- Article
CT Radiomics Models Did Not Outperform Experts in Predicting [68Ga]Ga-PSMA-PET Positivity in Prostate Cancer Lymph Node Staging
- Thula Cannon Walter-Rittel,
- Boris Gorodetski and
- Tobias Penzkofer
- + 6 authors
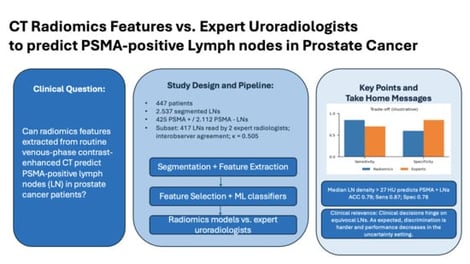
Background: The use of [68Ga]Ga-PSMA-PET/CT for prostate cancer (PCa) staging is limited by cost and availability. This study evaluates whether radiomic features from contrast-enhanced (CE) CT can predict PSMA-positive lymph nodes (LNs) as a surrogate for metastasis. Methods: A retrospective study of 447 patients included 2537 segmented LNs (425 PET-positive, 2112 PET-negative). Two uroradiologists assessed 417 LNs on CE-CT using a four-point Likert scale. Radiomic features were extracted, selected using four algorithms, and analyzed with six model-building methods. Model performance was compared to radiologist ratings. Results: Radiomic models achieved an accuracy of 0.77–0.85, sensitivity of 0.85–0.91, and specificity of 0.74–0.85. Compared to radiologists, models had higher NPV (0.97–0.98 vs. 0.96) and sensitivity (0.85–0.91 vs. 0.76), but radiologists had superior accuracy (0.95 vs. 0.77–0.85) and specificity (0.97–0.98 vs. 0.74–0.85). In a subanalysis of LNs rated as probably benign or malignant, expert radiologists outperformed the algorithm with greater specificity and PPV (p < 0.005). A density threshold of >27 HU predicted PSMA-positive LNs with 0.79 accuracy, 0.87 sensitivity, and 0.78 specificity. Conclusions: While radiomics did not outperform expert radiologists, the single first-order parameter CT density >27 HU was predictive of PSMA-positive LNs. Clinical Relevance Statement: Radiomic models did not outperform expert uroradiologists. However, in high-volume or resource-limited settings lacking access to [68Ga]Ga-PSMA-PET/CT, they may help improve LN assessment in PCa patients with CT alone.
2 March 2026