Systemic Metabolomics in a Framework of Genetics and Lifestyle in Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Evaluation of Metabolites
2.3. Evaluation of the Genetic and Lifestyle Determinants
2.4. Evaluation of AMD Phenotype
2.5. Statistical Analysis
3. Results
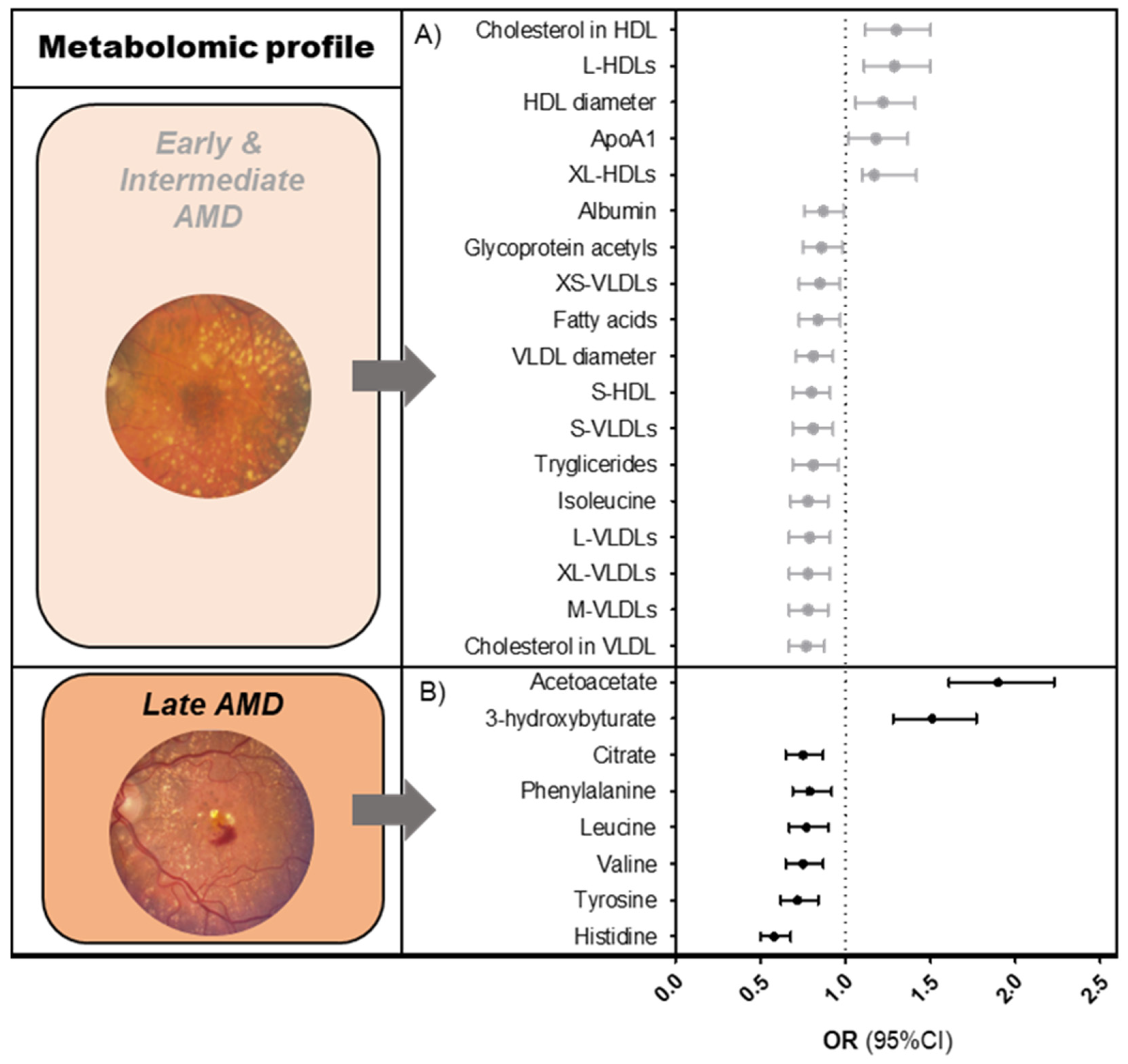
3.1. Metabolomic Profiles for AMD Disease Stages
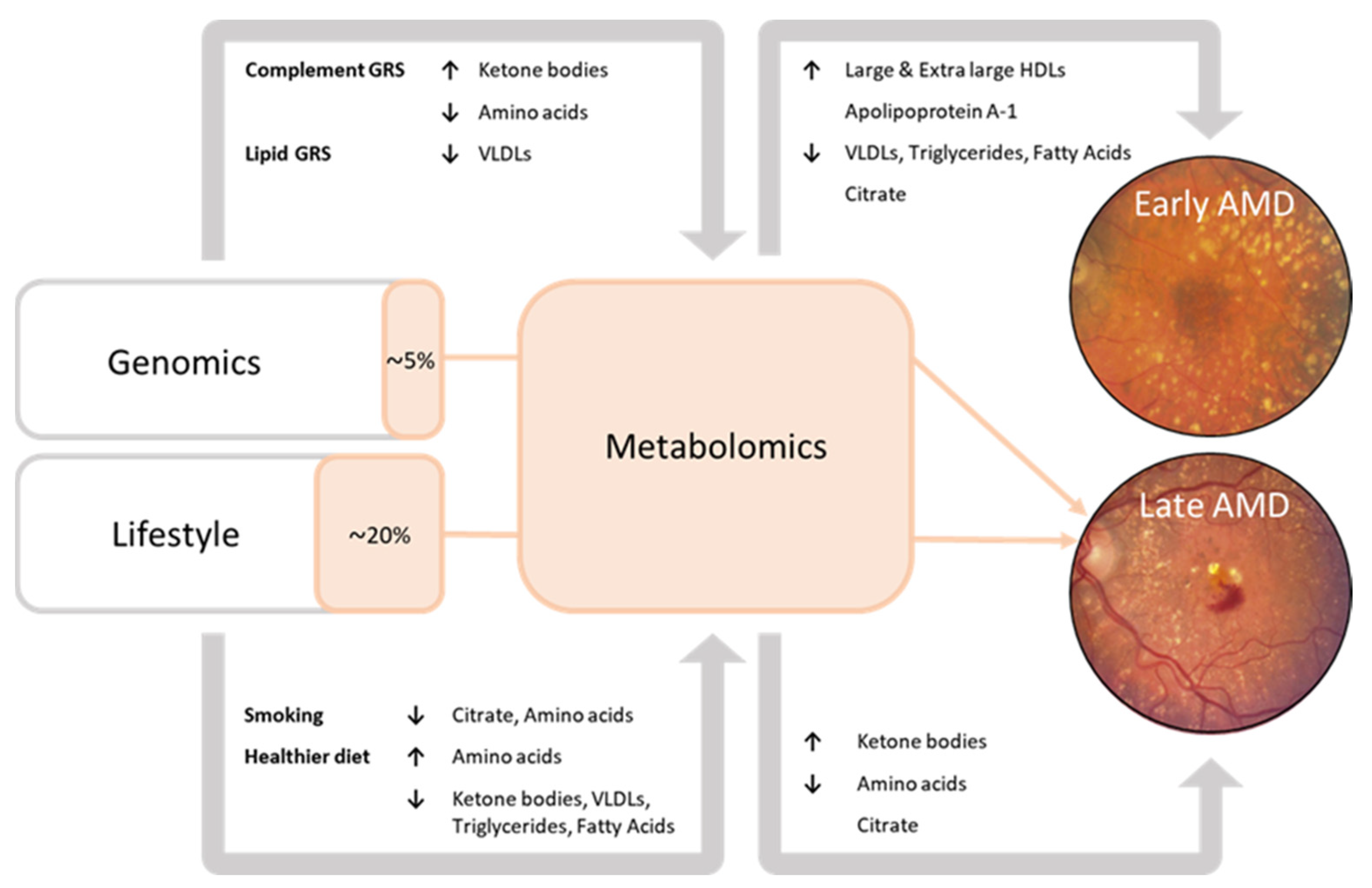
3.2. Association of Genetic and Lifestyle Factors with Metabolites
3.3. Interplay between Metabolite, Genetic, and Lifestyle Factors for Late AMD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blindness, G.B.D.; Vision Impairment, C.; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef]
- Geerlings, M.J.; de Jong, E.K.; den Hollander, A.I. The complement system in age-related macular degeneration: A review of rare genetic variants and implications for personalized treatment. Mol. Immunol. 2017, 84, 65–76. [Google Scholar] [CrossRef]
- Han, X.; Gharahkhani, P.; Mitchell, P.; Liew, G.; Hewitt, A.W.; MacGregor, S. Genome-wide meta-analysis identifies novel loci associated with age-related macular degeneration. J. Hum. Genet. 2020, 65, 657–665. [Google Scholar] [CrossRef]
- Colijn, J.M.; Meester-Smoor, M.; Verzijden, T.; de Breuk, A.; Silva, R.; Merle, B.M.J.; Cougnard-Gregoire, A.; Hoyng, C.B.; Fauser, S.; Coolen, A.; et al. Genetic Risk, Lifestyle, and Age-Related Macular Degeneration in Europe: The EYE-RISK Consortium. Ophthalmology 2021, 128, 1039–1049. [Google Scholar] [CrossRef]
- de Breuk, A.; Acar, I.E.; Kersten, E.; Schijvenaars, M.; Colijn, J.M.; Haer-Wigman, L.; Bakker, B.; de Jong, S.; Meester-Smoor, M.A.; Verzijden, T.; et al. Development of a Genotype Assay for Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmology 2020, 128, 1604–1617. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.; Silver, R.E.; Rosner, B.; Seddon, J.M. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: A prospective cohort study. Am. J. Clin. Nutr. 2015, 102, 1196–1206. [Google Scholar] [CrossRef]
- Merle, B.M.J.; Colijn, J.M.; Cougnard-Gregoire, A.; de Koning-Backus, A.P.M.; Delyfer, M.N.; Kiefte-de Jong, J.C.; Meester-Smoor, M.; Feart, C.; Verzijden, T.; Samieri, C.; et al. Mediterranean Diet and Incidence of Advanced Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmology 2019, 126, 381–390. [Google Scholar] [CrossRef]
