, G.; Qiu, X. Extrinsic Factors Influencing Gut Microbes, the Immediate Consequences and Restoring Eubiosis. AMB Express 2020, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef] [PubMed]
- Myhrstad, M.C.W.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation—Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- Opara, E.I.; Chohan, M. Culinary Herbs and Spices: Their Bioactive Properties, the Contribution of Polyphenols and the Challenges in Deducing Their True Health Benefits. Int. J. Mol. Sci. 2014, 15, 19183. [Google Scholar] [CrossRef] [PubMed]
- Galli, C. Bioactive Components in Mediterranean Diets. Nutrafoods 2012, 11, 11–17. [Google Scholar] [CrossRef]
- Oliver, A.; Chase, A.B.; Weihe, C.; Orchanian, S.B.; Riedel, S.F.; Hendrickson, C.L.; Lay, M.; Sewall, J.M.; Martiny, J.B.H.; Whiteson, K. High-Fiber, Whole-Food Dietary Intervention Alters the Human Gut Microbiome but Not Fecal Short-Chain Fatty Acids. Msystems 2021, 6, e00115-21. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef]
- Akagawa, S.; Akagawa, Y.; Nakai, Y.; Yamagishi, M.; Yamanouchi, S.; Kimata, T.; Chino, K.; Tamiya, T.; Hashiyada, M.; Akane, A.; et al. Fiber-Rich Barley Increases Butyric Acid-Producing Bacteria in the Human Gut Microbiota. Metabolites 2021, 11, 559. [Google Scholar] [CrossRef]
- Precup, G.; Vodnar, D.C. Gut Prevotella as a Possible Biomarker of Diet and Its Eubiotic versus Dysbiotic Roles: A Comprehensive Literature Review. Br. J. Nutr. 2019, 122, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The Immune Response to Prevotella Bacteria in Chronic Inflammatory Disease. Immunology 2017, 151, 363. [Google Scholar] [CrossRef]
- Nehlig, A. The Neuroprotective Effects of Cocoa Flavanol and Its Influence on Cognitive Performance. Br. J. Clin. Pharmacol. 2013, 75, 716. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Camps-Bossacoma, M.; Massot-Cladera, M.; Rigo-Adrover, M.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Effect of Cocoa’s Theobromine on Intestinal Microbiota of Rats. Mol. Nutr. Food Res. 2017, 61, 1700238. [Google Scholar] [CrossRef]
- O’neill Rothenberg, D.; Zhang, L. Mechanisms Underlying the Anti-Depressive Effects of Regular Tea Consumption. Nutrients 2019, 11, 1361. [Google Scholar] [CrossRef]
- Trefflich, I.; Marschall, H.U.; di Giuseppe, R.; Ståhlman, M.; Michalsen, A.; Lampen, A.; Abraham, K.; Weikert, C. Associations between Dietary Patterns and Bile Acids—Results from a Cross-Sectional Study in Vegans and Omnivores. Nutrients 2020, 12, 47. [Google Scholar] [CrossRef]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaź, P. Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int. J. Mol. Sci. 2020, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, X.; Zhang, D. Fish Consumption and Risk of Depression: A Meta-Analysis. J. Epidemiol. Community Health 2016, 70, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Klimova, B.; Novotny, M.; Valis, M. The Impact of Nutrition and Intestinal Microbiome on Elderly Depression—A Systematic Review. Nutrients 2020, 12, 710. [Google Scholar] [CrossRef]
- Ezra-Nevo, G.; Henriques, S.F.; Ribeiro, C. The Diet-Microbiome Tango: How Nutrients Lead the Gut Brain Axis. Curr. Opin. Neurobiol. 2020, 62, 122–132. [Google Scholar] [CrossRef]
- Koopman, M.; Daniels, J.K.; Spitzer, C.; Lampe, A.; El Aidy, S. Depressed Gut? The Microbiota-Diet-Inflammation Trialogue in Depression. Curr. Opin. Psychiatry 2017, 30, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, Diet and Stress as Modulators of Gut Microbiota: Implications for Neurodegenerative Diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-F.; Ferreira Rocha, N.; Paes, F.; Arias-Carrión, O.; Machado, S.; de Sá Filho, A. Neural Mechanisms of Exercise: Effects on Gut Miccrobiota and Depression. CNS Neurol. Disord. Drug Targets 2015, 14, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Wang, Y.; Jiang, A.; Zhou, Y.; Zhou, H. Effect of Regular Physical Exercise on Gut Microbiota and Depressive Behaviors in Rats. J. Food Qual. 2021, 2021, 1210089. [Google Scholar] [CrossRef]
- Tabone, M.; Bressa, C.; García-Merino, J.A.; Moreno-Pérez, D.; Van, E.C.; Castelli, F.A.; Fenaille, F.; Larrosa, M. The Effect of Acute Moderate-Intensity Exercise on the Serum and Fecal Metabolomes and the Gut Microbiota of Cross-Country Endurance Athletes. Sci. Rep. 2021, 11, 3558. [Google Scholar] [CrossRef] [PubMed]
- Proia, P.; di Liegro, C.M.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a Metabolite and a Regulator in the Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef]
- Nalbandian, M.; Takeda, M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-Omics Analysis of Elite Athletes Identifies a Performance-Enhancing Microbe That Functions via Lactate Metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Exercise-Induced Stress Behavior, Gut-Microbiota-Brain Axis and Diet: A Systematic Review for Athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef]
- Intlekofer, K.A.; Berchtold, N.C.; Malvaez, M.; Carlos, A.J.; McQuown, S.C.; Cunningham, M.J.; Wood, M.A.; Cotman, C.W. Exercise and Sodium Butyrate Transform a Subthreshold Learning Event into Long-Term Memory via a Brain-Derived Neurotrophic Factor-Dependent Mechanism. Neuropsychopharmacology 2013, 38, 2027–2034. [Google Scholar] [CrossRef]
- O’Sullivan, O.; Cronin, O.; Clarke, S.F.; Murphy, E.F.; Molloy, M.G.; Shanahan, F.; Cotter, P.D. Exercise and the Microbiota. Gut Microbes 2015, 6, 131. [Google Scholar] [CrossRef]
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep, Circadian Rhythm, and Gut Microbiota. Sleep Med. Rev. 2020, 53, 101340. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Y.; Fan, F.; Zhang, B. The Role of Microbiome in Insomnia, Circadian Disturbance and Depression. Front. Psychiatry 2018, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut Microbiome Diversity Is Associated with Sleep Physiology in Humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef]
- Zhang, Q.; Yun, Y.; An, H.; Zhao, W.; Ma, T.; Wang, Z.; Yang, F. Gut Microbiome Composition Associated With Major Depressive Disorder and Sleep Quality. Front. Psychiatry 2021, 12, 551. [Google Scholar] [CrossRef]
- Mortaş, H.; Bilici, S.; Karakan, T. The Circadian Disruption of Night Work Alters Gut Microbiota Consistent with Elevated Risk for Future Metabolic and Gastrointestinal Pathology. Chronobiol. Int. 2020, 37, 1067–1081. [Google Scholar] [CrossRef]
- Nutt, D.J.; Wilson, S.; Paterson, L. Sleep Disorders as Core Symptoms of Depression. Dialogues Clin. Neurosci. 2008, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Haynes, P.L.; Mcquaid, J.R.; Ancoli-Israel, S.; Martin, J.L. Disrupting Life Events and the Sleep-Wake Cycle in Depression. Psychol. Med. 2006, 36, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front. Nutr. 2019, 6, 48. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).