- de Koning-Backus, A.P.M.; Buitendijk, G.H.S.; Kiefte-de Jong, J.C.; Colijn, J.M.; Hofman, A.; Vingerling, J.R.; Haverkort, E.B.; Franco, O.H.; Klaver, C.C.W. Intake of Vegetables, Fruit, and Fish is Beneficial for Age-Related Macular Degeneration. Am. J. Ophthalmol. 2019, 198, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.W.; Wang, Y.; Pan, C.W. Metabolomics in Age-Related Macular Degeneration: A Systematic Review. Investig. Ophthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Lains, I.; Kelly, R.S.; Miller, J.B.; Silva, R.; Vavvas, D.G.; Kim, I.K.; Murta, J.N.; Lasky-Su, J.; Miller, J.W.; Husain, D. Human Plasma Metabolomics Study across All Stages of Age-Related Macular Degeneration Identifies Potential Lipid Biomarkers. Ophthalmology 2018, 125, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Lains, I.; Gantner, M.; Murinello, S.; Lasky-Su, J.A.; Miller, J.W.; Friedlander, M.; Husain, D. Metabolomics in the study of retinal health and disease. Prog. Retin. Eye Res. 2019, 69, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.N.; Green, B.D.; Thompson, R.B.; den Hollander, A.I.; Lengyel, I.; Eye-Risk Consortium. Metabolomics and Age-Related Macular Degeneration. Metabolites 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Osborn, M.P.; Park, Y.; Parks, M.B.; Burgess, L.G.; Uppal, K.; Lee, K.; Jones, D.P.; Brantley, M.A., Jr. Metabolome-wide association study of neovascular age-related macular degeneration. PLoS ONE 2013, 8, e72737. [Google Scholar] [CrossRef]
- Kersten, E.; Dammeier, S.; Ajana, S.; Groenewoud, J.M.M.; Codrea, M.; Klose, F.; Lechanteur, Y.T.; Fauser, S.; Ueffing, M.; Delcourt, C.; et al. Metabolomics in serum of patients with non-advanced age-related macular degeneration reveals aberrations in the glutamine pathway. PLoS ONE 2019, 14, e0218457. [Google Scholar] [CrossRef]
- Lambert, V.; Hansen, S.; Schoumacher, M.; Lecomte, J.; Leenders, J.; Hubert, P.; Herfs, M.; Blacher, S.; Carnet, O.; Yip, C.; et al. Pyruvate dehydrogenase kinase/lactate axis: A therapeutic target for neovascular age-related macular degeneration identified by metabolomics. J. Mol. Med. 2020, 98, 1737–1751. [Google Scholar] [CrossRef]
- Lains, I.; Zhu, S.; Han, X.; Chung, W.; Yuan, Q.; Kelly, R.S.; Gil, J.Q.; Katz, R.; Nigalye, A.; Kim, I.K.; et al. Genomic-Metabolomic Associations Support the Role of LIPC and Glycerophospholipids in Age-Related Macular Degeneration. Ophthalmol. Sci. 2021, 1, 100017. [Google Scholar] [CrossRef]
- Bergen, A.A.; Arya, S.; Koster, C.; Pilgrim, M.G.; Wiatrek-Moumoulidis, D.; van der Spek, P.J.; Hauck, S.M.; Boon, C.J.F.; Emri, E.; Stewart, A.J.; et al. On the origin of proteins in human drusen: The meet, greet and stick hypothesis. Prog. Retin. Eye Res. 2019, 70, 55–84. [Google Scholar] [CrossRef]
- Kersten, E.; Paun, C.C.; Schellevis, R.L.; Hoyng, C.B.; Delcourt, C.; Lengyel, I.; Peto, T.; Ueffing, M.; Klaver, C.C.W.; Dammeier, S.; et al. Systemic and ocular fluid compounds as potential biomarkers in age-related macular degeneration. Surv. Ophthalmol. 2018, 63, 9–39. [Google Scholar] [CrossRef]
- Acar, I.E.; Lores-Motta, L.; Colijn, J.M.; Meester-Smoor, M.A.; Verzijden, T.; Cougnard-Gregoire, A.; Ajana, S.; Merle, B.M.J.; de Breuk, A.; Heesterbeek, T.J.; et al. Integrating Metabolomics, Genomics, and Disease Pathways in Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmology 2020, 127, 1693–1709. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, C.; Korobelnik, J.F.; Buitendijk, G.H.; Foster, P.J.; Hammond, C.J.; Piermarocchi, S.; Peto, T.; Jansonius, N.; Mirshahi, A.; Hogg, R.E.; et al. Ophthalmic epidemiology in Europe: The “European Eye Epidemiology” (E3) consortium. Eur. J. Epidemiol. 2016, 31, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Heesterbeek, T.J.; de Jong, E.K.; Acar, I.E.; Groenewoud, J.M.M.; Liefers, B.; Sanchez, C.I.; Peto, T.; Hoyng, C.B.; Pauleikhoff, D.; Hense, H.W.; et al. Genetic risk score has added value over initial clinical grading stage in predicting disease progression in age-related macular degeneration. Sci. Rep. 2019, 9, 6611. [Google Scholar] [CrossRef]
- Biarnes, M.; Colijn, J.M.; Sousa, J.; Ferraro, L.L.; Garcia, M.; Verzijden, T.; Meester-Smoor, M.A.; Delcourt, C.; Klaver, C.C.W.; den Hollander, A.I.; et al. Genotype- and Phenotype-Based Subgroups in Geographic Atrophy Secondary to Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmol. Retin. 2020, 4, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Gattoussi, S.; Buitendijk, G.H.S.; Peto, T.; Leung, I.; Schmitz-Valckenberg, S.; Oishi, A.; Wolf, S.; Deak, G.; Delcourt, C.; Klaver, C.C.W.; et al. The European Eye Epidemiology spectral-domain optical coherence tomography classification of macular diseases for epidemiological studies. Acta Ophthalmol. 2019, 97, 364–371. [Google Scholar] [CrossRef]
- Guymer, R.H.; Rosenfeld, P.J.; Curcio, C.A.; Holz, F.G.; Staurenghi, G.; Freund, K.B.; Schmitz-Valckenberg, S.; Sparrow, J.; Spaide, R.F.; Tufail, A.; et al. Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy in Age-Related Macular Degeneration: Classification of Atrophy Meeting Report 4. Ophthalmology 2020, 127, 394–409. [Google Scholar] [CrossRef]
- Thee, E.F.; Meester-Smoor, M.A.; Luttikhuizen, D.T.; Colijn, J.M.; Enthoven, C.A.; Haarman, A.E.G.; Rizopoulos, D.; Klaver, C.C.W.; EyeNED Reading Center. Performance of Classification Systems for Age-Related Macular Degeneration in the Rotterdam Study. Transl. Vis. Sci. Technol. 2020, 9, 26. [Google Scholar] [CrossRef]
- Han, X.; Ong, J.S.; Hewitt, A.W.; Gharahkhani, P.; MacGregor, S. The effects of eight serum lipid biomarkers on age-related macular degeneration risk: A Mendelian randomization study. Int. J. Epidemiol. 2021, 50, 325–336. [Google Scholar] [CrossRef]
- Lains, I.; Chung, W.; Kelly, R.S.; Gil, J.; Marques, M.; Barreto, P.; Murta, J.N.; Kim, I.K.; Vavvas, D.G.; Miller, J.B.; et al. Human Plasma Metabolomics in Age-Related Macular Degeneration: Meta-Analysis of Two Cohorts. Metabolites 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Lains, I.; Duarte, D.; Barros, A.S.; Martins, A.S.; Carneiro, T.J.; Gil, J.Q.; Miller, J.B.; Marques, M.; Mesquita, T.S.; Barreto, P.; et al. Urine Nuclear Magnetic Resonance (NMR) Metabolomics in Age-Related Macular Degeneration. J. Proteome Res. 2019, 18, 1278–1288. [Google Scholar] [CrossRef]
- Kettunen, J.; Tukiainen, T.; Sarin, A.P.; Ortega-Alonso, A.; Tikkanen, E.; Lyytikainen, L.P.; Kangas, A.J.; Soininen, P.; Wurtz, P.; Silander, K.; et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat. Genet. 2012, 44, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Durante, W. Amino Acids in Circulatory Function and Health. Adv. Exp. Med. Biol. 2020, 1265, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Kalloniatis, M.; Loh, C.S.; Acosta, M.L.; Tomisich, G.; Zhu, Y.; Nivison-Smith, L.; Fletcher, E.L.; Chua, J.; Sun, D.; Arunthavasothy, N. Retinal amino acid neurochemistry in health and disease. Clin. Exp. Optom. 2013, 96, 310–332. [Google Scholar] [CrossRef] [PubMed]
- Adijanto, J.; Du, J.; Moffat, C.; Seifert, E.L.; Hurle, J.B.; Philp, N.J. The retinal pigment epithelium utilizes fatty acids for ketogenesis. J. Biol. Chem. 2014, 289, 20570–20582. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Reveles, J.; Dhingra, A.; Alexander, D.; Bragin, A.; Philp, N.J.; Boesze-Battaglia, K. Phagocytosis-dependent ketogenesis in retinal pigment epithelium. J. Biol. Chem. 2017, 292, 8038–8047. [Google Scholar] [CrossRef]
- Wurtz, P.; Havulinna, A.S.; Soininen, P.; Tynkkynen, T.; Prieto-Merino, D.; Tillin, T.; Ghorbani, A.; Artati, A.; Wang, Q.; Tiainen, M.; et al. Metabolite profiling and cardiovascular event risk: A prospective study of 3 population-based cohorts. Circulation 2015, 131, 774–785. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G. Mendelian Randomization Implicates High-Density Lipoprotein Cholesterol-Associated Mechanisms in Etiology of Age-Related Macular Degeneration. Ophthalmology 2017, 124, 1165–1174. [Google Scholar] [CrossRef]
- Colijn, J.M.; den Hollander, A.I.; Demirkan, A.; Cougnard-Gregoire, A.; Verzijden, T.; Kersten, E.; Meester-Smoor, M.A.; Merle, B.M.J.; Papageorgiou, G.; Ahmad, S.; et al. Increased High-Density Lipoprotein Levels Associated with Age-Related Macular Degeneration: Evidence from the EYE-RISK and European Eye Epidemiology Consortia. Ophthalmology 2019, 126, 393–406. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; DiGirolamo, M.; Tuvlin, J.A.; Isaacs, S.D.; Bhoola, S.M.; Kokko, J.P. Differences in metabolic and hormonal milieu in diabetic- and alcohol-induced ketoacidosis. J. Crit. Care 2000, 15, 52–59. [Google Scholar] [CrossRef]
- Lin, J.B.; Halawa, O.A.; Husain, D.; Miller, J.W.; Vavvas, D.G. Dyslipidemia in age-related macular degeneration. Eye 2022, 36, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Artigas, A.; Wernerman, J.; Arroyo, V.; Vincent, J.L.; Levy, M. Role of albumin in diseases associated with severe systemic inflammation: Pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J. Crit. Care 2016, 33, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.R.; Lee, S.B.; Hong, K.W.; Jung, D.H. C-reactive protein-to-albumin ratio and 8-year incidence of type 2 diabetes: The Korean genome and epidemiology study. Acta Diabetol. 2021, 58, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Newgard, C.B. Integrated metabolomics and genomics: Systems approaches to biomarkers and mechanisms of cardiovascular disease. Circ. Cardiovasc. Genet. 2015, 8, 410–419. [Google Scholar] [CrossRef]
- Scalbert, A.; Brennan, L.; Manach, C.; Andres-Lacueva, C.; Dragsted, L.O.; Draper, J.; Rappaport, S.M.; van der Hooft, J.J.; Wishart, D.S. The food metabolome: A window over dietary exposure. Am. J. Clin. Nutr. 2014, 99, 1286–1308. [Google Scholar] [CrossRef]
| Subjects in the Analysis * (n = 5923) | |||||
|---|---|---|---|---|---|
| Controls (n = 3850) | Early-Intermediate AMD (n = 1114) | Late AMD (n = 959) | p-Value Early-Intermediate AMD | p-Value Late AMD | |
| Baseline age (mean ± SD) | 72.4 ± 7.2 | 74.7 ± 8.1 | 77.7 ± 8.2 | <0.0001 | <0.0001 |
| Sex (% male) | 41.8 | 40 | 41.5 | 0.287 | 0.842 |
| BMI | n = 3526 | n = 970 | n = 805 | ||
| mean ± SD | 26.0 ± 3.6 | 25.5 ± 3.5 | 26.0 ± 3.8 | <0.0001 | 0.772 |
| Smoking | n = 3583 | n = 990 | n = 808 | ||
| Former | 47.5 | 47.1 | 49.6 | 0.317 | 0.07 |
| Current | 14.9 | 11.9 | 15.2 | 0.04 | 0.017 |
| Hypertension | n = 3610 | n = 1011 | n = 878 | ||
| % yes | 43.7 | 41.9 | 34.3 | 0.055 | <0.0001 |
| Diabetes type II | n = 3450 | n = 992 | n = 869 | ||
| % yes | 9.8 | 9.3 | 13 | 0.346 | 0.088 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thee, E.F.; Acar, İ.E.; Colijn, J.M.; Meester-Smoor, M.A.; Verzijden, T.; Baart, S.J.; Jarboui, M.A.; Fauser, S.; Hoyng, C.B.; Ueffing, M.; et al. Systemic Metabolomics in a Framework of Genetics and Lifestyle in Age-Related Macular Degeneration. Metabolites 2023, 13, 701. https://doi.org/10.3390/metabo13060701
Thee EF, Acar İE, Colijn JM, Meester-Smoor MA, Verzijden T, Baart SJ, Jarboui MA, Fauser S, Hoyng CB, Ueffing M, et al. Systemic Metabolomics in a Framework of Genetics and Lifestyle in Age-Related Macular Degeneration. Metabolites. 2023; 13(6):701. https://doi.org/10.3390/metabo13060701
Chicago/Turabian StyleThee, Eric F., İlhan E. Acar, Johanna M. Colijn, Magda A. Meester-Smoor, Timo Verzijden, Sara J. Baart, Mohamed A. Jarboui, Sascha Fauser, Carel B. Hoyng, Marius Ueffing, and et al. 2023. "Systemic Metabolomics in a Framework of Genetics and Lifestyle in Age-Related Macular Degeneration" Metabolites 13, no. 6: 701. https://doi.org/10.3390/metabo13060701
APA StyleThee, E. F., Acar, İ. E., Colijn, J. M., Meester-Smoor, M. A., Verzijden, T., Baart, S. J., Jarboui, M. A., Fauser, S., Hoyng, C. B., Ueffing, M., den Hollander, A. I., & Klaver, C. C. W., for the European Eye Epidemiology Consortium and EYE-RISK Project. (2023). Systemic Metabolomics in a Framework of Genetics and Lifestyle in Age-Related Macular Degeneration. Metabolites, 13(6), 701. https://doi.org/10.3390/metabo13060701






